Stress-Protective Role of Dietary α-Tocopherol Supplementation in Longfin Yellowtail (Seriola rivoliana) Juveniles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rearing, Sampling, and Growth Measurements
2.2. Growth Evaluation and Sampling
2.3. Histological Analyses
2.4. Antioxidant Enzymatic Activities
2.5. Immune System Gene Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Evaluation
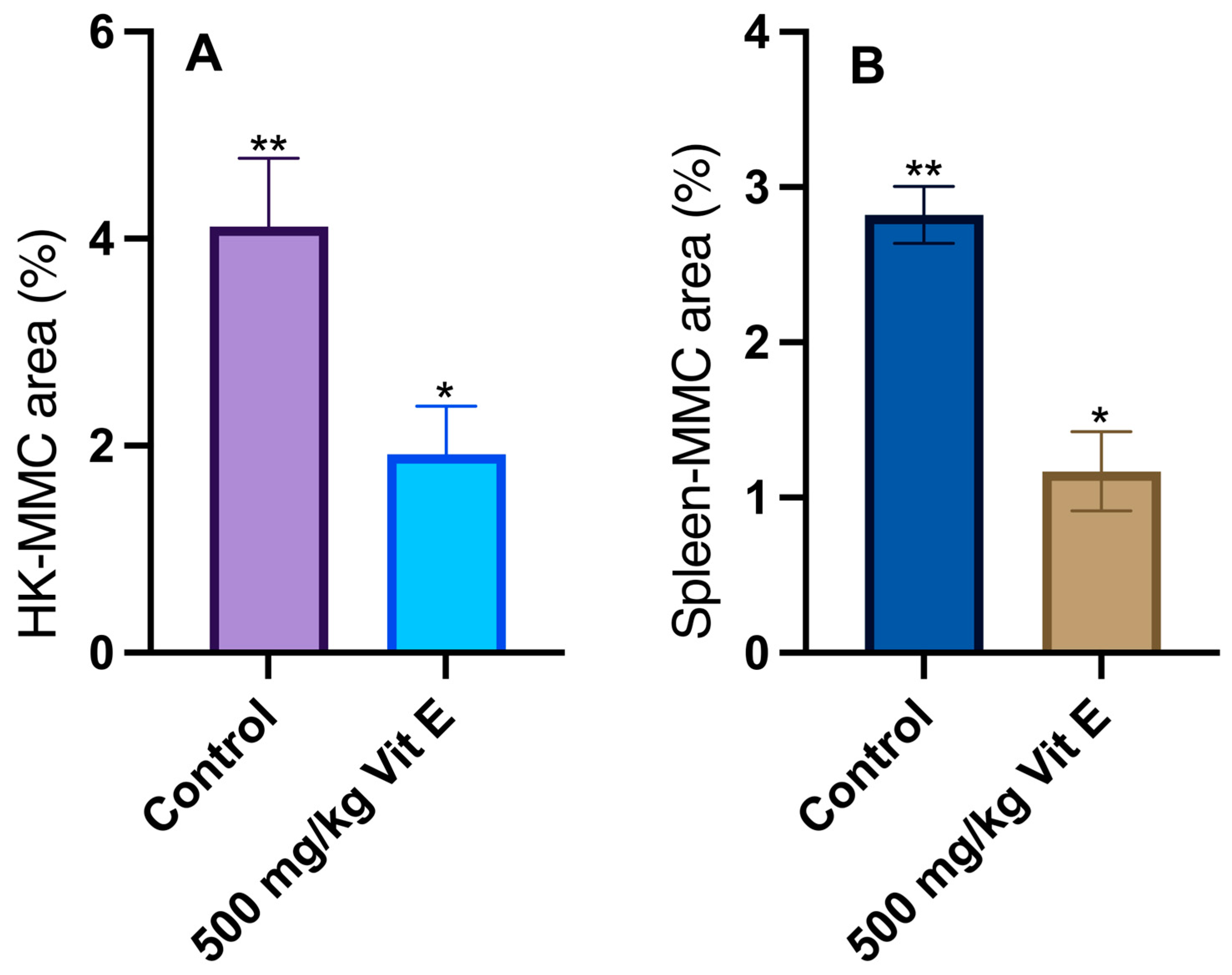
3.2. Histological Analyses
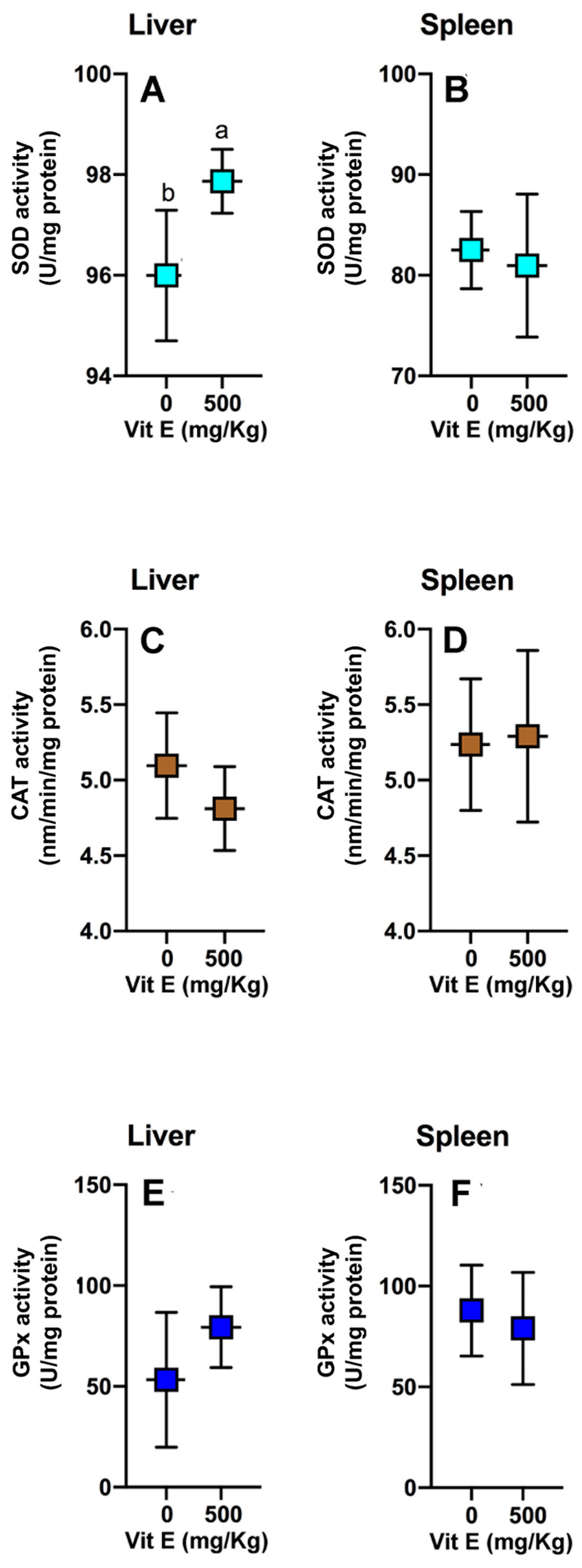
3.3. Antioxidant Enzymatic Activities
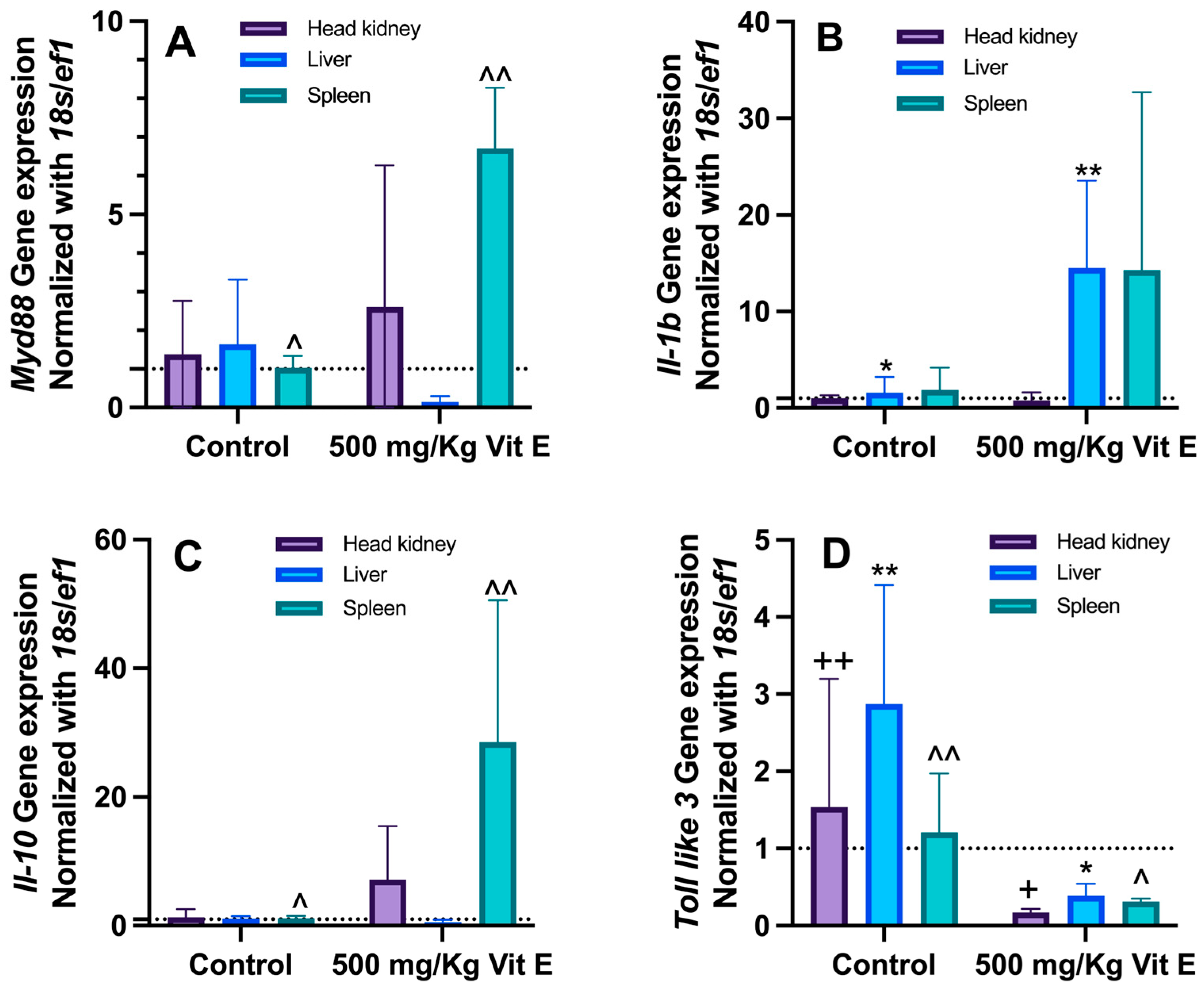
3.4. Immune System Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture: Toward Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Roo, J.; Fernández-Palacios, H.; Hernández-Cruz, C.M.; Mesa-Rodriguez, A.; Schuchardt, M.; Izquierdo, M. First results of spawning and larval rearing of longfin yellowtail Seriola rivoliana as a fast-growing candidate for European marine finfish aquaculture diversification. Aquac. Res. 2014, 45, 689–700. [Google Scholar] [CrossRef]
- Viader-Guerrero, M.; Guzmán-Villanueva, L.T.; Spanopoulos-Zarco, M.; Estrada-Godínez, J.A.; Maldonado-García, D.; Gracia-López, V.; Omont, A.; Maldonado-García, M. Effects of temperature on hatching rate and early larval development of longfin yellowtail Seriola rivoliana. Aquac. Rep. 2021, 21, 100843. [Google Scholar] [CrossRef]
- Blacio, E.; Darquea, J.; Rodríguez, S. Advances in Growing Huayaipe, Seriola rivoliana (Valeciennes 1833) Facilities. Cenaim. El Mundo Acuícola 2003, 9, 21–24. [Google Scholar]
- Laidley, C.W.; Shields, R.J.; Ostrowksi, A.O. Amberjack culture Progress at Oceanic Institute in Hawaii. Glob. Aqua. Advoc. 2004, 7, 42–43. [Google Scholar]
- Mesa-Rodríguez, A.; Hernández-Cruz, C.M.; Socorro, J.A.; Fernández-Palacios, H.; Izquierdo, M.S.; Roo, J. Skeletal Development and Mineralization Pattern of the Vertebral, Column, Dorsal, Anal and Caudal Fin Complex in Seriola rivoliana (Valenciennes, 1833) Larvae. J. Aquac. Res. Develop. 2014, 5, 266. [Google Scholar] [CrossRef]
- Teles, A.; Salas-Leiva, J.; Alvarez-González, C.A.; Gisbert, E.; Ibarra, L.; Pèrez-Urbiola, J.C.; Tovar-Ramìrez, D. Histological study of the gastrointestinal tract in longfin yellowtail (Seriola rivoliana) larvae. Fish. Physiol. Biochem. 2014, 43, 1613–1628. [Google Scholar] [CrossRef]
