Abstract
Overnutrition in high-density aquaculture can negatively affect the health of farmed fish. The Chinese herbal medicine Siberian ginseng (Acanthopanax senticosus, AS) can promote animal growth and immunity, and regulate lipid metabolism. Therefore, we conducted an 8-week experiment, in which Oreochromis niloticus was fed with a diet supplemented with different concentrations of AS water extract (ASW) (0‰, 0.1‰, 0.2‰, 0.4‰, 0.8‰, and 1.6‰). The ASW improved the growth performance and increased the specific growth rate (SGR). Linear regression analysis based on the SGR estimated that the optimal ASW amount was 0.74‰. Dietary supplementation with 0.4–0.8‰ ASW reduced the triglyceride and total cholesterol levels in the serum and liver, and regulated lipid transport by increasing the high-density lipoprotein cholesterol concentration and lowering the low-density lipoprotein cholesterol concentration. Dietary supplementation with ASW increased the activities of superoxide dismutase and catalase in the liver, thereby improving the antioxidant capacity. Moreover, ASW modulated the transcription of genes in the peroxisome proliferator-activated receptor signaling pathway in the liver (upregulation of PPARα, APOA1b, and FABP10a and downregulation of PPARγ), thereby regulating fatty acid synthesis and metabolism and slowing fat deposition. These results showed that 0.4–0.8‰ ASW can slow fat deposition and protected the liver from cell damage and abnormal lipid metabolism.
1. Introduction
As essential nutrients, lipids play an important physiological role in maintaining the normal function of the body, promoting fish growth, and improving the survival rate [1,2]. In addition, they comprise the main structure of biofilms. Moreover, they serve as carriers of fat-soluble vitamins and as cofactors of hormones and enzymes [3,4]. However, in recent years, with the development of intensive farming models, excessive energy intake [5] and environmental pollution [6] at high densities can result in excessive fat deposition. This can eventually cause fatty liver disease, which damages liver cells and affects liver metabolism, leading to slow growth and weakened immunity of farmed fish [7,8,9]. Therefore, the excessive deposition of fish fat is one of the urgent problems to be solved in aquaculture.
Tilapia is one of the main freshwater farmed fish in China, with a production of 1,655,410 tons in 2020 [10]. Genetically improved farmed tilapia (Oreochromis niloticus, GIFT) is bred from selected tilapia populations from African and Asian strains [11]. Owing to its excellent growth performance and disease resistance, it has extremely high market value and broad market potential [12,13], and currently accounts for nearly 75% of the total farmed tilapia in China. Therefore, from both economic and biological perspectives, it is particularly important to better understand the potential mechanism of fatty liver in GIFT to develop prevention or treatment strategies against excessive fatty deposits.
Lipid metabolism research can no longer be simply based on experimental observations and descriptions; however, it should involve genomic data in-depth analysis. The peroxisome proliferator-activated receptor (PPAR) signal transduction pathway is one of the main pathways of lipid metabolism. It has a wide range of biological effects in animals, and regulates important physiological processes, such as lipid metabolism, energy homeostasis, and cell division and differentiation [14]. At present, studies on PPARα and PPARγ in fish are mainly focused on lipid metabolism. PPARα regulates lipid metabolism by regulating target genes encoding enzymes involved in peroxisome and mitochondrial fatty acid beta-oxidation [15,16]. PPARγ is a superfamily of nuclear receptors that regulate lipid metabolism in adipose tissue and control the differentiation, proliferation, and adipogenesis of adipocytes [17,18]. The regulatory effects of PPARα and PPARγ on lipid metabolism have been verified in zebrafish (Danio rerio) [19] and large yellow croaker (Pseudosciaena crocea R.) [20]. Apolipoprotein A1 (ApoA1) is the main protein component of high-density lipoprotein (HDL). In an animal model study, it was found that the body fat content of ApoA1-knockout mice was significantly higher than normal mice [21]. Fatty acid-binding protein (FABP) belongs to the family of intracellular lipid-binding proteins (ILBPs) and is mainly involved in the transport of fatty acids and in fat metabolism in cells [22]. FABP can specifically bind to intracellular fatty acids and reduce the cell membrane damage caused by unsaturated fatty acids.
Traditional Chinese herbal medicines have considerable potential as aquafeed additives since they are natural products, innocuous, easy to prepare, inexpensive, and have few side effects for the fish or the environment [23]. Acanthopanax senticosus (AS) is a typical oriental folk medicinal herb, and several studies have shown that it is rich in the nutrients and trace elements that are required by animals, as well as a variety of active ingredients, such as polysaccharides, saponins, and flavonoids [24,25]. This herb has been demonstrated to have a positive effect on animal growth [26], improve antioxidant capacity [27], enhance immunity [28], treat inflammation [29,30], and regulate lipid metabolism [31]. The physiological role of AS in lipid metabolism has been shown by many experimental studies. For example, dietary supplementation of AS saponins alleviated fatty liver changes caused by a high-fat diet in mice [32]. Ethanol extracts from the stem bark of AS enhanced the insulin effect in the liver, reduced the synthesis of triglycerides, and improved liver steatosis in mice on a high-fat diet [31]. A metabolomics analysis of mice with Parkinson’s disease showed that the therapeutic effect of AS extract may involve regulating metabolic pathways, such as mitochondrial β-oxidation of long chain saturated fatty acids and fatty acid metabolism [33]. Therefore, in this study, GIFT was fed with diets containing different concentrations of AS water extract (ASW), and then its effects were determined on biochemical and physiological parameters and on the transcript levels of lipid metabolism-related genes in the PPAR pathway (PPARα, PPARγ, APOA1b, and FABP10a).
To explore the effects of ASW on fat deposition, serum biochemical parameters and liver enzyme activities were measured under different levels of ASW supplementation. In addition, differences in lipid metabolism-related gene transcript levels among the different ASW supplementation groups were detected using quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis. We aimed to explore the method in which ASW regulates lipid metabolism in GIFT at the physiological and molecular levels, and to provide a theoretical basis for the application of ASW as a green feed additive.
2. Materials and Methods
2.1. Ethics Statement
The study protocols were approved by the Ethics Committee at the Freshwater Fisheries Research Centre of the Chinese Academy of Fishery Sciences (FFRC, Wuxi, China). All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals in China.
2.2. Experimental Diets
In Saito’s research [34], 0.5‰ and 1.0‰ AS fruit supplementation improved obesity-associated insulin resistance and hepatic lipid accumulation in high-fat diet-fed mice. Therefore, in this study, six groups supplemented with 0‰, 0.1‰, 0.2‰, 0.4‰, 0.8‰, and 1.6‰ ASW were prepared (Table 1). We used commercially available ASW, which was provided by the Beijing Yujing Biotechnology Co., Ltd. (Beijing, China). The feed preparation method was in accordance with Tao, Qiang [35]. The nutrient composition of the experimental diets was determined using previously published methods [36,37]. The moisture content was determined by weighing the samples, drying them at 105 °C for 24 h, and then reweighing them. The crude protein content was determined using the Kjeldahl method after acid digestion. In addition, the crude lipid content was determined by the ether extraction method. Moreover, the ash content was determined by combusting the dry samples in a muffle furnace (Thermolyne Corporation, Dubuque, IA, USA) at 550 °C for 6 h, and then weighing the residue.

