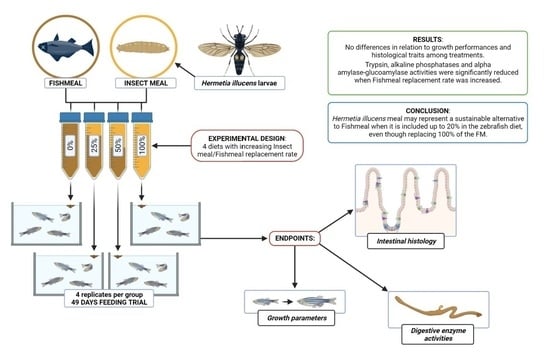
Fishmeal Replacement with Hermetia illucens Meal in Aquafeeds: Effects on Zebrafish Growth Performances, Intestinal Morphometry, and Enzymology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rearing System and Experimental Conditions
2.2. Fish Management
2.3. Experimental Design and Diet Formulations
2.4. Experimental Procedures and Measurements
2.5. Intestine Sampling
2.6. Digestive Enzyme Activities
2.6.1. Protein Extraction
2.6.2. Enzyme Assays
- Trypsin activity was determined according to Hassanabatar et al. [54] using 0.1 mM N-α-benzoyl-DL-arginine-p-nitroanilide hydrochloride (BAPNA) as the substrate in 50 mM tris-HCl pH 7.5 and 20 mM CaCl2. Samples were added to the substrate and incubated at 37 °C. The absorbance was recorded at 410 nm for 3 min with an Ultrospec 2100 UV pro spectrophotometer (Amersham Biosciences©, Little Chalfont, UK). The results of the trypsin assay were expressed as unit/mg protein;
- Alkaline phosphatase activity was determined according to Hassanabatar et al. [54,55] using 2 mM 4-nitrophenyl phosphate (4 pNPP) as the substrate in 30 mM Na2CO3 buffer (pH 9.8). Samples were added to the substrate and incubated at 37 °C. The reaction was stopped by the addition of 0.1N NaOH. Absorbance was recorded at 407 nm for 20 min with an Ultrospec 2100 UV pro spectrophotometer (Amersham Biosciences©, Little Chalfont, UK). Results of the alkaline phosphatase assay were expressed as µg BTEE (N-Benzoyl-L-tyrosine ethyl ester)/min mL µg protein;
- Alpha-amylase and glucoamylase activities were measured according to Xiao et al. [56] using soluble starch (2 g/L) as the substrate. Samples were added to the substrate and incubated at 50 °C. The reaction was stopped by the addition of 1M HCl. After the addition of iodine reagent (5 mM I2 and 5 mM KI), absorbance of samples was recorded at 580 nm with an Ultrospec 2100 UV pro spectrophotometer (Amersham Biosciences©, Little Chalfont, UK). Results of the alpha-amylase and glucoamylase assay were expressed as unit/mL mg protein.
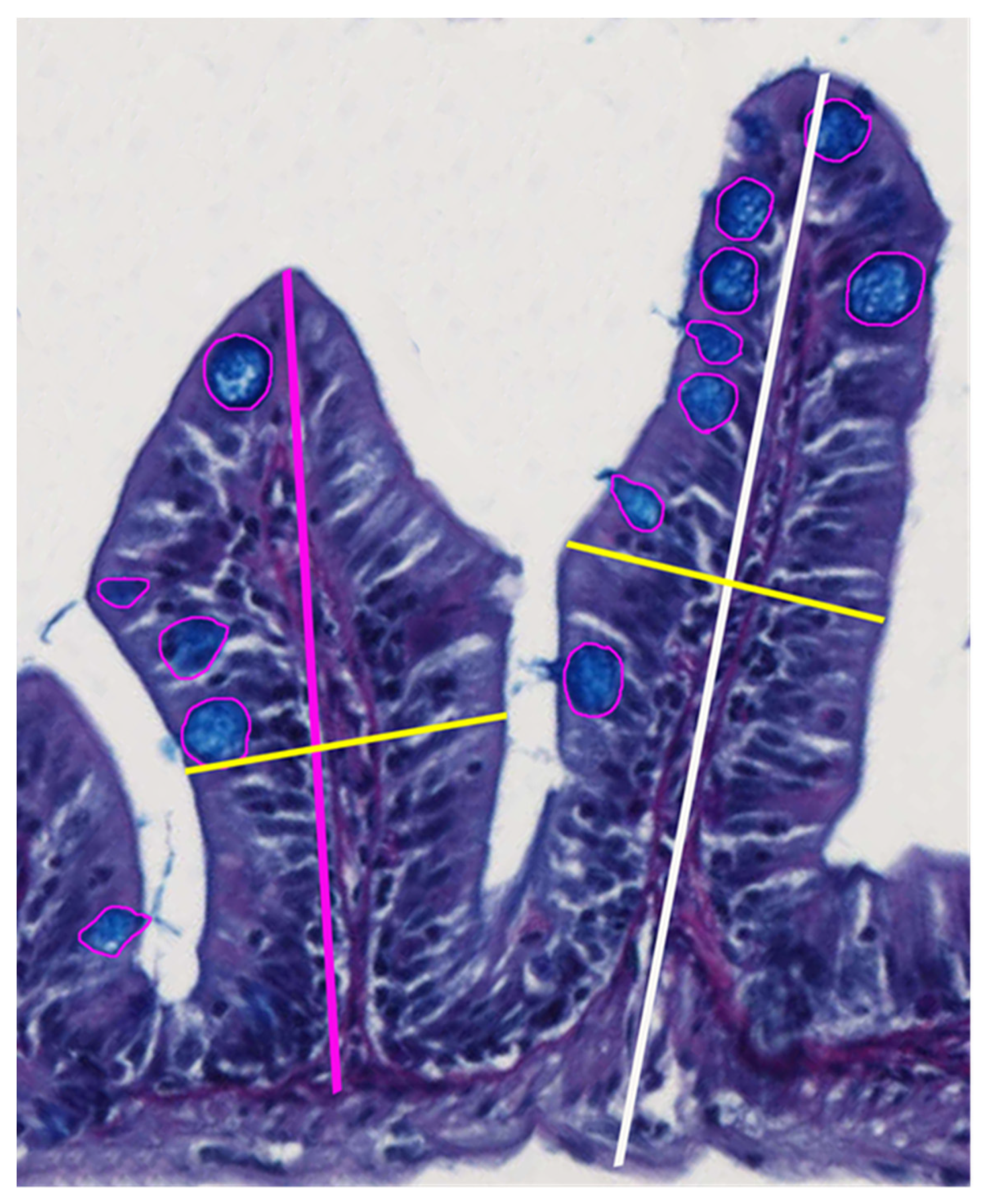
2.7. Histological Examination and Morphometry
2.8. Statistical Analysis
3. Results
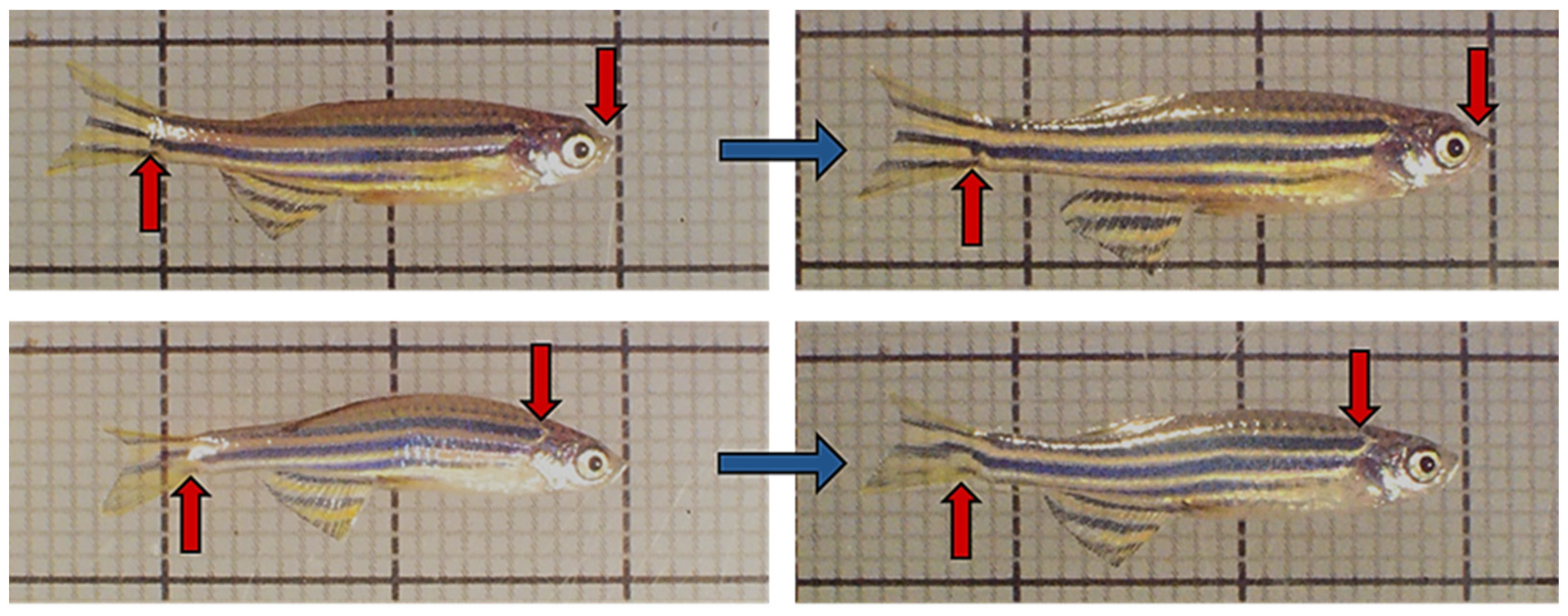
3.1. Survival Rate and Growth Performances
3.2. Intestinal Morphometry
3.3. Digestive Enzyme Activities
4. Discussion
4.1. Growth Parameters
4.2. Intestinal Morphometry
