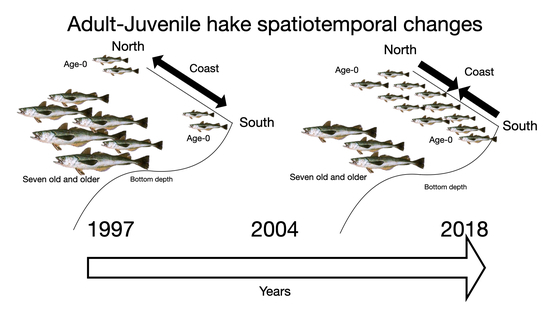
Juvenile Hake Merluccius gayi Spatiotemporal Expansion and Adult-Juvenile Relationships in Chile
Abstract
1. Introduction
2. Materials and Methods
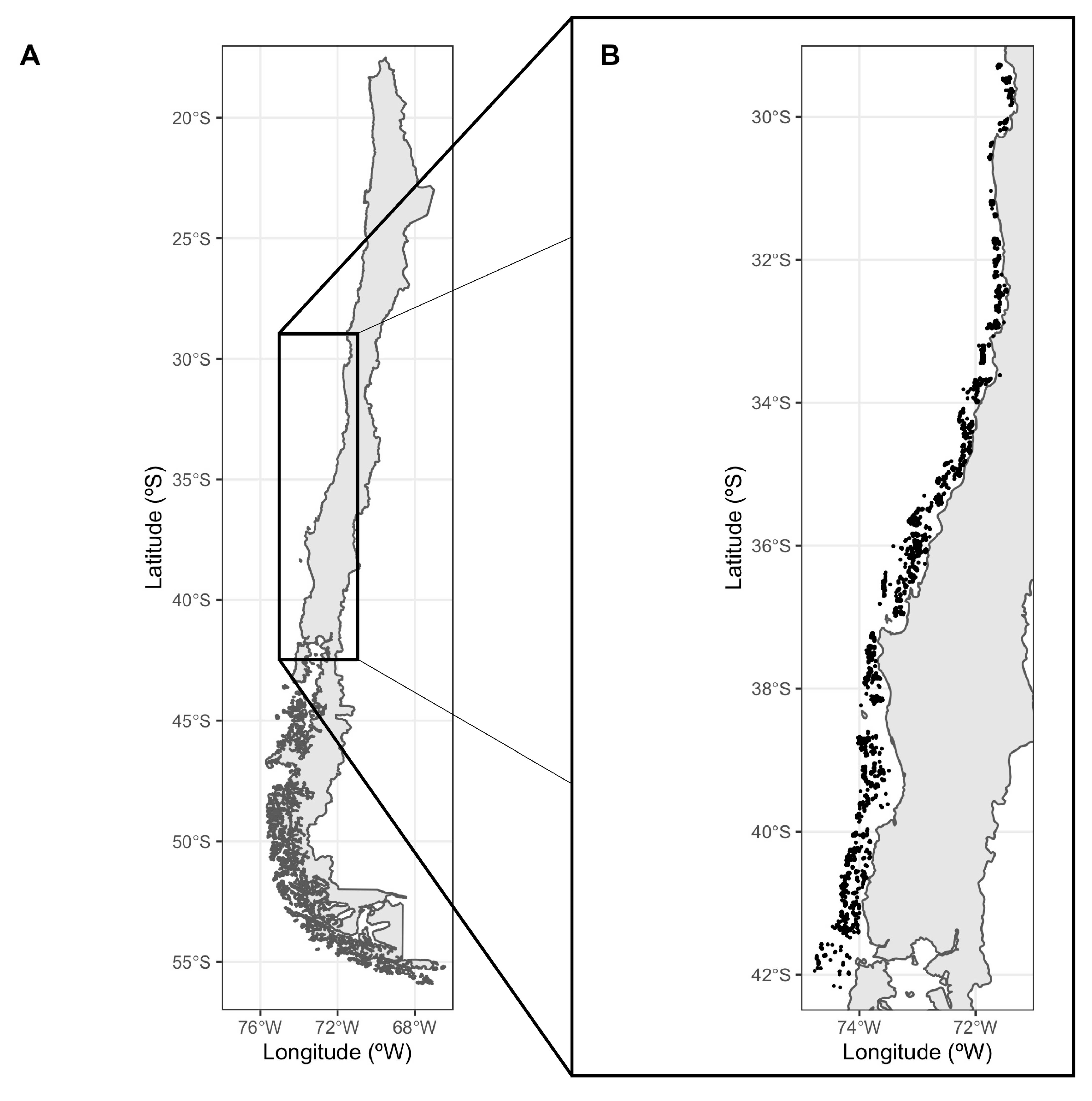
2.1. Study Area and Data
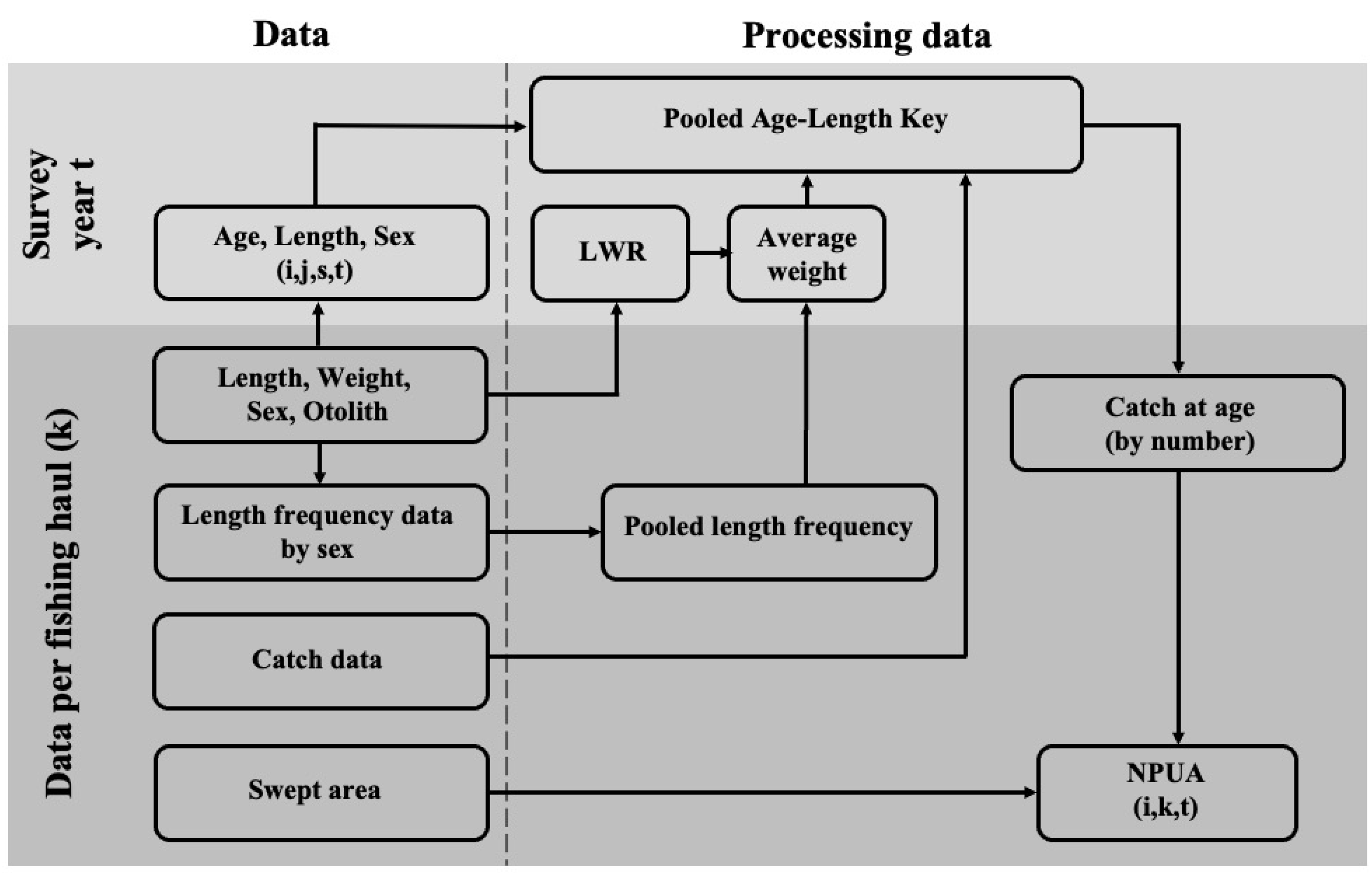
2.2. Age Composition and Abundance per Unit Area
2.3. Spatial Distribution and Juvenile–Adult Relationships
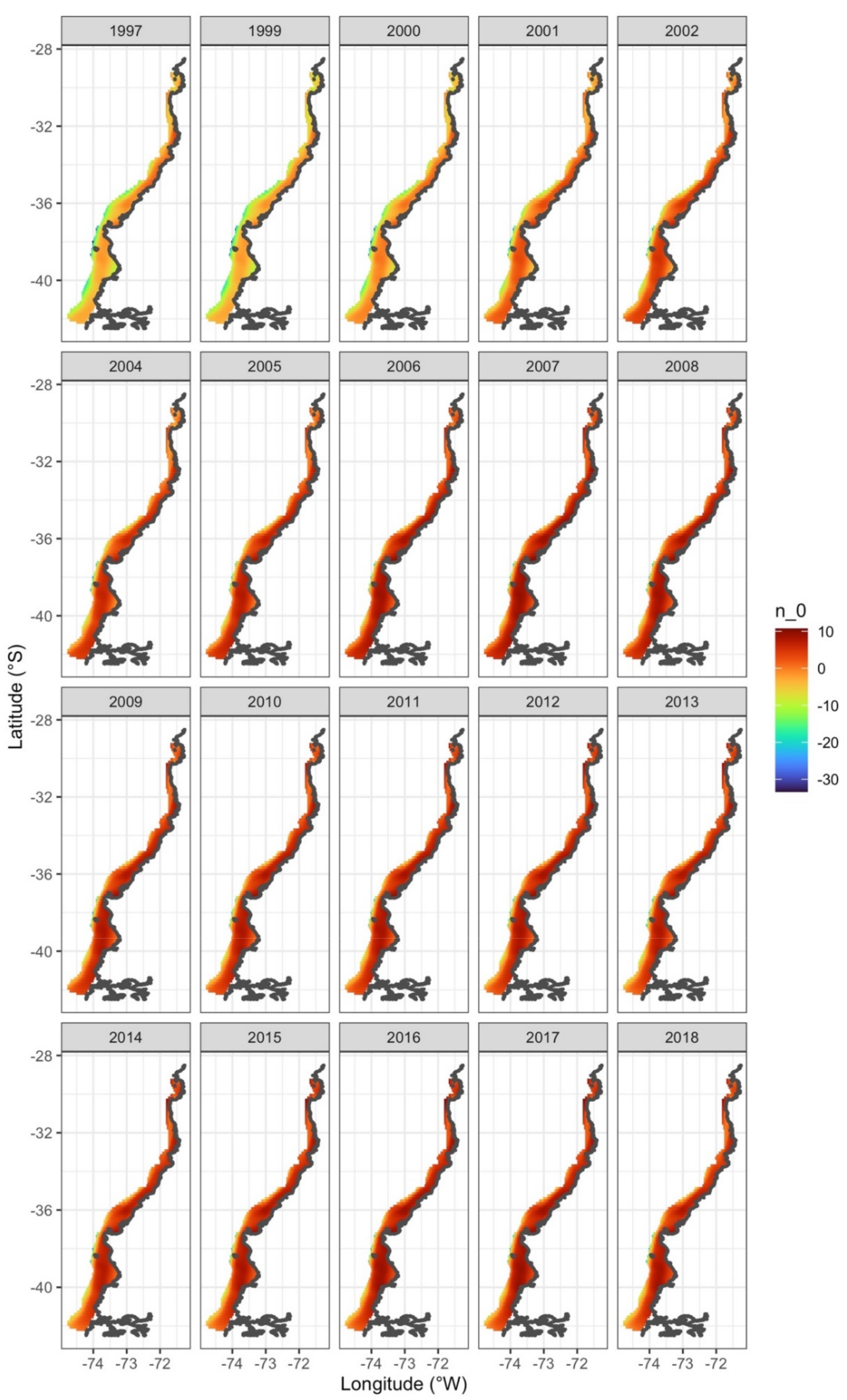
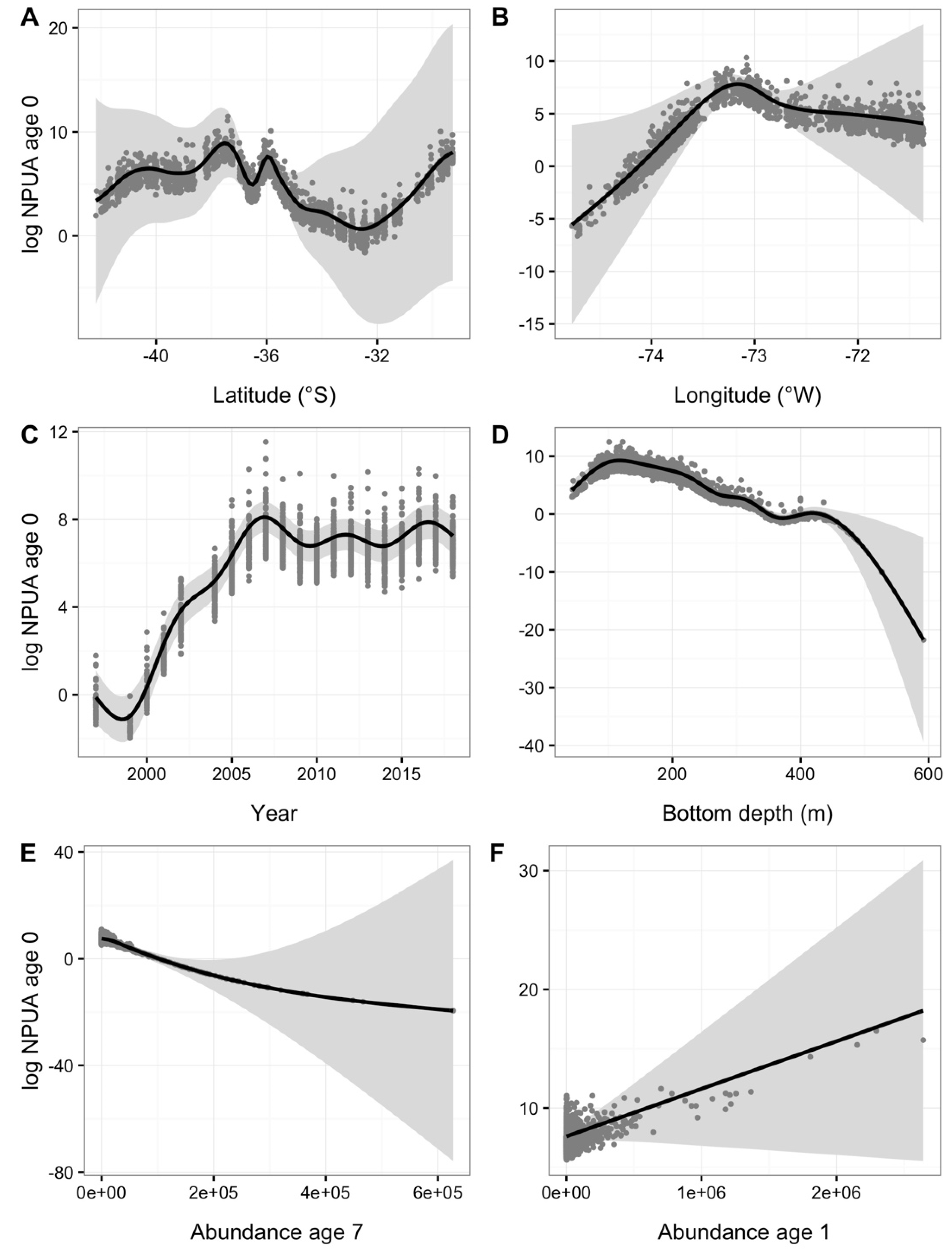
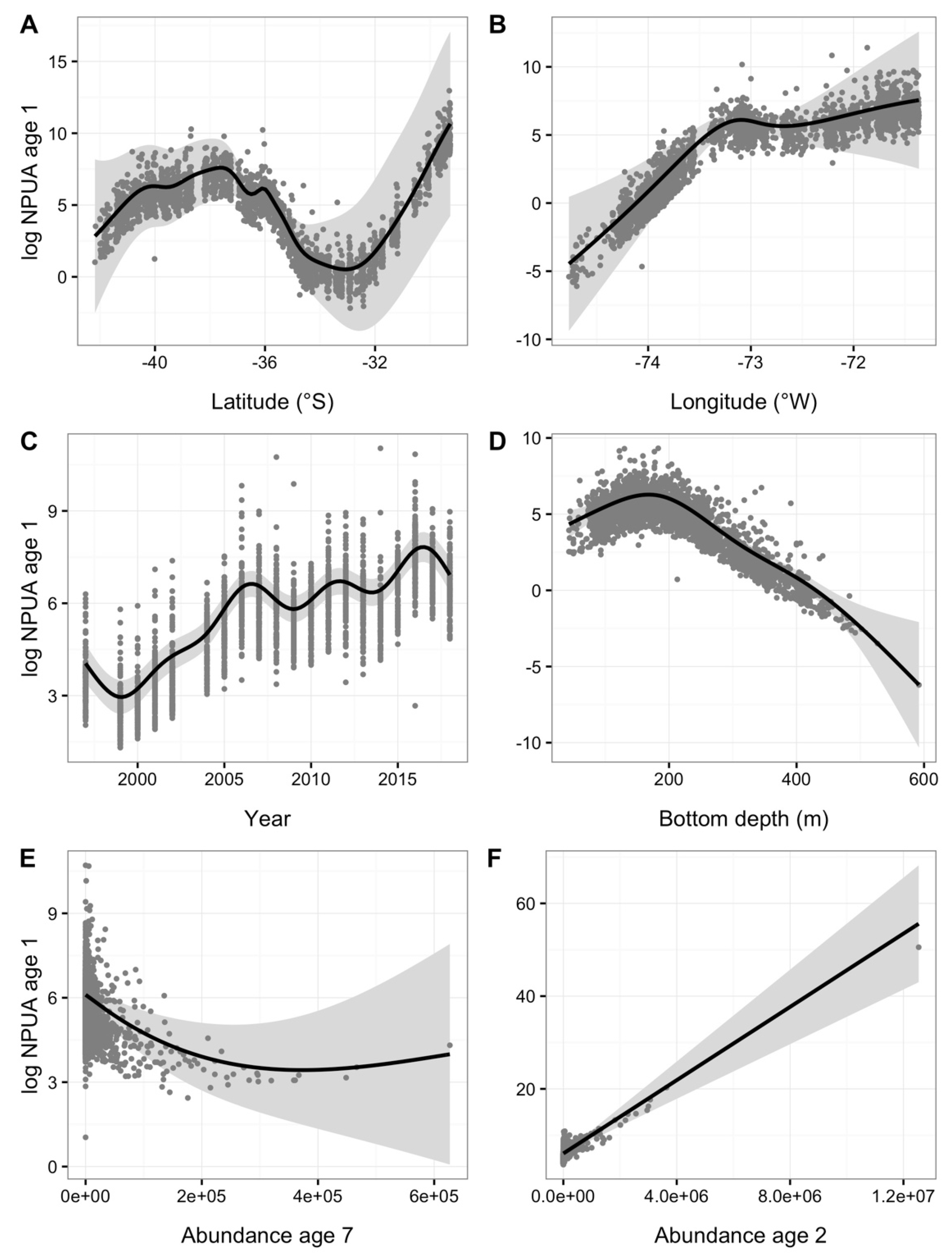
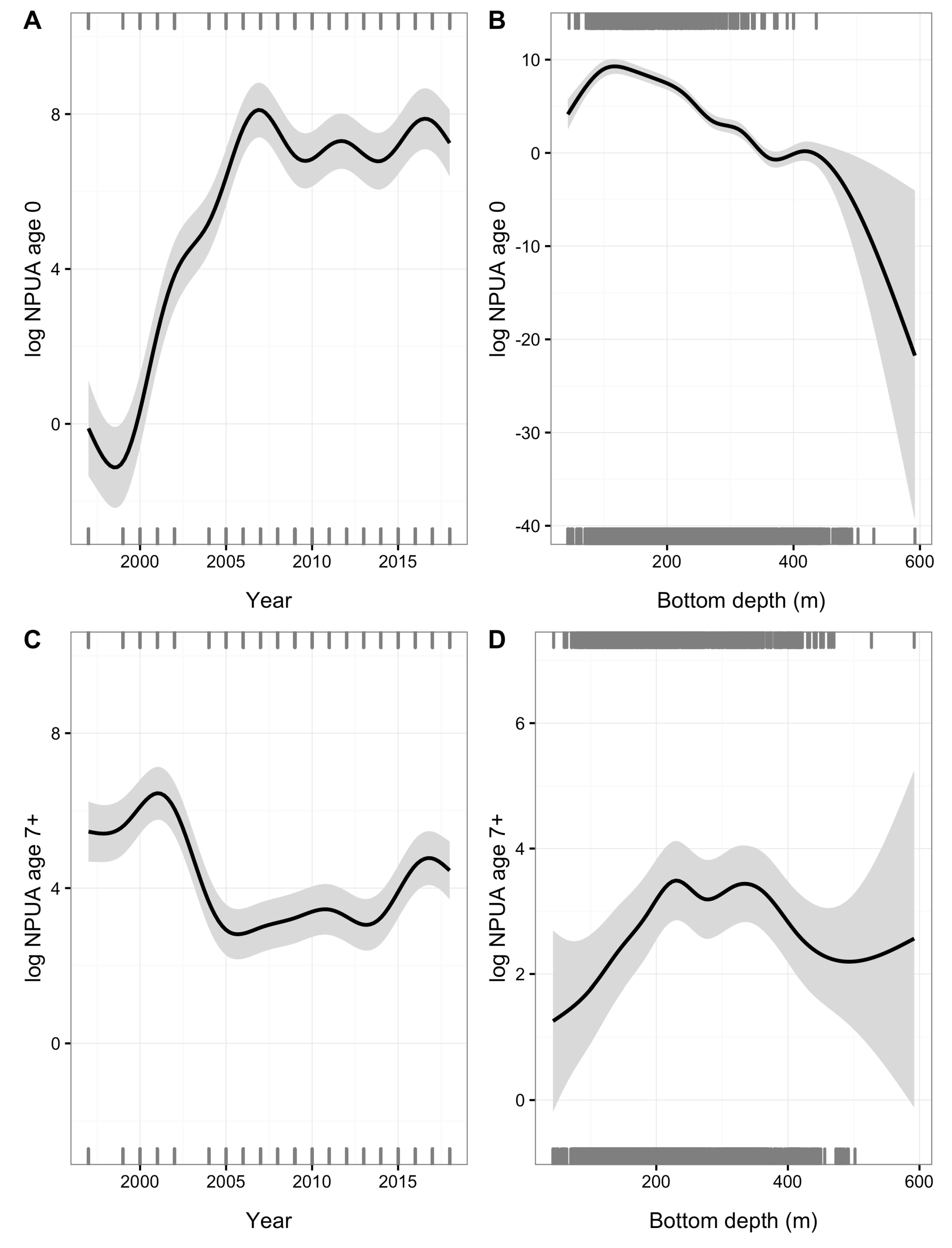
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Link, J.S.; Nye, J.A.; Hare, J.A. Guidelines for incorporating fish distribution shifts into a fisheries management context. Fish Fish. 2011, 12, 461–469. [Google Scholar] [CrossRef]
- Ward, E.J.; Jannot, J.E.; Lee, Y.-W.; Ono, K.; Shelton, A.O.; Thorson, J.T. Using spatiotemporal species distribution models to identify temporally evolving hotspots of species co-occurrence. Ecol. Appl. 2015, 25, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Ciannelli, L.; Bailey, K.; Olsen, E.M. Evolutionary and ecological constraints of fish spawning habitats. ICES J. Mar. Sci. 2015, 72, 285–296. [Google Scholar] [CrossRef]