Table 1.
Feed formulae of experimental diets (containing 0‰, 0.1‰, 0.2‰, 0.4‰, 0.8‰, and 1.6‰ ASW).
2.3. Experimental Facility and Fish Rearing
The experimental GIFT juveniles were selected from the Yixing experimental base of the Freshwater Fisheries Research Center. Juveniles were acclimated in an aerated flow-through system and fed with commercial feed for 1 week. The breeding experiment was carried out in a circulating water system. A total of 540 healthy GIFT juveniles of similar size (2.00 ± 0.02 g) were selected and randomly divided into six groups, each with three replicate tanks (30 fish/tank). The tanks were filled with 600 L of dechlorinated freshwater. The fish were fed with experimental feed twice per day (08:00 and 17:00) to apparent satiation for 56 days. During the entire experimental period, the water temperature was maintained at 28 ± 0.5 °C, the pH was 7.4, and dissolved oxygen was ≥5 mg/L. A third of the water was exchanged every 3 days.
2.4. Sample Collection
Following 56 days of feeding, the fish were starved for 24 h. Then, they were quickly and deeply anesthetized with 100 mg/L MS-222 (Argent Chemical Laboratories, Redmond, WA, USA) and the number and total weight of all fish in each tank were recorded. Three fish were randomly captured from each tank and blood samples were taken from the tail vein and divided into two parts, one for blood cell determination and the other, which was for serum analysis, was immediately centrifuged as described by Ma, Qiang [38]. The serum was stored at −80 °C for further biochemical analyses. Three fish per tank were weighed and dissected, and then their liver tissues were weighed. In addition, nine fish were picked from six groups, and the liver tissues were collected, quickly frozen in liquid nitrogen, and stored at −80 °C until analysis. The liver samples were separated into three portions, one was used to determine physiological indexes, one was used for Oil Red O staining for section examination, and one for RNA extraction.
2.5. Fish Growth Performance
Differences in the growth performance of GIFT among the six groups were determined by comparing the initial body weight (IBW), final body weight (FBW), specific growth rate (SGR), and hepatosomatic index (HSI). During feeding, the feed consumption was calculated daily and the number of dead fish was recorded. The feed conversion ratio (FCR) was calculated as an indicator of feed use. These parameters were calculated as described elsewhere [39].
2.6. Blood Biochemical Analysis
Red blood cells (RBC, 1012/L), white blood cells (WBC, 109/L), hemoglobin concentration (Hb, g/L), and hematocrit levels (Ht, %) were determined using an automatic blood cell analyzer (bc-5300, MINDRAY, Shenzhen, China), in accordance with the instructions of each test kit. A fully automatic biochemical analyzer (bs-400, MINDRAY, Shenzhen, China) was used to measure serum total protein (TP), triglyceride (TG), total cholesterol (TC), glucose, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) contents, as well as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities. All test kits were purchased from MINDRAY.
2.7. Hepatic Biochemical Analysis
Liver samples (about 1.0 g) were homogenized in ice-cold phosphate-buffered saline (PBS, 50 mmol/L, pH 7.3), and then centrifuged for 15 min at 5000× g at 4 °C [39]. Liver TG, TC, malondialdehyde (MDA), free fatty acid (FFA) concentrations, and superoxide dismutase (SOD) and catalase (CAT) activities were measured using enzyme-linked immunosorbent assay (ELISA) kits. All kits were purchased from Shanghai Lengton Bioscience Co., Ltd. (Shanghai, China).
2.8. Liver Tissue Sections
The preparation and analysis of liver sections was conducted as described previously [35]. Liver samples collected from fish were washed with physiological saline. The samples were flash-frozen in liquid nitrogen, and then cut into 10-mm frozen sections using a freezing microtome (Model 3050S, Leica, Wetzlar, Germany). The sections were stained with Oil Red O solution for 15 min, rinsed with distilled water for 30 s, counterstained with Mayer’s hematoxylin for 3 min, and then viewed and photographed under an optical microscope. Images were analyzed using Image-Pro plus (v6.0.0.260, Media Cybernetics Corporation, Silver Spring, MD, USA), and at least six different microscope fields per group were analyzed to calculate the area of lipid droplets. The reagents used for histological staining were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.9. Quantitative Reverse Transcription PCR Analyses of Lipid Metabolism-Related Genes
We selected four genes related to lipid metabolism in the PPAR signaling pathway for qRT-PCR analysis: PPARα, PPARγ, APOA1b, and FABP10a. All primers used to amplify the genes (Table 2) were synthesized by Suzhou GeneWiz Biotechnology Co., Ltd. (Suzhou, China). Total RNA was extracted from liver samples using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and was reverse transcribed into cDNA using Prime Script™ RT Master Mix (Takara, Dalian, China). The qRT-PCR analyses were performed on a CFX96™ Real-time PCR System (Bio-Rad, Hercules, CA, USA) in accordance with the instructions of the SYBR® Premix Ex Taq kit (Takara). The internal reference gene was β-actin. Each PCR mixture (25 μL) contained 8 μL of RNase-free water, 12.5 μL of SYBR Premix Ex Taq II (2×), 0.5 μL of ROX Dye (50×), 2 μL of forward and reverse primers (10 μM), and 2 μL of cDNA working solution. The amplification conditions were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s [40]. Each reaction was repeated three times. The relative gene transcript levels in the different treatments were calculated using the 2−ΔΔCT method [41].

Table 2.
Sequences of primers used for qRT-PCR.
2.10. Data Analysis
The results are reported as mean ± standard error of the mean (SEM). The Shapiro-Wilk test and the Levene test were used to analyze the data results for normal distribution and homogeneity of variance. One-way analysis of variance (ANOVA) with post-hoc Duncan’s multiple range test was used to determine the significance level among the groups. p < 0.05 was considered significant. Statistical analyses were conducted using SPSS v22.0 (IBM Corp., Armonk, NY, USA). The relationship between SGR and dietary ASW levels was analyzed using a two-slope broken-line model [42].
3. Results
3.1. Growth Performance, FCR, and Survival
As shown in Table 3 and Figure 1, the FBW and SGR of the 0.4–0.8‰ ASW treatment groups were significantly higher than those of the control group, with the 0.8‰ ASW group showing the highest performance (p < 0.05). Compared with the control, the 0.4–0.8‰ ASW treatment groups showed significantly lower HSI levels (p < 0.05). However, ASW supplementation did not significantly affect the FCR or survival rate of GIFT (p > 0.05).

Table 3.
Effect of ASW on growth performance of GIFT (mean ± SEM).
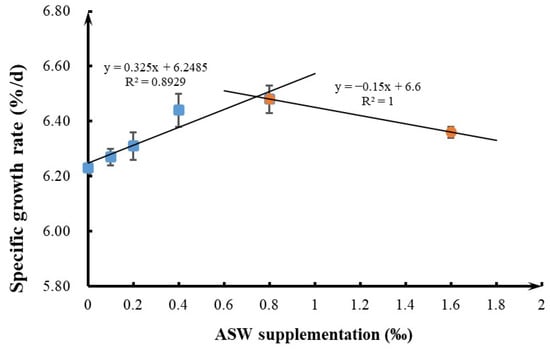
Figure 1.