- Olmos, S.; Paniagua-Michel, J.; Lopez, J.D.J.; Ochoa, L. Functional Feeds in Aquaculture. In Springer Handbook of Marine Biotechnology; Kim, S.K., Ed.; Springer Handbooks; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Arshad, N.; Samat, N.; Lee, L.K. Insight Into the Relation Between Nutritional Benefits of Aquaculture Products and its Consumption Hazards: A Global Viewpoint. Front. Mar. Sci. 2022, 9, 925463. [Google Scholar] [CrossRef]
- Gatlin, D.M., III; Yamamoto, F.Y. Nutritional supplements and fish health. In Fish Nutrition, 4th ed.; Hardy, R.W., Kaushik, S.J., Eds.; Academic Press: Cambridge, MA, USA, 2022; Chapter 11; pp. 745–773. [Google Scholar] [CrossRef]
- Wang, K.; Wang, E.; Qin, Z.; Zhou, Z.; Geng, Y.; Chen, D. Effects of dietary vitamin E deficiency on systematic pathological changes and oxidative stress in fish. Oncotarget 2016, 7, 83869–83879. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radical Res. 2012, 46, 382–419. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Betancor, M. Vitamin E. In Dietary Nutrients, Additives, and Fish Health; Lee, C.S., Lim, C., Gatlin, D.M., III, Webster, C.D., Eds.; Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; Chapter 8; pp. 173–193. [Google Scholar] [CrossRef]
- Schmölz, L.; Birringer, M.; Lorkowski, S.; Wallert, M. Complexity of vitamin E metabolism. World J. Biol. Chem. 2016, 26, 14–43. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Bruno, R.S. Vitamin E. Present Knowledge in Nutrition, 11th ed.; Marriott, B.P., Birt, D.F., Stallings, V.A., Yates, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 7; pp. 115–136. [Google Scholar] [CrossRef]
- Niki, E.; Abe, K. Vitamin E: Chemistry and Nutritional Benefits. In Food Chemistry, Function and Analysis; Niki, E., Ed.; The Royal Society of Chemistry: London, UK, 2019; Chapter 1; pp. 1–11. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M.; Izquierdo, M. The importance of vitamin E for farmed fish—A review. Rev. Aquac. 2021, 14, 688–703. [Google Scholar] [CrossRef]
- Le, K.T.; Fotedar, R.; Partridge, G. Selenium and vitamin E interaction in the nutrition of yellowtail kingfish (Seriola lalandi): Physiological and immune responses. Aquacul. Nutr. 2019, 20, 303–313. [Google Scholar] [CrossRef]