- Ohlberger, J.; Rogers, L.A.; Stenseth, N.C. Stochasticity and determinism: How density-independent and density-dependent processes affect population variability. PLoS ONE 2014, 9, 98940. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, R.N.M.; Walters, C.J.; Christensen, V. Foraging arena theory. Fish Fish. 2012, 13, 41–59. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Heithaus, M.R.; Serafy, J.E. Influence of predation risk and food supply on nocturnal fish foraging distributions along a mangrove-seagrass ecotone. Mar. Ecol. Prog. Ser. 2010, 414, 223–235. [Google Scholar] [CrossRef]
- Dalpadado, P.; Bogstad, B.; Eriksen, E.; Rey, L. Distribution and diet of 0-group cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) in the Barents Sea in relation to food availability and temperature. Polar Biol. 2019, 32, 1583–1596. [Google Scholar] [CrossRef]
- Nøttestad, L.; Giske, J.; Holst, J.C.; Huse, G. A length-based hypothesis for feeding migrations in pelagic fish. Can. J. Fish. Aquat. Sci. 1999, 56, 26–34. [Google Scholar] [CrossRef]
- Eriksen, E.; Ingvaldsen, R.; Stiansen, J.E.; Johansen, G.O. Thermal habitat for 0-group fish in the Barents Sea; how climate variability impacts their density, length, and geographic distribution. ICES J. Mar. Sci. 2012, 69, 870–879. [Google Scholar] [CrossRef]
- Thorson, J.T.; Ianelli, J.N.; Kotwicki, S. The relative influence of temperature and size-structure on fish distribution shifts: A case-study on Walleye pollock in the Bering Sea. Fish Fish. 2017, 18, 1073–1084. [Google Scholar] [CrossRef]
- Brook, B.W.; Bradshaw, C.J.A. Strength of evidence for density dependence in abundance time series of 1198. Ecology 2006, 87, 1445–1451. [Google Scholar] [PubMed]
- Herrando-Pérez, S.; Delean, S.; Brook, B.W.; Bradshaw, C.J.A. Density dependence: An ecological Tower of Babel. Oecologia 2012, 170, 585–603. [Google Scholar] [PubMed]
- van Gemert, R.; Andersen, K.H. Challenges to fisheries advice and management due to stock recovery. ICES J. Mar. Sci. 2018, 75, 1864–1870. [Google Scholar]