Relationship between specific growth rate and dietary supplementation with ASW (mean ± SEM). Relationship was determined using a two-slope broken-line model [42].
3.2. Hematological Indexes
The WBC count differed significantly between the control group and the GIFT fed with 0.4–0.8‰ ASW supplementation, with the highest performance observed in the 0.8‰ diet group (p < 0.05; Table 4). However, we found that the RBC, Hb, and Ht were not significantly different between the treatment groups and the control group (p > 0.05).

Table 4.
Effect of ASW on blood parameters of GIFT (mean ± SEM).
3.3. Serum Biochemical Parameters
Dietary supplementation with ASW did not significantly affect the TP and Glu contents in the serum of GIFT (p > 0.05) (Table 5). However, the TG and TC levels significantly decreased with the increasing dietary ASW in a dose-dependent manner. The TG and TC levels were significantly lower in the 0.4–0.8‰ treatment groups than the control group (p < 0.05). Similarly, ALT and AST activities were significantly lower in the 0.4–0.8‰ treatment groups (p < 0.05) than the control. The HDL-C and LDL-C contents showed opposite trends. To be specific, compared with the control group, the groups supplemented with 0.2–1.6‰ ASW showed significantly increased HDL-C contents and significantly decreased LDL-C contents (p < 0.05).

Table 5.
Effect of ASW on serum biochemical parameters of GIFT (mean ± SEM).
3.4. Hepatic Biochemical Parameters
There were significant differences in liver TG and TC levels between the control group and the 0.4–0.8‰ ASW treatments groups, with the lowest TG and TC levels in the 0.8‰ treatment group (p < 0.05) (Table 6). In addition, compared with the control group and the 0.1‰, 0.2‰, and 1.6‰ ASW treatment groups, the 0.4–0.8‰ ASW treatment groups showed significantly decreased FFA levels in the liver (p < 0.05). The activities of CAT and SOD in the GIFT liver were significantly higher in the 0.4–0.8‰ treatment groups than the other groups and the control (p < 0.05). The liver MDA content was significantly lower in the 0.4–0.8‰ ASW treatment groups than the control group (p < 0.05).

Table 6.
Effect of ASW on liver lipids and antioxidant enzymes in GIFT (mean ± SEM).
3.5. Histological Structure of Liver
The lipid droplets area stained with Oil Red O occupied almost the entire area of the liver section of the GIFT in the control group (Figure 2A), whereas it did not in the fish supplemented with ASW in the diet (Figure 2B,C), with the 0.4‰ supplementation group showing the least lipid droplets (Table 7).
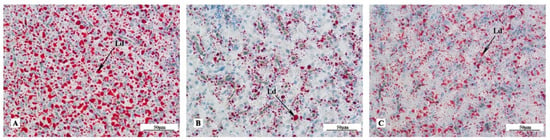
Figure 2.
Oil Red O staining of liver tissues of GIFT fed with different ASW concentrations. Magnification 200×. (A) Fish fed the diet with no ASW; (B) fish fed the diet with 0.4‰ ASW; (C) fish fed the diet with 1.6‰ ASW. Ld: Lipid droplet.

Table 7.
Effect of ASW on the area of hepatic lipid droplets in GIFT (mean ± SEM).
3.6. Expression Level of Lipid Metabolism Genes in the PPAR Signaling Pathway
As shown in Figure 3, ASW at a low concentration (0–0.2 and 1.6‰) did not have a significant effect on lipid metabolism-related genes in the PPAR signaling pathway (p > 0.05); however, the expression level of PPARα, PPARγ, APOA1b, and FABP10a mRNA under 0.4–0.8‰ ASW supplementation was significantly changed (p < 0.05). Compared with the control group, the expression levels of PPARα, APOA1b, and FABP10a mRNA in the GIFT liver with 0.4–0.8‰ ASW added to the diet were significantly increased (p < 0.05). Among these genes, the expression of PPARα, APOA1b, and FABP10a was the highest in the 0.4‰ ASW group. In addition, compared with the control, in the 0.4–0.8‰ ASW diet groups, the PPARγ mRNA expression level was significantly downregulated (p < 0.05), with the lowest expression observed in the 0.4‰ supplementation group.
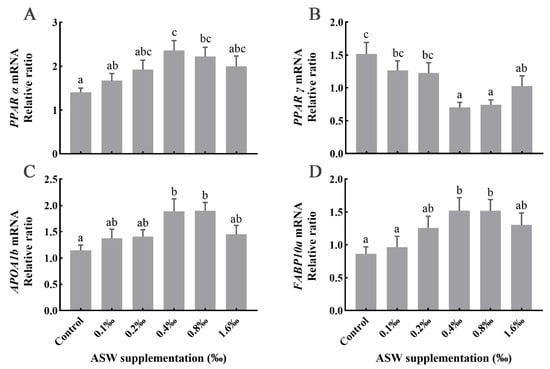
Figure 3.
Expression levels of (A) PPARα; (B) PPARγ; (C) APOA1b; and (D) FABP10a mRNA in the liver of GIFT fed with different ASW concentrations. Different superscript lowercase letters show a significant difference (p < 0.05).
4. Discussion
Chinese herbal medicines are rich in a variety of nutritional elements and biologically active substances, which can promote the synthesis of proteins and enzymes, accelerate the absorption and use of nutrients, stimulate the metabolism of animals, and thus be beneficial to animal growth [43,44]. In recent years, AS has been proven to play an active role in the growth of animals, such as yellow catfish (Pelteobagrus fulvidraco) [45], swamp eels (Monopterus albus) [46], weaned piglets [26], and broiler chickens [47]. In this study, we found that adding 0.4–0.8‰ ASW to the diet significantly promoted the growth of GIFT, and that 0.74‰ of ASW was the optimum concentration.
The fish blood composition is affected by metabolism, nutritional status, and immune response. The levels of various parameters, such as RBC, WBC, Hb, and Ht are considered to be effective physiological indicators, and thus are commonly used to evaluate fish nutrition, health, and the stress response to the environment [48,49]. We found that supplementation of 0.4‰ and 0.8‰ ASW significantly increased the WBC level in GIFT, which includes granulocytes, monocytes, and lymphocytes that are closely related to the fish immunity [50]. Therefore, we considered that the fish in these two groups may have better non-specific immunity.