4.3. Digestive Enzyme Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.R.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of World Fisheries and Aquaculture: Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; pp. 1–210. [Google Scholar]
- Arru, B.; Furesi, R.; Gasco, L.; Madau, F.A.; Pulina, P. The Introduction of Insect Meal into Fish Diet: The First Economic Analysis on European Sea Bass Farming. Sustainability 2019, 11, 1697. [Google Scholar] [CrossRef] [Green Version]
- Moniruzzaman, M.; Damusaru, J.H.; Won, S.; Cho, S.J.; Chang, K.H.; Bai, S.C. Effects of partial replacement of dietary fish meal by bioprocessed plant protein concentrates on growth performance, hematology, nutrient digestibility and digestive enzyme activities in juvenile Pacific white shrimp, Litopenaeus, Vannamei. J. Sci. Food Agric. 2020, 100, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Song, W.; Shao, Q.; Peng, X.; Xiao, J.; Hua, Y.; Owari, B.N.; Zhang, T.; Ng, W.K. Partial Replacement of Fish Meal by Fermented Soybean Meal in Diets for Black Sea Bream, Acanthopagrus Schlegelii Juveniles. J. World Aquac. Soc. 2011, 42, 184–197. [Google Scholar] [CrossRef]
- Nishshanka, K.M.; Radampola, K.; Bulugahapitiya, V. Effects of partial replacement of dietary fishmeal using plant-protein sources on the growth performance, coloration and liver histology of guppy fry (Poecilia reticulata) in outdoor farming conditions. J. Appl. Aquac. 2021, 2021, 1–19. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Glencross, B.D.; Booth, M.; Allan, G.L. A feed is only as good as its ingredients—A review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Barroso, F.G.; de Haro, C.; Sanchez-Muros, M.J.; Venegas, E.; Martinez-Sanchez, A.; Perez-Banon, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422–423, 193–201. [Google Scholar] [CrossRef]
- Rossi, L.; Bibbiani, C.; Fierro-Sañudo, J.F.; Maibam, C.; Incrocci, L.; Pardossi, A.; Fronte, B. Selection of marine fish for integrated multi-trophic aquaponic production in the Mediterranean area using DEXi multi-criteria analysis. Aquaculture 2021, 535, 736402. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO Forestry Paper: Rome, Italy, 2013; pp. 153–160. [Google Scholar]
- Lock, E.; Arsiwalla, T.; Waagbo, R. Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquac. Nutr. 2016, 22, 1202–1213. [Google Scholar] [CrossRef]