- Andersen, K.H.; Jacobsen, N.S.; Jansen, T.; Beyer, J.E. When in life does density dependence occur in fish populations? Fish Fish. 2017, 18, 656–667. [Google Scholar]
- Lorenzen, K.; Camp, E.V. Density-dependence in the life history of fishes: When is a fish recruited? Fish. Res. 2019, 217, 5–10. [Google Scholar]
- Morfin, M.; Fromentin, J.-M.; Jadaud, A.; Bez, N. Spatio-temporal patterns of key exploited marine species in the Northwestern Mediterranean sea. PLoS ONE 2012, 7, e37907. [Google Scholar]
- Bartolino, V.; Ottavi, A.; Colloca, F.; Ardizzone, G.D.; Stefánsson, G. Bathymetric preferences of juvenile European hake (Merluccius merluccius). ICES J. Mar. Sci. 2008, 65, 963–969. [Google Scholar]
- Jansen, T.; Kristensen, K.; Kainge, P.; Durholtz, D.; Strømme, T.; Thygesen, U.H.; Wilhelm, M.R.; Kathena, J.; Fairweather, T.P.; Paulus, S.; et al. Migration, distribution and population (stock) structure of shallow-water hake (Merluccius capensis) in the Benguela Current Large Marine Ecosystem inferred using a geostatistical population model. Fish Res. 2016, 179, 156–167. [Google Scholar]
- Tamdrari, H.; Castonguay, M.; Brêthes, J.C.; Duplisea, D. Density-independent and -dependent habitat selection of Atlantic cod (Gadus morhua) based on geostatistical aggregation curves in the northern Gulf of St Lawrence. ICES J. Mar. Sci. 2010, 67, 1676–1686. [Google Scholar]
- Izquierdo, F.; Paradinas, I.; Cerviño, S.; Conesa, D.; Alonso-Fernández, A.; Velasco, F.; Preciado, I.; Punzón, A.; Saborido-Rey, F.; Pennino, M.G. Spatio-temporal assessment of the European hake (Merluccius merluccius) Recruits in the Northern Iberian Peninsula. Frontiers Mar. Sci. 2021, 8, 614675. [Google Scholar]
- Aguayo-Hernández, M. Biology and fisheries of Chilean hakes (M. gayi and M. australis). In Hake: Biology, Fisheries and Markets; Alheit, J., Pitcher, T.J., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 305–337. [Google Scholar]
- Evaluación Directa De Merluza Común. 2016. Instituto de Fomento Pesquero, Valparaíso. Available online: https://www.ifop.cl (accessed on 22 February 2022).