As an important metabolic transport system, the blood participates in the regulation of fish lipid metabolism. The levels of TG and TC in the serum reflect the fat metabolism [51]. Fu, Fu [52], Li, Qiang [53], and Nishida, Kondo [54] have reported that AS reduced the concentration of TC and TG in animals, and improved lipid metabolism in the blood. This is consistent with our results that the serum TG and TC concentrations were significantly reduced in the 0.4–0.8‰ ASW treatment groups. In addition, we observed that the 0.4‰ ASW group had the highest HDL-C concentration, which was significantly higher than the control group, but the LDL-C concentration was the lowest in 0.8‰ ASW group. Relevant studies have shown that HDL-C transports excess blood lipids to the liver, which are excreted after processing and decomposing [55]. When fish consume excessive energy, high concentrations of fatty acids will lead to a decrease in HDL-C content [56]. LDL-C transports lipids from the liver to other tissues; when the LDL-C content is too high, the lipids accumulate on the arterial wall, which can easily cause arteriosclerosis and vascular damage [57]. Therefore, we speculated that dietary supplementation with 0.4–0.8‰ ASW can regulate the lipid transport in fish by affecting the concentration of lipoproteins, thereby improving lipid metabolism in the blood. Previous studies have shown that AS extracts have a hypoglycemic effect [58,59]. In our study, the serum Glu content did not differ significantly between the ASW treatment groups and the control group, but there was a decreasing trend with the lowest Glu content in the 0.8‰ ASW group.
The liver is an important organ for lipid synthesis and storage [60]. In this study, the liver FFAs concentration in GIFT in the control group was the highest and significantly higher than the 0.4–0.8‰ ASW treatment groups. This finding is consistent with the results of the increase in the serum HDL-C content and the decrease in the serum LDL-C content in the ASW supplementation groups. Furthermore, studies have shown that a high concentration of FFAs in the liver may cause sever cytotoxicity and lead to liver cell damage and the induction of abnormal lipid metabolism [61]. We found that adding 0.4–0.8‰ ASW to the diet significantly reduced the HSI in GIFT. In this case, high levels of HSI represent liver fat deposition and liver cell damage. The liver TG and TC concentrations were similar to those in the serum, and significantly decreased under 0.4–0.8‰ ASW supplementation. These results indicate that ASW reduced lipid synthesis, increased catabolism by regulating lipid transport, and reduced fat deposition in the body, and thereby improved liver steatosis. In addition, histopathological analysis further confirmed the lipid-lowering effect of ASW in GIFT. The group of fish that did not receive ASW clearly showed fat deposition in the liver, and the Oil Red O staining showed that almost all of the cytoplasm in the control group was filled with red lipid droplets, whereas the liver fat deposition was significantly improved under 0.4‰ ASW supplementation, which is similar to the results reported in mice [54].
During the formation of a fatty liver, the massive production of active oxygen destroys the antioxidant defense system and oxidative stress occurs [62]. Studies have shown that several oxygen free radicals, such as superoxide and hydroxyl radicals, as well as hydrogen peroxide (H2O2), caused oxidative stress in the body, which may lead to structural alterations in the cellular and intracellular organelle membranes [63]. SOD and CAT are the first line of enzymatic antioxidant defense. In this study, the activities of liver SOD and CAT enzymes were significantly increased by 0.4–0.8‰ ASW addition. Therefore, we believe that ASW can significantly increase the activity of antioxidant enzymes in GIFT liver to maintain the intracellular redox balance, and thus protect the body from oxidative stress. Consistently, the MDA content, which is the end product of lipid peroxidation and an indirect indicator of the degree of cell damage [64], was lower in the 0.4–0.8‰ ASW treatment groups than the control group.
PPARs are members of the nuclear hormone receptor superfamily, which can be activated by various xenobiotics and natural fatty acids [65]. This pathway regulates fatty acid synthesis and metabolism, adipocyte proliferation and differentiation, and inflammation through specific target genes [66]. PPARs play an important role in lipid metabolism and homeostasis. Therefore, to further examine the effect of ASW on the regulation of GIFT lipid metabolism, we selected four genes related to lipid metabolism in the PPARs signal transduction pathways for qRT-PCR analysis. PPARα is highly expressed in liver cells, integrates carbohydrate and lipid signals in liver cells, and stimulates the transcription of genes essential for fatty acid oxidation [67]. The lipid-lowering effect of PPARα activation has been confirmed in carp (Cyprinus carpio) [68], yellow catfish [69], and Nile tilapia (Oreochromis niloticus) [70]. PPARγ is a ligand-activated transcription factor that plays a key role in controlling the expression of genes related to various physiological processes. Adipocytes are the core cells that control the energy balance and systemic lipid homeostasis. When preadipocytes differentiate into adipocytes, PPARγ is induced and highly expressed in white adipose tissue [71], resulting in the accumulation of TG droplets [72]. Studies have shown that PPARγ mutants with dominant-negative activity inhibit adipogenesis in cultured preadipocytes [73]. In our study, the liver PPARα mRNA expression in GIFT was significantly upregulated by 0.4–0.8‰ ASW supplementation, which stimulated the oxidative metabolism of lipids in the liver. In contrast, the PPARγ gene was significantly downregulated, indicating that ASW effectively reduced adipogenesis and lipid droplet accumulation.
Growth and energy transfer depend on the efficient transport of lipid molecules between tissues and cells. FABPs are a class of small molecule proteins, whose physiological functions include the uptake and use of fatty acids, the metabolism of fatty acids, and protection of cell structures from the effects of fatty acids [74,75]. FABP10 is specifically expressed in the liver [76], and the ablation of this gene greatly induces the accumulation of liver TC and TG, as well as weight gain [77]. We found that the expression level of FABP10a mRNA in GIFT that did not receive an ASW diet was significantly lower than the 0.4–0.8‰ ASW groups. The control fish showed severe fat deposition, which was consistent with the above biochemical indicators and liver histology results. Apolipoprotein is an important part of plasma lipoproteins, which are involved in the regulation of enzyme activity, fat transport, absorption, and energy storage [78]. ApoA1 is considered as the main protein in HDL, and plays an important role in reversing TC transport by extracting TC and phospholipids from various cell types, and then transferring them to the liver [79]. In our study, compared with the control group, the 0.4–0.8‰ ASW treatment groups had higher transcript levels of APOA1b, indicating that ASW can promote the transcription of APOA1b to regulate fat transport.
5. Conclusions
The Chinese herbal medicine Siberian ginseng (Acanthopanax senticosus, AS) contains a variety of nutrients and active ingredients. In this study, we found that when feeding the genetically improved farmed tilapia (Oreochromis niloticus, GIFT) with different AS water extract (ASW) concentrations, 0.4–0.8‰ of ASW effectively promoted the growth of GIFT, reduced the levels of triglyceride (TG) and total cholesterol (TC) in the blood and liver, adjusted lipid transport by changing the lipoproteins’ concentrations, as well as modulated the transcription of specific target genes in the PPAR signaling pathways to regulate fatty acid synthesis and metabolism and reduce the size of the liver. In addition, certain ASW concentrations reduced the concentration of malondialdehyde (MDA), and enhanced the activity of antioxidant enzymes in the liver, thereby improving antioxidant capacity and reducing stress hazards. Therefore, based on the current research, aiming at the high-lipid load of overnutrition under intensive aquaculture, the addition of 0.4–0.8‰ ASW to the diet of GIFT may effectively slow down fat deposition and protect the liver from cell damage and abnormal lipid metabolism (Figure 4).