- Stadtlander, T.; Stamer, A.; Buser, A.; Wohlfahrt, J.; Leiber, F.; Sandrock, C. Hermetia illucens meal as fish meal replacement for rainbow trout on farm. J. Insects Food Feed 2017, 3, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The Potential Role of Insects as Feed: A Multi-Perspective Review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fy prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Fronte, B.; Abramo, F.; Brambilla, F.; De Zoysa, M.; Miragliotta, V. Effect of hydrolysed fish protein and autolysed yeast as alternative nitrogen sources on gilthead sea bream (Sparus aurata) growth performances and gut morphology. Ital. J. Anim. Sci. 2019, 18, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Ginés, R.; Sweetman, J.; Izquierdo, M. Improved feed utilization, intestinal mucus production and immune parameters in sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS). Aquac. Nutr. 2011, 17, 223–233. [Google Scholar] [CrossRef]
- Langeland, M.; Lindberg, J.E.; Lundh, T. Digestive Enzyme Activity in Eurasian Perch (Perca Fluviatilis) and Arctic Charr (Salvelinus Alpinus). J. Aquac. Res. Dev. 2013, 5, 1–15. [Google Scholar] [CrossRef]
- Guerrera, M.C.; De Pasquale, F.; Muglia, U.; Caruso, G. Digestive enzymatic activity during ontogenetic development in zebrafish (Danio rerio). J. Exp. Zool. Part B 2015, 324B, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, H.; Blier, P. Trypsin Activity Measurement in Fish and Mammals. J. Aquat. Food Prod. Technol. 2007, 16, 13–26. [Google Scholar] [CrossRef]
- Yang, Y.; Wandler, A.M.; Postlethwait, J.H.; Guillemin, K. Dynamic evolution of the LPS-detoxifying enzyme intestinal alkaline phosphatase in zebrafish and other vertebrates. Front. Immunol. 2012, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Leigh, S.C.; Nguyen-Phuc, B.Q.; German, D.P. The effects of protein and fiber content on gut structure and function in zebrafish (Danio rerio). J. Comp. Physiol. B 2018, 188, 237–253. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Sheikhzadeh, N.; Roshanaei, K.; Dargahi, N.; Faggio, C. Can dietary ginger (Zingiber officinale) alter biochemical and immunological parameters and gene expression related to growth, immunity and antioxidant system in zebrafish (Danio rerio)? Aquaculture 2019, 507, 341–348. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, Z.; Ding, Q.; Yang, Y.; Bindelle, J.; Ran, C.; Zhou, Z. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fawley, J.; Gourlay, D.M. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J. Surg. Res. 2016, 202, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Ding, Q.; Wang, A.; Liu, Y.; Teame, T.; Ran, C.; Yang, Y.; He, S.; Zhou, W.; Olsen, R.E.; et al. Effects of dietary sodium acetate on food intake, weight gain, intestinal digestive enzyme activities, energy metabolism and gut microbiota in cultured fish: Zebrafish as a model. Aquaculture 2020, 523, 735188. [Google Scholar] [CrossRef]