- Evaluación Directa De Merluza Común. 2018. Instituto de Fomento Pesquero, Valparaíso. Available online: https://www.ifop.cl (accessed on 22 February 2022).
- Gatica, C.; Neira, S.; Arancibia, H.; Vásquez, S. The biology, fishery and market of Chilean hake (Merluccius gayi gayi) in the Southeastern Pacific Ocean. In Hakes; Arancibia, H., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 126–153. [Google Scholar]
- Instituto de Fomento Pesquero. Estatus Y Posibilidades De Explotación Biológicamente Sustentables De Los Principales Recursos Pesqueros Nacionales, Año 2018: Merluza común; Instituto de Fomento Pesquero: Valparaíso, Chile, 2018. [Google Scholar]
- Arancibia, H.; Neira, S. Overview of the Chilean hake (Merluccius gayi) stock. A biomass forecast, and the jumbo squid (Dosidicus gigas) predator-prey relationship off Central Chile (33 °S– 39°S). CalCOFI Rep. 2008, 49, 104–115. [Google Scholar]
- Alarcón-Muñoz, R.; Cubillos, L.; Gatica, C. Jumbo squid (Dosidicus gigas) biomass off central Chile: Effects on chilean hake (Merluccius gayi). CalCOFI Rep. 2008, 49, 157–166. [Google Scholar]
- San Martín, M.A.; Cubillos, L.A.; Saavedra, J.C. The spatio-temporal distribution of juvenile hake (Merluccius gayi gayi) off central southern Chile (1997–2006). Aquat. Living Resour. 2011, 24, 161–168. [Google Scholar] [CrossRef][Green Version]
- San Martín, M.A.; Wiff, R.; Saavedra-Nievas, J.C.; Cubillos, L.A.; Lillo, S. Relationship between Chilean hake (Merluccius gayi gayi) abundance and environmental conditions in the central-southern zone of Chile. Fish. Res. 2013, 143, 89–97. [Google Scholar] [CrossRef]
- Cubillos, L.A.; Alarcón, C.; Arancibia, H. Selectividad por tamaño de las presas en merluza común (Merluccius gayi gayi), zona centro-sur de Chile (1992–1997). Investig. Mar. 2007, 35, 55–69. [Google Scholar] [CrossRef]
- Link, J.S.; Lucey, S.M.; Melgey, J.H. Examining cannibalism in relation to recruitment of silver hake Merluccius bilinearis in the U.S. northwest Atlantic. Fish. Res. 2012, 114, 31–41. [Google Scholar] [CrossRef]
- Jurado-Molina, J.; Gatica, C.; Cubillos, L.A. Incorporating cannibalism into an age-structured model for the Chilean hake. Fish. Res. 2006, 82, 30–40. [Google Scholar] [CrossRef]
- Kimura, D.K. Statistical Assessment of the Age–Length Key. J. Fish. Board Can. 1977, 34, 317–324. [Google Scholar] [CrossRef]
- Wood, S. Generalized Additive Models: An Introduction with R, 2nd ed.; CRC/Taylor & Francis: New York, NY, USA, 2017. [Google Scholar]
- Cran. Available online: https://cran.r-project.org/web/packages/mgcv/index.html (accessed on 22 February 2022).
- R-project. Available online: https://www.R-project.org/ (accessed on 22 February 2022).
- Simpson, G.L. Modelling palaeoecological time series using Generalised Additive Models. Frontiers Ecol. Evol. 2018, 6, 149. [Google Scholar] [CrossRef]
- Paradinas, I.; Conesa, D.; López-Quílez, A.; Bellido, J.M. Spatio-temporal model structures with shared components for semi-continuous species distribution modelling. Spat. Stat. 2017, 22, 434–450. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE T. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Buckland, S.T.; Burnham, K.P.; Augustin, N.H. Model selection: An integral part of inference. Biometrics 1997, 53, 603–618. [Google Scholar] [CrossRef]
- Sobarzo, M.; Bravo, L.; Donoso, D.; Garcés-Vargas, J.; Schneider, W. Coastal upwelling and seasonal cycles that influence the water column over the continental shelf off central Chile. Prog. Oceanogr. 2007, 75, 363–382. [Google Scholar] [CrossRef]
- Contreras, M.; Pizarro, O.; Dewitte, B.; Sepulveda, H.H.; Renault, L. Subsurface mesoscale eddy generation in the ocean off Central Chile. J. Geophys. Res. Oceans 2019, 124, 5700–5722. [Google Scholar] [CrossRef]
- Silva, N.; Neshyba, S. On the Southernmost Extension of the Peru-chile Undercurrent. Deep-Sea Res. Part I Oceanogr. Res. Pap. 1979, 26, 1387–1393. [Google Scholar]
- Biología Reproductiva de Merluza Común. FIPA 2006-16. Available online: https://www.subpesca.cl/fipa/613/w3-article-89139.html (accessed on 22 February 2022).