- Pan, J.-H.; Feng, L.; Jiang, W.-D.; Wu, P.; Kuang, S.-Y.; Tang, L.; Zhang, Y.-A.; Zhou, X.-Q.; Liu, Y. Vitamin E deficiency depressed fish growth, disease resistance, and the immunity and structural integrity of immune organs in grass carp (Ctenopharyngodon idella): Referring to NF-κB, TOR and Nrf2 signaling. Fish Shellfish Immunol. 2017, 60, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Armenta-López, G.E.; Sumaya-Martínez, M.T.; Spanopoulos-Hernández, M.; Balois-Morales, R.; Sánchez-Herrera, M.; Jiménez-Ruíz, E. Inclusion of natural antioxidant compounds in fish feeds to counteract oxidative stress. Rev. Bio. Cien. 2015, 3, 68–78. [Google Scholar]
- Liu, H.P.; Wen, B.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.; Zhang, Y.C.; Wang, Z.X.; Peng, Y. Effects of dietary vitamin C and vitamin E on the growth, antioxidant defense and digestive enzyme activities of juvenile discus fish (Symphysodon haraldi). Aquacult. Nutr. 2019, 25, 176–183. [Google Scholar] [CrossRef]
- Welker, T.; Congleton, J.L. Effect of dietary alpha-tocopherol+ascorbic acid, selenium and iron on oxidative stress in sub-yearling Chinook salmon. J. Phys. Nutr. 2009, 93, 15–25. [Google Scholar]
- Naderi, M.; Keyvanshokooh, S.; Salati, A.P.; Ghaedi, A. Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquaculture 2017, 474, 40–47. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hess, D.; Keller, H.E.; Oberlin, B.; Bonfanti, R.; Schüep, W. Simultaneous determination of retinol, tocopherols, carotenes and lycopene in plasma by means of high-performance liquid chromatography on reversed phase. Int. J. Vitam. Nutr. Res. 1991, 61, 232–238. [Google Scholar]
- Rodríguez-Jaramillo, M.C.; Hurtado, M.A.; Romero-Vivas, E.; Ramírez, J.L.; Manzano, J.L.; Palacios, E. Gonadal development and histochemistry of the tropical oyster, Crassostrea corteziensis (Hertlein, 1951) during an annual reproductive cycle. J. Shellfish Res. 2008, 27, 1129–1141. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.W.; Truong, L.; Barton, C.L.; Labut, E.M.; Lebold, K.M.; Traber, M.G.; Tanguay, R.L. The influences of parental diet and vitamin E intake on the embryonic zebrafish transcriptome. Comp. Biochem. Phys. D Genom. Proteom. 