- Arani, M.; Salati, A.P.; Safari, O.; Keyvanshokooh, S. Dietary supplementation effects of Pediococcus acidilactici as probiotic on growth performance, digestive enzyme activities and immunity response in zebrafish (Danio rerio). Aquac. Nutr. 2019, 25, 854–861. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Naef, V.; Mero, S.; Fichi, G.; D’Amore, A.; Ogi, A.; Gemignani, F.; Santorelli, F.M.; Marchese, M. Swimming in Deep Water: Zebrafish Modeling of Complicated Forms of Hereditary Spastic Paraplegia and Spastic Ataxia. Front. Neurosci. 2019, 13, 1311. [Google Scholar] [CrossRef] [PubMed]
- Ogi, A.; Licitra, R.; Naef, V.; Marchese, M.; Fronte, B.; Gazzano, A.; Santorelli, F.M. Social Preference Tests in Zebrafish: A Systematic Review. Front. Vet. Sci. 2021, 7, 590057. [Google Scholar] [CrossRef] [PubMed]
- Licitra, R.; Marchese, M.; Brogi, L.; Fronte, B.; Pitto, L.; Santorelli, F.M. Nutraceutical Screening in a Zebrafish Model of Muscular Dystrophy: Gingerol as a Possible Food Aid. Nutrients 2021, 13, 998. [Google Scholar] [CrossRef]
- Alestrom, P.; Holter, J.L.; Nourizadeh-Lillabadi, R. Zebrafish in functional genomics and aquatic biomedicine. Trends Biotechnol. 2006, 24, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Dahm, R.; Geisler, R. Learning from Small Fry: The Zebrafish as a Genetic Model Organism for Aquaculture Fish Species. Mar. Biotechnol. 2006, 8, 329–345. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Iturra, P.; Neira, R. Zebrafish as a model organism for nutrition and growth: Towards comparative studies of nutritional genomics applied to aquacultured fishes. Rev. Fish Biol. Fish. 2011, 21, 649–666. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Medrano, J.F.; Feijoo, C.G. Zebrafish as animal model for aquaculture nutrition research. Front. Genet. 2014, 5, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yossa, R.; Sarker, P.K.; Karanth, S.; Ekker, M.; Vandenberg, G.W. Effects of dietary biotin and avidin on growth, survival, feed conversion, biotin status and gene expression of zebrafish Danio rerio. Comp. Biochem. Physiol. B 2011, 160, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Ribas, L.; Piferrer, F. The zebrafish (Danio rerio) as a model organism, with emphasis on applications for finfish aquaculture research. Rev. Aquac. 2013, 5, 1–32. [Google Scholar] [CrossRef]