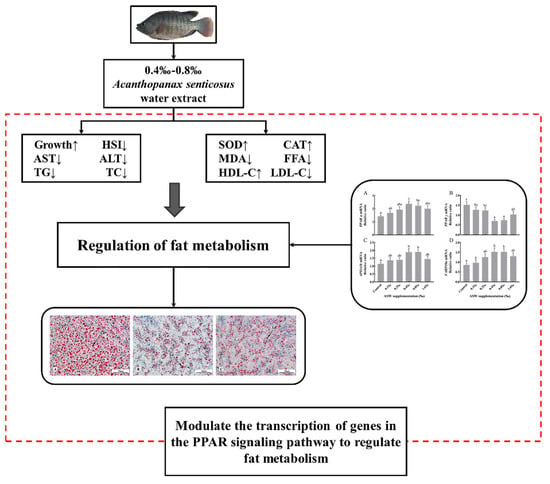
Figure 4.
Pathways related to growth performance and fat metabolism in GIFT (Oreochromis niloticus) potentially affected by ASW supplementation in the diet.
Author Contributions
J.Q. and X.Z. conceived and designed the experiments; M.L., J.Q., X.Z., J.B., Y.T. and H.Z. performed the experiments; M.L. and J.B. analyzed the data; M.L. wrote the paper with contributions from J.Q., X.Z., J.B., Y.T. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Guangdong South China Sea Key Laboratory of Aquaculture for Aquatic Economic Animals, Guangdong Ocean University (No. KFKT2019ZD06).
Institutional Review Board Statement
The study protocols were approved by the Ethics Committee at the Freshwater Fisheries Research Centre of the Chinese Academy of Fishery Sciences (FFRC, Wuxi, China). All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals in China.
Acknowledgments
We thank Michal Bell and Jennifer Smith from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac, accessed on 13 June 2022) for editing the English text of a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, L.; Zhu, X.; Yu, X.; Huyan, Z.; Wang, X. Rapid and Simultaneous Determination of the Iodine Value and Saponification Number of Edible Oils by FTIR Spectroscopy. Eur. J. Lipid Sci. Technol. 2018, 120, 1700396. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Ding, Z. Polyunsaturated Fatty Acid Supplements Could Considerably Promote the Breeding Performance of Carp. Eur. J. Lipid Sci. Technol. 2017, 119, 1600183. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, X.; Li, S.; Li, W.; Zhu, D. Effects of Dietary Lipid Levels on Growth, Feed Utilization, Body Composition, Fatty Acid Profiles and Antioxidant Parameters of Juvenile Chu’s Croaker Nibea Coibor. Aquacult. Int. 2016, 24, 1229–1245. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, X.; Li, S.; Zhu, D.; Li, Y. Effects of Dietary Lipid Levels on Growth, Feed Utilization, Body Composition and Antioxidants of Juvenile Mud Crab Scylla paramamosain (Estampador). Aquaculture 2015, 435, 200–206. [Google Scholar] [CrossRef]
- Du, Z.Y.; Liu, Y.J.; Tian, L.X.; He, J.G.; Cao, J.M.; Liang, G.Y. The Influence of Feeding Rate on Growth, Feed Efficiency and Body Composition of Juvenile Grass Carp (Ctenopharyngodon idella). Aquacult. Int. 2006, 14, 247–257. [Google Scholar] [CrossRef]
- Chen, Q.L.; Luo, Z.; Liu, X.; Song, Y.F.; Liu, C.X.; Zheng, J.L.; Zhao, Y.-H. Effects of Waterborne Chronic Copper Exposure on Hepatic Lipid Metabolism and Metal-Element Composition in Synechogobius hasta. Arch. Environ. Contam. Toxicol. 2013, 64, 301–315. [Google Scholar] [CrossRef]
- Du, Z.Y.; Clouet, P.; Zheng, W.H.; Degrace, P.; Tian, L.X.; Liu, Y.J. Biochemical Hepatic Alterations and Body Lipid Composition in the Herbivorous Grass Carp (Ctenopharyngodon idella) Fed High-Fat Diets. Br. J. Nutr. 2006, 95, 905–915. [Google Scholar] [CrossRef]
- Lu, K.; Xu, W.; Li, J.; Li, X.; Huang, G.; Liu, W. Alterations of Liver Histology and Blood Biochemistry in Blunt Snout Bream Megalobrama amblycephala Fed High-Fat Diets. Fish Sci. 2013, 79, 661–671. [Google Scholar] [CrossRef]
- Li, D.; Liu, L. Introduction to diagnosis and treatment of fish fatty liver disease. Prog. Vet. Med. 2016, 37, 114–117. [Google Scholar] [CrossRef]
- China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2021.
- Tendencia, E.A.; Fermin, A.C.; dela Peña, M.R.; Choresca, C.H. Effect of Epinephelus coioides, Chanos, and GIFT Tilapia in Polyculture with Penaeus monodon on the Growth of the Luminous Bacteria Vibrio harveyi. Aquaculture 2006, 253, 48–56. [Google Scholar] [CrossRef]
- Qiang, J.; Yang, H.; Wang, H.; Kpundeh, M.D.; Xu, P. Interacting Effects of Water Temperature and Dietary Protein Level on Hematological Parameters in Nile Tilapia Juveniles, Oreochromis niloticus (L.) and Mortality under Streptococcus iniae Infection. Fish Shellfish Immunol. 2013, 34, 8–16. [Google Scholar] [CrossRef]
- Ng, W.K.; Romano, N. A Review of the Nutrition and Feeding Management of Farmed Tilapia throughout the Culture Cycle. Rev. Fish. Sci. 2013, 5, 220–254. [Google Scholar] [CrossRef]
- Luquet, S.; Gaudel, C.; Holst, D.; Lopez-Soriano, J.; Jehl-Pietri, C.; Fredenrich, A.; Grimaldi, P.A. Roles of PPAR Delta in Lipid Absorption and Metabolism: A New Target for the Treatment of Type 2 Diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 313–317. [Google Scholar] [CrossRef]
- Kersten, S.; Desvergne, B.; Wahli, W. Roles of PPARs in Health and Disease. Nature 2000, 405, 421–424. [Google Scholar] [CrossRef]
- Peters, J.M.; Lee, S.S.; Li, W.; Ward, J.M.; Gavrilova, O.; Everett, C.; Reitman, M.L.; Hudson, L.D.; Gonzalez, F.J. Growth, Adipose, Brain, and Skin Alterations Resulting from Targeted Disruption of the Mouse Peroxisome Proliferator-Activated Receptor Beta (Delta). Mol. Cell Biol. 2000, 20, 5119–5128. [Google Scholar] [CrossRef]
- Park, Y.; Pariza, M.W. Mechanisms of Body Fat Modulation by Conjugated Linoleic Acid (CLA). Food Res. Int. 2007, 40, 311–323. [Google Scholar] [CrossRef]
- Walczak, R.; Tontonoz, P. PPARadigms and PPARadoxes: Expanding Roles for PPAR Gamma in the Control of Lipid Metabolism. J. Lipid Res. 2002, 43, 177–186. [Google Scholar] [CrossRef]