2014, 10, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.; Gavaia, P.; Darias, M.J.; Gisbert, E. Fat-soluble vitamins in fish: A transcriptional tissue-specific crosstalk that remains to be unveiled and characterized. In Emerging Issues in Fish Larvae Research; Yúfera, M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 159–208. [Google Scholar]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. Gamma-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 2007, 27, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, A. Absorption, transport and tissue delivery of vitamin E. Mol. Aspects Med. 2007, 28, 423–436. [Google Scholar] [CrossRef]
- Jiang, Q. Metabolism of natural forms of vitamin E and biological actions of vitamin E metabolites. Free Radic. Biol. Med. 2022, 179, 375–387. [Google Scholar] [CrossRef]
- Manor, D.; Morley, S. The alpha-tocopherol transfer protein. Vitam. Horm. 2007, 76, 45–65. [Google Scholar] [CrossRef]
- Combs, D.F.; McClung, J.P. The Vitamins, Fundamental Aspects in Nutrition and Health; Academic Press: London, UK, 2017; ISBN 9780128029831. [Google Scholar]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Tovar-Ramírez, D.; Mazurais, D.; Gatesoupe, J.F.; Quazuguel, P.; Cahu, C.L.; Zambonino-Infante, J.L. Dietary probiotic live yeast modulates antioxidant enzyme activities and gene expression of sea bass (Dicentrarchus labrax) larvae. Aquaculture 2010, 300, 142–147. [Google Scholar] [CrossRef]
- Liu, C.H.; Tseng, M.C.; Cheng, W. Identification and cloning of the antioxidant enzyme, glutathione peroxidase, of white shrimp, Litopenaeus vannamei, and its expression following Vibrio alginolyticus infection. Fish Shellfish Immu. 2007, 23, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Shakouri, M.; Van Doan, H.; Shafiei, S.; Yousefi, M.; Raeisi, M.; Yousefi, S.; Harikrishnan, R.; Reverter, M. Dietary supplementation of lemon verbena (Aloysia citrodora) improved immunity, immune-related genes expression and antioxidant enzymes in rainbow trout (Oncorhyncus mykiss). Fish Shellfish Immun. 2020, 99, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Agius, C.; Roberts, J. Melano-macrophage centers and their role in fish pathology. J. Fish Path. 2003, 26, 499–509. [Google Scholar] [CrossRef]
- Evans, D.; Nowak, B. Effect of ranching time on melanomacrophage centres in anterior kidney and spleen of southern bluefin tuna, Thunnus maccoyii. Fish Shellfish Immu. 2016, 59, 358–364. [Google Scholar] [CrossRef]