- Cantafaro, A.; Ardizzone, G.; Enea, M.; Ligas, A.; Colloca, F. Assessing the importance of nursery areas of European hake (Merluccius merluccius) using a body condition index. Ecol. Ind. 2017, 81, 383–389. [Google Scholar] [CrossRef]
- Carlucci, R.; Giuseppe, L.; Porzia, M.; Francesca, C.; Alessandra, M.C.; Letizia, S.; Teresa, S.M.; Nicola, U.; Angelo, T.; D’Onghia, G. Nursery areas of red mullet (Mullus barbatus), hake (Merluccius merluccius) and deep-water rose shrimp (Parapenaeus longirostris) in the Eastern-Central Mediterranean Sea. Estuar. Coast. Shelf Sci. 2009, 83, 529–538. [Google Scholar] [CrossRef]
- Abella, A.; Serena, F.; Ria, M. Distributional response to variations in abundance over spatial and temporal scales for juveniles of European hake (Merluccius merluccius) in the Western Mediterranean Sea. Fish. Res. 2005, 71, 295–310. [Google Scholar] [CrossRef]
- Fiorentino, F.; Garofalo, G.; Santi, A.D.; Bono, G.; Giusto, G.B.; Norrito, G. Spatio-Temporal distribution of recruits (0 group) of Merluccius merluccius and Phycis blennoides (Pisces, Gadiformes) in the Strait of Sicily (Central Mediterranean). In Migrations and Dispersal of Marine Organisms; Developments in Hydrobiology; Jones, M.B., Ingólfsson, A., Ólafsson, E., Helgason, G.V., Gunnarsson, K., Svavarsson, J., Eds.; Springer: Dordrecht, The Netherlands, 2003; Volume 174, pp. 223–236. [Google Scholar]
- Maynou, F.; Lleonart, J.; Cartes, J.E. Seasonal and spatial variability of hake (Merluccius merluccius L.) recruitment in the NW Mediterranean. Fish. Res. 2003, 60, 65–78. [Google Scholar] [CrossRef]
- Queirolo, D.; Gaete, E.; Ahumada, M. Gillnet selectivity for Chilean hake (Merluccius gayi gayi Guichenot, 1848) in the bay of Valparaíso. J. Appl. Ichthyol. 2013, 29, 775–781. [Google Scholar] [CrossRef]
- Queirolo, D.; Flores, A. Seasonal variability of gillnet selectivity in Chilean hake Merluccius gayi gayi (Guichenot, 1848). J. Appl. Ichthyol. 2017, 33, 699–708. [Google Scholar] [CrossRef]
- Capuzzo, E.; Lynam, C.P.; Barry, J.; Stephens, D.; Forster, R.M.; Greenwood, N.; McQuatters-Gollop, A.; Silva, T.; van Leeuwen, S.M.; Engelhard, G.H. A decline in primary production in the North Sea over 25 years, associated with reductions in zooplankton abundance and fish stock recruitment. Glob. Change Biol. 2018, 24, e352–e364. [Google Scholar] [CrossRef] [PubMed]
- Malick, M.; Hunsicker, M.; Haltuch, M.; Parker-Stetter, S.; Berger, A.; Marshall, K. Relationships between temperature and Pacific hake distribution vary across latitude and life-history stage. Mar. Ecol. Progr. Ser. 2020, 639, 185–197. [Google Scholar] [CrossRef]
- Thiaw, M.; Auger, P.A.; Ngom, F.; Brochier, T.; Faye, S.; Diankha, O.; Brehmer, P. Effect of environmental conditions on the seasonal and inter-annual variability of small pelagic fish abundance off North-West Africa: The case of both Senegalese sardinella. Fish. Oceanogr. 2017, 26, 583–601. [Google Scholar] [CrossRef]
- Kathena, J.N.; Yemane, D.; Bahamon, N.; Jansen, T. Population abundance and seasonal migration patterns indicated by commercial catch-per-unit-effort of hakes (Merluccius capensis and M. paradoxus) in the northern Benguela Current Large Marine Ecosystem. Afr. J. Mar. Sci. 2018, 40, 197–209. [Google Scholar] [CrossRef]
- Vine, J.R.; Kanno, Y.; Holbrook, S.C.; Post, W.C.; Peoples, B.K. Using side-scan sonar and n-mixture modeling to estimate Atlantic sturgeon spawning migration abundance. N. Am. J. Fish. Manag. 2019, 39, 939–950. [Google Scholar] [CrossRef]
- Camara, M.L.; Mérigot, B.; Leprieur, F.; Tomasini, J.A.; Diallo, I.; Diallo, M.; Jouffre, D. Structure and dynamics of demersal fish assemblages over three decades (1985–2012) of increasing fishing pressure in Guinea. Afr. J. Mar. Sci. 2016, 38, 189–206. [Google Scholar] [CrossRef]