- Li, X.; Xue, Y.; Pang, L.; Len, B.; Lin, Z.; Huang, J.; ShangGuan, Z.; Pan, Y. Agaricus Bisporus-Derived β-Glucan Prevents Obesity through PPAR γ Downregulation and Autophagy Induction in Zebrafish Fed by Chicken Egg Yolk. Int. J. Biol. Macromol. 2019, 125, 820–828. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Wu, T.X.; Tang, H.G.; Pan, X.D.; Zhang, J.Z. Effect of Conjugated Linoleic Acid on Growth, Lipid Metabolism and Liver Peroxisome Proliferator-Activated Receptor Expression of Large Yellow Croaker (Pseudosciaenacrocea R.). J. Food Lipids 2008, 15, 534–554. [Google Scholar] [CrossRef]
- Wang, N.; Wang, W.; Breslow, J.L.; Tall, A.R. Scavenger Receptor BI (SR-BI) Is Up-Regulated in Adrenal Gland in Apolipoprotein A-I and Hepatic Lipase Knock-out Mice as a Response to Depletion of Cholesterol Stores: In Vivo Evidence That SR-BI Is a Functional High Density Lipoprotein Receptor under Feedback Control. J. Biol. Chem. 1996, 271, 21001–21004. [Google Scholar] [CrossRef]
- Esteves, A.; Knoll-Gellida, A.; Canclini, L.; Silvarrey, M.C.; André, M.; Babin, P.J. Fatty Acid Binding Proteins Have the Potential to Channel Dietary Fatty Acids into Enterocyte Nuclei. J. Lipid Res. 2016, 57, 219–232. [Google Scholar] [CrossRef]
- Pu, H.; Li, X.; Du, Q.; Cui, H.; Xu, Y. Research Progress in the Application of Chinese Herbal Medicines in Aquaculture: A Review. Engineering 2017, 3, 731–737. [Google Scholar] [CrossRef]
- Yi, J.M.; Kim, M.S.; Seo, S.W.; Lee, K.N.; Yook, C.S.; Kim, H.-M. Acanthopanax senticosus Root Inhibits Mast Cell-Dependent Anaphylaxis. Clin. Chim. Acta 2001, 312, 163–168. [Google Scholar] [CrossRef]
- Yi, J.-M.; Hong, S.H.; Kim, J.H.; Kim, H.K.; Song, H.J.; Kim, H.M. Effect of Acanthopanax senticosus Stem on Mast Cell-Dependent Anaphylaxis. J. Ethnopharmacol. 2002, 79, 347–352. [Google Scholar] [CrossRef]
- Fang, J.; Yan, F.Y.; Kong, X.F.; Ruan, Z.; Liu, Z.Q.; Huang, R.L.; Li, T.J.; Geng, M.M.; Yang, F.; Zhang, Y.Z.; et al. Dietary Supplementation with Acanthopanax senticosus Extract Enhances Gut Health in Weanling Piglets. Livest. Prod. Sci. 2009, 123, 268–275. [Google Scholar] [CrossRef]
- Lee, S.; Son, D.; Ryu, J.; Lee, Y.S.; Jung, S.H.; Kang, J.; Lee, S.Y.; Kim, H.-S.; Shin, K.H. Anti-Oxidant Activities Ofacanthopanax senticosus Stems and Their Lignan Components. Arch. Pharm. Res. 2004, 27, 106–110. [Google Scholar] [CrossRef]
- Chen, R.; Liu, Z.; Zhao, J.; Chen, R.; Meng, F.; Zhang, M.; Ge, W. Antioxidant and Immunobiological Activity of Water-Soluble Polysaccharide Fractions Purified from Acanthopanax senticosu. Food Chem. 2011, 127, 434–440. [Google Scholar] [CrossRef]
- Yamazaki, T.; Shimosaka, S.; Sasaki, H.; Matsumura, T.; Tukiyama, T.; Tokiwa, T. (+)-Syringaresinol-Di-O-β-d-Glucoside, a Phenolic Compound from Acanthopanax senticosus Harms, Suppresses Proinflammatory Mediators in SW982 Human Synovial Sarcoma Cells by Inhibiting Activating Protein-1 and/or Nuclear Factor-ΚB Activities. Toxicol. Vitr. 2007, 21, 1530–1537. [Google Scholar] [CrossRef]
- Jung, H.J.; Park, H.J.; Kim, R.G.; Shin, K.M.; Ha, J.; Choi, J.W.; Kim, H.J.; Lee, Y.S.; Lee, K.-T. In Vivo Anti-Inflammatory and Antinociceptive Effects of Liriodendrin Isolated from the Stem Bark of Acanthopanax senticosus. Planta Med. 2003, 69, 610–616. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.G.; Kang, S.K.; Chung, S.H. Acanthopanax senticosus Reverses Fatty Liver Disease and Hyperglycemia in Ob/Ob Mice. Arch. Pharm. Res. 2006, 29, 768. [Google Scholar] [CrossRef]
- Yue, B.; Xu, L.; Li, Y. Protection and mitochondria mechanism of Aacanthopanax senticosus saponins on high-fat diet induced fatty liver in mice. Cent. South Pharm. 2018, 16, 1725–1728. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Lu, F.; Liu, C.; Wang, Y.; Bai, Y.; Wang, N.; Liu, S. Cerebral Metabonomics Study on Parkinson’s Disease Mice Treated with Extract of Acanthopanax senticosus Harms. Phytomedicine 2013, 20, 1219–1229. [Google Scholar] [CrossRef]
- Saito, T.; Nishida, M.; Saito, M.; Tanabe, A.; Eitsuka, T.; Yuan, S.-H.; Ikekawa, N.; Nishida, H. The Fruit of Acanthopanax senticosus (Rupr. Et Maxim.) Harms Improves Insulin Resistance and Hepatic Lipid Accumulation by Modulation of Liver Adenosine Monophosphate–Activated Protein Kinase Activity and Lipogenic Gene Expression in High-Fat Diet–Fed Obese Mice. Nutr. Res. 2016, 36, 1090–1097. [Google Scholar] [CrossRef]
- Tao, Y.F.; Qiang, J.; Bao, J.W.; Chen, D.J.; Yin, G.J.; Xu, P.; Zhu, H.-J. Changes in Physiological Parameters, Lipid Metabolism, and Expression of MicroRNAs in Genetically Improved Farmed Tilapia (Oreochromis niloticus) With Fatty Liver Induced by a High-Fat Diet. Front. Physiol. 2018, 9, 1521. [Google Scholar] [CrossRef]
- Berntssen, M.H.G.; Lundebye, A.-K.; Maage, A. Effects of Elevated Dietary Copper Concentrations on Growth, Feed Utilisation and Nutritional Status of Atlantic Salmon (Salmo salar L.) Fry. Aquaculture 1999, 174, 167–181. [Google Scholar] [CrossRef]
- Qiang, J.; Yang, H.; Ma, X.Y.; He, J.; Wang, H.; Kpundeh, M.D.; Xu, P. Comparative Studies on Endocrine Status and Gene Expression of Hepatic Carbohydrate Metabolic Enzymes in Juvenile GIFT Tilapia (Oreochromis niloticus) Fed High-Carbohydrate Diets. Aquac. Res. 2016, 47, 758–768. [Google Scholar] [CrossRef]
- Ma, X.Y.; Qiang, J.; He, J.; Gabriel, N.N.; Xu, P. Changes in the Physiological Parameters, Fatty Acid Metabolism, and SCD Activity and Expression in Juvenile GIFT Tilapia (Oreochromis niloticus) Reared at Three Different Temperatures. Fish Physiol. Biochem. 2015, 41, 937–950. [Google Scholar] [CrossRef]