- Latoszek, E.; Kamaszewski, M.; Milczarek, K.; Puppel, H.; Adamski, A.; Bury-Burzymski, P.; Ostaszewska, T. Histochemical Characteristics of Macrophages of Butterfly Splitfin Ameca splendens. Folia Biologica 2019, 67, 53–60. [Google Scholar] [CrossRef]
- Steinel, N.C.; Bolnick, D.I. Melanomacrophage Centers As a Histological Indicator of Immune Function in Fish and Other Poikilotherms. Front Immunol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Jordanova, M.; Miteva, N.; Rocha, E. A Qualitative and Quantitative Study of the Hepatic Pigmented Macrophage Aggregates During the Breeding Cycle of Ohrid Trout, Salmo letnica Kar. (Teloestei, Salmonidae). Micros. Res. Technol. 2008, 71, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Blazer, V.S.; Socorro, J.; Izquierdo, M.S.; Tort, L. Dietary and culture influences on macrophage aggregate parameters in gilthead seabream (Sparus aurata) juveniles. Aquaculture 1999, 179, 523–534. [Google Scholar] [CrossRef]
- Caballero, M.J.; Izquierdo, M.S.; Kjørsvik, E.; Fernandez, A.J.; Rosenlund, G. Histological alterations in the liver of sea bream, Sparus aurata L., caused by short- or long-term feeding with vegetable oils. Recovery of normal morphology after feeding fish oil as the sole lipid source. J. Fish Dis. 2004, 27, 531–541. [Google Scholar] [CrossRef]
- Broeg, K. The activity of macrophage aggregates in the liver of flounder (Platichthys flesus) and wrasse (Symphodus melops) is associated with tissue damage. Mar. Environ. Res. 2010, 69, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.H.; Younes, H.A.M. Melanomacrophage centers in Clarias gariepinus as an immunological biomarker for toxicity of silver nanoparticles. J. Microsc. Ultrastruct. 2017, 5, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Dixon, B. Understanding acute stress-mediated immunity in teleost fish. Fish Shellfish Immu. Rep. 2021, 2, 100010. [Google Scholar] [CrossRef]
- Sveen, L.; Karlsen, C.; Ytteborg, E. Mechanical induced wounds in fish—A review on models and healing mechanisms. Rev. Aquac. 2020, 12, 2446–2465. [Google Scholar] [CrossRef]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000 Prime Rep. 2014, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, M.; Zhu, H.; Yang, C.; Xia, H.; Yang, X.; Yang, L.; Sun, G. MyD88 determines the protective effects of fish oil and perilla oil against metabolic disorders and inflammation in adipose tissue from mice fed a high-fat diet. Nutr. Diabetes 2021, 11, 23. [Google Scholar] [CrossRef]