- Kuparinen, A.; Boit, A.; Valdovinos, F.S.; Lassaux, H.; Martinez, N.D. Fishing-induced life-history changes degrade and destabilize harvested ecosystems. Sci. Rep. 2016, 6, 22245. [Google Scholar] [CrossRef]
- Ponce, T.; Cubillos, L.A.; Ciancio, J.; Castro, L.R.; Araya, M. Isotopic niche and niche overlap in benthic crustacean and demersal fish associated to the bottom trawl fishing in south-central Chile. J. Sea Res. 2021, 173, 102059. [Google Scholar] [CrossRef]
- Olsson, K.H.; Andersen, K.H. Cannibalism as a selective force on offspring size in fish. Oikos 2018, 127, 1264–1271. [Google Scholar] [CrossRef]


| Year | Code | ALK (n) | WLD (n) | LFD (n) | Tows (n) |
|---|---|---|---|---|---|
| 1997 | 1997-12 | 972 | 3754 | 23,497 | 133 |
| 1999 | 1999-04 | 999 | 3699 | 15,035 | 135 |
| 2000 | 2000-04 | 1011 | 2216 | 21,952 | 124 |
| 2001 | 2001-18 | 1045 | 2693 | 26,427 | 141 |
| 2002 | 2002-03 | 1138 | 3778 | 29,210 | 153 |
| 2004 | 2004-09 | 1013 | 3624 | 17,570 | 137 |
| 2005 | 2005-05 | 726 | 3321 | 16,516 | 138 |
| 2006 | 2006-03 | 1117 | 3599 | 17,819 | 134 |
| 2007 | 2007-16 | 997 | 4669 | 28,080 | 171 |
| 2008 | 2008-14 | 993 | 4240 | 26,648 | 153 |
| 2009 | 2009-13 | 572 | 4335 | 16,673 | 149 |
| 2010 | 2010-10 | 544 | 3226 | 12,102 | 125 |
| 2011 | 2011-03 | 647 | 3800 | 12,310 | 138 |
| 2012 | 2012-04 | 659 | 3648 | 11,375 | 138 |
| 2013 | 2013-12 | 635 | 3679 | 13,645 | 146 |
| 2014 | 682-020 | 627 | 3387 | 13,397 | 136 |
| 2015 | 682-032 | 621 | 2995 | 9419 | 104 |
| 2016 | 682-042 | 653 | 4155 | 13,914 | 145 |
| 2017 | 682-046 | 644 | 3548 | 11,947 | 127 |
| 2018 | 682-056 | 625 | 3813 | 11,935 | 135 |
| Model | Linear Predictor | NB(θ) | Deviance Explained | Log-Likelihood | AIC | ΔAIC | AIC Weight |
|---|---|---|---|---|---|---|---|
| M0 | 0.138 | 55.5 | −9675.9 | 18,961.87 | 572.29 | 0.0 | |
| M0.1 | 0.147 | 58.6 | −9645.5 | 18,874.29 | 484.71 | 0.0 | |
| M0.2 | 0.160 | 60.8 | −9392.1 | 18,606.05 | 216.47 | 0.0 | |
| M0.3 | 0.163 | 61.5 | −9386.8 | 18,584.78 | 195.20 | 0.0 | |
| M0.4 | 0.179 | 76.7 | −9333.4 | 18,420.24 | 30.66 | 0.0 | |
| M0.5 | 0.179 | 75.6 | −9327.5 | 18,418.53 | 28.95 | 0.0 | |
| M0.6 | 0.164 | 61.9 | −9381.0 | 18,571.10 | 181.52 | 0.0 | |
| M0.7 | 0.181 | 75.9 | −9315.8 | 18,389.58 | 0.00 | 61.3 | |
| M0.8 | 0.182 | 76.8 | −9314.5 | 18,390.50 | 0.92 | 38.7 | |
| M1 | 0.230 | 40.5 | −13,664.0 | 26,955.16 | 1333.84 | 0.0 | |
| M1.1 | 0.244 | 44.1 | −13,619.0 | 26,822.37 | 1201.05 | 0.0 | |
| M1.2 | 0.288 | 51.8 | −13,242.0 | 26,256.11 | 634.79 | 0.0 | |
| M1.3 | 0.295 | 52.9 | −13,227.0 | 26,198.38 | 577.06 | 0.0 | |
| M1.4 | 0.365 | 95.1 | −12,936.0 | 25,641.25 | 19.93 | 0.0 | |
| M1.5 | 0.368 | 95.3 | −12,932.0 | 25,631.72 | 10.40 | 0.5 | |
| M1.6 | 0.289 | 52.0 | −13,240.0 | 26,251.62 | 630.30 | 0.0 | |
| M1.7 | 0.365 | 95.1 | −12,938.0 | 25,644.33 | 23.01 | 0.0 | |
| M1.8 | 0.369 | 95.3 | −12,926.0 | 25,621.32 | 0.00 | 99.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepsen, D.V.; Cubillos, L.A.; Arancibia, H. Juvenile Hake Merluccius gayi Spatiotemporal Expansion and Adult-Juvenile Relationships in Chile. Fishes 2022, 7, 88. https://doi.org/10.3390/fishes7020088
Yepsen DV, Cubillos LA, Arancibia H. Juvenile Hake Merluccius gayi Spatiotemporal Expansion and Adult-Juvenile Relationships in Chile. Fishes. 2022; 7(2):88. https://doi.org/10.3390/fishes7020088
Chicago/Turabian StyleYepsen, Daniela V., Luis A. Cubillos, and Hugo Arancibia. 2022. "Juvenile Hake Merluccius gayi Spatiotemporal Expansion and Adult-Juvenile Relationships in Chile" Fishes 7, no. 2: 88. https://doi.org/10.3390/fishes7020088
APA StyleYepsen, D. V., Cubillos, L. A., & Arancibia, H. (2022). Juvenile Hake Merluccius gayi Spatiotemporal Expansion and Adult-Juvenile Relationships in Chile. Fishes, 7(2), 88. https://doi.org/10.3390/fishes7020088