- Qiang, J.; Wasipe, A.; He, J.; Tao, Y.-F.; Xu, P.; Bao, J.-W.; Chen, D.; Zhu, J.-H. Dietary Vitamin E Deficiency Inhibits Fat Metabolism, Antioxidant Capacity, and Immune Regulation of Inflammatory Response in Genetically Improved Farmed Tilapia (GIFT, Oreochromis niloticus) Fingerlings Following Streptococcus Iniae Infection. Fish Shellfish Immunol. 2019, 92, 395–404. [Google Scholar] [CrossRef]
- Tao, Y.F.; Qiang, J.; Bao, J.W.; Li, H.X.; Yin, G.J.; Xu, P.; Chen, D.J. MiR-205-5p Negatively Regulates Hepatic Acetyl-CoA Carboxylase β MRNA in Lipid Metabolism of Oreochromis Niloticus. Gene 2018, 660, 1–7. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Qiang, J.; Khamis, O.A.M.; Jiang, H.J.; Cao, Z.M.; He, J.; Tao, Y.F.; Xu, P.; Bao, J.W. Effects of Dietary Supplementation with Apple Peel Powder on the Growth, Blood and Liver Parameters, and Transcriptome of Genetically Improved Farmed Tilapia (GIFT, Oreochromis niloticus). PLoS ONE 2019, 14, e0224995. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Lin, H.; Yang, Y. Effects of dietary Bacillus spp. and traditional Chinese medicines on growth and intestinal bacterial flora of shrimp Litopenaeus vannamei. J. Trop. Oceanogr. 2010, 29, 132–137. [Google Scholar] [CrossRef]
- Xie, L.; Cao, J.; Yang, S.; Zhao, C.; Ren, L. The Impact of Dietary Chinese Herbal Medicines on Growth Performance and Muscular Composition in Juvenile Tilapia. Fish Sci. 2009, 28, 11–14. [Google Scholar] [CrossRef]
- Song, Z.; Jiao, C.; Chen, B.; Xu, W.; Wang, M.; Zou, J.; Xu, W.; Xu, Z.; Wang, Q. Dietary Acanthopanax senticosus Extracts Modulated the Inflammatory and Apoptotic Responses of Yellow Catfish to Protect against Edwardsiella ictaluri Infection. Aquacult. Res. 2021, 52, 5078–5092. [Google Scholar] [CrossRef]
- Ruan, G.; Yang, D.; Wang, J. Effects of Herbal Feed Addtives on Immunity and Growth of Monopterus albus. Feed Ind. 2005, 26, 34–36. [Google Scholar] [CrossRef]
- Long, L.N.; Zhang, H.H.; Wang, F.; Yin, Y.X.; Yang, L.Y.; Chen, J.S. Research Note: Effects of Polysaccharide-Enriched Acanthopanax senticosus Extract on Growth Performance, Immune Function, Antioxidation, and Ileal Microbial Populations in Broiler Chickens. Poult. Sci. 2021, 100, 101028. [Google Scholar] [CrossRef]
- Ram Bhaskar, B.; Srinivasa Rao, K. Influence of Environmental Variables on Haematology, and Compendium of Normal Haematological Ranges of Milkfish, Chanos (Forskal), in Brackishwater Culture. Aquaculture 1989, 83, 123–136. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, M.C.; Kim, J.-S.; Balasundaram, C.; Heo, M.-S. Protective Effect of Herbal and Probiotics Enriched Diet on Haematological and Immunity Status of Oplegnathus fasciatus (Temminck & Schlegel) against Edwardsiella tarda. Fish Shellfish Immunol. 2011, 30, 886–893. [Google Scholar] [CrossRef]
- Li, M.X.; Li, H.X.; Qiang, J.; Xu, P.; Bao, J.W.; Chen, D.J.; Tao, Y.F.; Zhu, H.J. Effects of Acanthopanax senticosus on Growth Performance, Fat Deposition and Proinflammatory Cytokine Expression of Genetically Improved Farmed Tilapia (GIFT, Oreochromis niloticus). J. Anim. Nutr. 2019, 31, 5801–5812. [Google Scholar]
- Qiang, J.; Tao, Y.F.; Bao, J.W.; Chen, D.J.; Li, H.X.; He, J.; Xu, P. High Fat Diet-Induced MiR-122 Regulates Lipid Metabolism and Fat Deposition in Genetically Improved Farmed Tilapia (GIFT, Oreochromis niloticus) Liver. Front. Physiol. 2018, 9, 1422. [Google Scholar] [CrossRef]
- Fu, J.; Fu, J.; Liu, Y.; Li, R.; Gao, B.; Zhang, N.; Wang, B.; Cao, Y.; Guo, K.; Tu, Y. Modulatory Effects of One Polysaccharide from Acanthopanax senticosus in Alloxan-Induced Diabetic Mice. Carbohydr. Polym. 2012, 87, 2327–2331. [Google Scholar] [CrossRef]
- Li, H.X.; Qiang, J.; Song, C.Y.; Xu, P. Acanthopanax senticosus Promotes Survival of Tilapia Infected With Streptococcus Iniae by Regulating the PI3K/AKT and Fatty Acid Metabolism Signaling Pathway. Front. Physiol. 2021, 12, 699247. [Google Scholar] [CrossRef]
- Nishida, M.; Kondo, M.; Shimizu, T.; Saito, T.; Sato, S.; Hirayama, M.; Konishi, T.; Nishida, H. Antihyperlipidemic Effect of Acanthopanax senticosus (Rupr. et Maxim) Harms Leaves in High-Fat-Diet Fed Mice. J. Sci. Food Agric. 2016, 96, 3717–3722. [Google Scholar] [CrossRef]
- Vilella, E.; Joven, J.; Fernández, M.; Vilaró, S.; Brunzell, J.; Olivecrona, T.; Bengtsson-Olivecrona, G. Lipoprotein Lipase in Human Plasma Is Mainly Inactive and Associated with Cholesterol-Rich Lipoproteins. J. Lipid Res. 1993, 34, 1555–1564. [Google Scholar] [CrossRef]
- De Smet, H.; Blust, R.; Moens, L. Absence of Albumin in the Plasma of the Common Carp Cyprinus carpio: Binding of Fatty Acids to High Density Lipoprotein. Fish Physiol. Biochem. 1998, 19, 71–81. [Google Scholar] [CrossRef]
- Babin, P.J.; Vernier, J.M. Plasma Lipoproteins in Fish. J. Lipid Res. 1989, 30, 467–489. [Google Scholar] [CrossRef]
- Bhathena, S.J.; Velasquez, M.T. Beneficial Role of Dietary Phytoestrogens in Obesity and Diabetes. Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [CrossRef]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wähälä, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In Vitro Metabolism of Plant Lignans: New Precursors of Mammalian Lignans Enterolactone and Enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef]