- Palti, Y. Toll-like receptors in bony fish: From genomics to function. Dev. Comp. Immunol. 2011, 35, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Danilova, N.; Wilkes, M.; Bibikova, E.; Youn, M.-Y.; Sakamoto, K.M.; Lin, S. Innate immune system activation in zebrafish and cellular models of Diamond Blackfan Anemia. Sci. Rep. 2018, 8, 5165. [Google Scholar] [CrossRef]
- Atalah, E.; Hernández-Cruz, C.M.; Ganga, R.; Ganuza, E.; Benítez-Santana, T.; Roo, J.; Fernández-Palacios, H.; Izquierdo, M.S. Enhancement of gilthead seabream (Sparus aurata) larval growth by dietary vitamin E in relation to two different levels of essential fatty acids. Aquac. Res. 2012, 43, 1816–1827. [Google Scholar] [CrossRef]
- Ruiz, M.A.; Hernández-Cruz, C.M.; Caballero, M.J.; Fernández-Palacios, H.; Saleh, R.; Izquierdo, M.S.; Betancor, M.B. Appearance of systemic granulomatosis is modulated by the dietary supplementation of vitamin E and C in meagre (Argyrosomus regius) larvae fed inert microdiets. Aquaculture 2019, 506, 139–147. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Wang, L.G.; Wang, H.L.; Wang, T.; Elmada, C.Z.; Xie, F.J. Dietary vitamin E could improve growth performance, lipid peroxidation and non-specific immune responses for juvenile cobia (Rachycentron canadum). Aquac. Nutr. 2013, 19, 421–429. [Google Scholar] [CrossRef]

| Treatment | Proteins * % | Lipids % | Humidity % | Ash % | NFE ** % | ∝-Tocopherol ** |
|---|---|---|---|---|---|---|
| Control | 47.85 | 16.01 | 10.1 | 10.38 | 15.66 | 9.95 |
| 500 mg/kg of ∝-tocopherol | 46.72 | 14.83 | 12.66 | 10.29 | 15.5 | 508.35 |
| Gene Name | Symbol | Primer Sequence (5′-3′) | Amplicon Size (pb) |
|---|---|---|---|
| β-actin | 18s | Fw 5′CTGAACTGGGGCCATGATTAAGAG Rev 5′GGTATCTGATCGTCGTCGAACCTC | 165 |
| Elongation factor 1 | ef1α | Fw 5′ TGGTGTTGGTGAGTTTGAGG 3′ Rev 5′ CGCTCACTTCCTTGGTGATT 3′ | 173 |
| Interleukin-10 receptor subunit beta | il-10 | Fw 5′ACAGTGGTATCAGGGATCCTCA Rev 5′CCGACTGTGTAGGGTATGACTG | 155 |
| Interleukin-1β | il-1b | Fw 5′AGCCAGCAGAGACACTTAG Rev 5′TGGGTAAAGGTGGCAAGTAG | 124 |
| Toll-like receptor 3 | Toll-like 3 | Fw 5′CAAATGTTACCAGATTGCCAAACC 3′ Rev 5′ TTACCATCAGCATCGGGACAAC 3′ | 168 |
| Myeloid differentiation primary response 88 | myd88 | Fw 5′ATGAAGCGACGAAAAACCCC 3′ Rev 5′AAGACTGAAGATCCTCCACAATGTC 3′ | 135 |
| Treatment | W0 (g) | Wf (g) | WG (g) | SGR | FCR | Survival (%) |
|---|---|---|---|---|---|---|
| Control | 3.17 ± 0.53 | 70.80 ± 9.48 | 68.50 ± 1.72 | 2.71 ± 0.02 | 0.72 ± 0.15 | 100 |
| 500 mg/kg of ∝-tocopherol | 3.06 ± 0.45 | 68.81 ± 8.81 | 64.47 ± 1.39 | 2.66 ± 0.02 | 1.36 ± 0.17 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asencio-Alcudia, G.G.; Sepúlveda-Quiroz, C.A.; Pérez-Urbiola, J.C.; Rodríguez-Jaramillo, M.d.C.; Teles, A.; Salas-Leiva, J.S.; Martínez-García, R.; Jiménez-Martínez, L.D.; Galaviz, M.; Tovar-Ramírez, D.; et al. Stress-Protective Role of Dietary α-Tocopherol Supplementation in Longfin Yellowtail (Seriola rivoliana) Juveniles. Fishes 2023, 8, 526. https://doi.org/10.3390/fishes8100526
Asencio-Alcudia GG, Sepúlveda-Quiroz CA, Pérez-Urbiola JC, Rodríguez-Jaramillo MdC, Teles A, Salas-Leiva JS, Martínez-García R, Jiménez-Martínez LD, Galaviz M, Tovar-Ramírez D, et al. Stress-Protective Role of Dietary α-Tocopherol Supplementation in Longfin Yellowtail (Seriola rivoliana) Juveniles. Fishes. 2023; 8(10):526. https://doi.org/10.3390/fishes8100526
Chicago/Turabian StyleAsencio-Alcudia, Gloria Gertrudys, Cesar Antonio Sepúlveda-Quiroz, Juan Carlos Pérez-Urbiola, María del Carmen Rodríguez-Jaramillo, Andressa Teles, Joan Sebastián Salas-Leiva, Rafael Martínez-García, Luis Daniel Jiménez-Martínez, Mario Galaviz, Dariel Tovar-Ramírez, and et al. 2023. "Stress-Protective Role of Dietary α-Tocopherol Supplementation in Longfin Yellowtail (Seriola rivoliana) Juveniles" Fishes 8, no. 10: 526. https://doi.org/10.3390/fishes8100526
APA StyleAsencio-Alcudia, G. G., Sepúlveda-Quiroz, C. A., Pérez-Urbiola, J. C., Rodríguez-Jaramillo, M. d. C., Teles, A., Salas-Leiva, J. S., Martínez-García, R., Jiménez-Martínez, L. D., Galaviz, M., Tovar-Ramírez, D., & Alvarez-González, C. A. (2023). Stress-Protective Role of Dietary α-Tocopherol Supplementation in Longfin Yellowtail (Seriola rivoliana) Juveniles. Fishes, 8(10), 526. https://doi.org/10.3390/fishes8100526