- Komprda, T.; Zornikova, G.; Knoll, A.; Vykoukalova, Z.; Rozikova, V.; Skultety, O.; Krobot, R. Effect of Dietary Eicosapentaenoic and Docosahexaenoic Acid on Expression of Rat Liver Genes Controlling Cholesterol Homeostasis and on Plasma Cholesterol Level. Czech J. Anim. Sci. 2014, 59, 391–398. [Google Scholar] [CrossRef]
- Feldstein, A.E. Novel Insights into the Pathophysiology of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2010, 30, 391–401. [Google Scholar] [CrossRef]
- Du, Z.Y.; Ma, T.; Liaset, B.; Keenan, A.H.; Araujo, P.; Lock, E.J.; Demizieux, L.; Degrace, P.; Frøyland, L.; Kristiansen, K.; et al. Dietary Eicosapentaenoic Acid Supplementation Accentuates Hepatic Triglyceride Accumulation in Mice with Impaired Fatty Acid Oxidation Capacity. Biochim. Biophys. Acta Lipids Lipid Metab. 2013, 1831, 291–299. [Google Scholar] [CrossRef]
- Lim, E.J.; Do, G.-M.; Shin, J.H.; Kwon, O. Protective Effects of Acanthopanax divaricatus Vat. Albeofructus and Its Active Compound on Ischemia–Reperfusion Injury of Rat Liver. Biochem. Biophys. Res. Commun. 2013, 432, 599–605. [Google Scholar] [CrossRef]
- Pirinccioglu, A.G.; Gökalp, D.; Pirinccioglu, M.; Kizil, G.; Kizil, M. Malondialdehyde (MDA) and Protein Carbonyl (PCO) Levels as Biomarkers of Oxidative Stress in Subjects with Familial Hypercholesterolemia. Clin. Biochem. 2010, 43, 1220–1224. [Google Scholar] [CrossRef]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauça, M.; Wahli, W. Differential Expression of Peroxisome Proliferator-Activated Receptors (PPARs): Tissue Distribution of PPAR-Alpha, -Beta, and -Gamma in the Adult Rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef]
- Gandarillas, M.; Matus, J.T.; Márquez-Hernández, R.I.; Vargas-Bello-Pérez, E. Development of Insulin Resistance in Horses (Equus caballus): Etiologic and Molecular Aspects. Cienc. Investig. Agrogenom. 2015, 42, 125–137. [Google Scholar] [CrossRef]
- Chakravarthy, M.V.; Pan, Z.; Zhu, Y.; Tordjman, K.; Schneider, J.G.; Coleman, T.; Turk, J.; Semenkovich, C.F. “New” Hepatic Fat Activates PPARα to Maintain Glucose, Lipid, and Cholesterol Homeostasis. Cell Metab. 2005, 1, 309–322. [Google Scholar] [CrossRef]
- Corcoran, J.; Winter, M.J.; Lange, A.; Cumming, R.; Owen, S.F.; Tyler, C.R. Effects of the Lipid Regulating Drug Clofibric Acid on PPARα-Regulated Gene Transcript Levels in Common Carp (Cyprinus carpio) at Pharmacological and Environmental Exposure Levels. Aquat. Toxicol. 2015, 161, 127–137. [Google Scholar] [CrossRef]
- Zheng, J.L.; Zhuo, M.Q.; Luo, Z.; Pan, Y.X.; Song, Y.F.; Huang, C.; Zhu, Q.L.; Hu, W.; Chen, Q.L. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) in Yellow Catfish Pelteobagrus fulvidraco: Molecular Characterization, MRNA Expression and Transcriptional Regulation by Insulin In Vivo and In Vitro. Gen. Comp. Endocrinol. 2015, 212, 51–62. [Google Scholar] [CrossRef]
- Ning, L.J.; He, A.Y.; Li, J.M.; Lu, D.L.; Jiao, J.G.; Li, L.Y.; Li, D.L.; Zhang, M.L.; Chen, L.Q.; Du, Z.Y. Mechanisms and Metabolic Regulation of PPARα Activation in Nile Tilapia (Oreochromis niloticus). Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2016, 1861, 1036–1048. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The Diverse Biology of PPAR Gamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of Adipogenesis in Fibroblasts by PPARγ2, a Lipid-Activated Transcription Factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Barroso, I.; Gurnell, M.; Crowley, V.E.F.; Agostini, M.; Schwabe, J.W.; Soos, M.A.; Maslen, G.L.; Williams, T.D.M.; Lewis, H.; Schafer, A.J.; et al. Dominant Negative Mutations in Human PPARγ Associated with Severe Insulin Resistance, Diabetes Mellitus and Hypertension. Nature 1999, 402, 880–883. [Google Scholar] [CrossRef]
- Córsico, B.; Liou, H.L.; Storch, J. The α-Helical Domain of Liver Fatty Acid Binding Protein Is Responsible for the Diffusion-Mediated Transfer of Fatty Acids to Phospholipid Membranes. Biochemistry 2004, 43, 3600–3607. [Google Scholar] [CrossRef]
- Murota, K.; Storch, J. Uptake of Micellar Long-Chain Fatty Acid and Sn-2-Monoacylglycerol into Human Intestinal Caco-2 Cells Exhibits Characteristics of Protein-Mediated Transport. J. Nutr. 2005, 135, 1626–1630. [Google Scholar] [CrossRef]
- Murai, A.; Furuse, M.; Kitaguchi, K.; Kusumoto, K.; Nakanishi, Y.; Kobayashi, M.; Horio, F. Characterization of Critical Factors Influencing Gene Expression of Two Types of Fatty Acid-Binding Proteins (L-FABP and Lb-FABP) in the Liver of Birds. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, 216–223. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, Y.X.; Liu, Z.M.; Yang, L.Y.; Li, H.; Li, Z.J.; Li, G.X.; Wang, Y.-B.; Tian, Y.-D.; Kang, X.-T.; et al. Genome-Wide Analysis of the FABP Gene Family in Liver of Chicken (Gallus gallus): Identification, Dynamic Expression Profile, and Regulatory Mechanism. Int. J. Mol. Sci. 2019, 20, 5948. [Google Scholar] [CrossRef]
- Lamon-Fava, S.; Diffenderfer, M.R.; Barrett, P.H.R.; Buchsbaum, A.; Nyaku, M.; Horvath, K.V.; Asztalos, B.F.; Otokozawa, S.; Ai, M.; Matthan, N.R.; et al. Extended-Release Niacin Alters the Metabolism of Plasma Apolipoprotein (Apo) A-I and ApoB-Containing Lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1672–1678. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.; Park, S.W.; Kim, K.; Lee, M.W.; Paik, S.; Jang, A.S.; Kim, D.J.; Uh, S.; Kim, Y.; et al. Attenuation of Cigarette Smoke–Induced Emphysema in Mice by Apolipoprotein A-1 Overexpression. Am. J. Respir. Cell Mol. Biol. 2016, 54, 91–102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).