- Paige, C.; Hill, B.; Canterbury, J.; Sweitzer, S.; Romero-Sandoval, E.A. Construction of an affordable and easy-to-build zebrafish facility. J. Vis. Exp. 2014, 93, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book, 5th ed.; University of Oregon Press: Eugene, OR, USA, 2007; p. 45. [Google Scholar]
- National Research Council. Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Fronte, B.; Kim, C.H.; Bagliacca, M.; Casini, L.; De Zoysa, M. 1,3-1-6 ß-glucans enhance tissue regeneration in zebrafish (Danio rerio): Potential advantages for aquaculture applications. Aquac. Res. 2019, 50, 3163–3170. [Google Scholar] [CrossRef]
- Silva, F.C.; Nicoli, J.R.; Zambonino-Infante, J.L.; Le Gall, M.M.; Kaushik, S.; Gatesoupe, F.J. Influence of partial substitution of dietary fish meal on the activity of digestive enzymes in the intestinal brush border membrane of gilthead sea bream, Sparus aurata and goldfish, Carassius auratus. Aquaculture 2010, 306, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, M.; Fronte, B.; Sagona, S.; Carrozza, M.L.; Forzan, M.; Pizzurro, F.; Bibbiani, C.; Miragliotta, V.; Abramo, F.; Millanta, F.; et al. Effect of 1,3-1,6 β-Glucan on Natural and Experimental Deformed Wing Virus Infection in Newly Emerged Honeybees (Apis mellifera ligustica). PLoS ONE 2016, 11, e0166297. [Google Scholar] [CrossRef] [PubMed]
- Hassantabar, F.; Fereidouni, A.E.; Ouraji, H.; Babaei, S.; Jafarpour, A. Comparison of growth and digestive enzymes activity of kutum (Rutilus kutum) during ontogeny fed with live prey and artificial feed. Aquac. Int. 2015, 23, 597–612. [Google Scholar] [CrossRef]
- Hassanatabar, F.; Ouraji, H.; Esmaeili, A.; Babaei, S.S. Study of the Activities of Digestive Enzymes, Amylase and Alkaline Phosphatase, in Kutum Larvae, Rutilusfrisiikutum Fed Artemia Nauplii. World J. Fish Mar. Sci. 2013, 5, 266–270. [Google Scholar] [CrossRef]
- Xiao, Z.; Storms, R.; Tsang, A. A quantitative starch–iodine method for measuring alpha-amylase and glucoamylase activities. Anal. Biochem. 2006, 351, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Muin, H.; Taufek, N.M.; Kamarudin, M.S.; Razak, S.A. Growth performance, feed utilization and body composition of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed with different levels of black soldier fly, Hermetia illucens (Linnaeus, 1758) maggot meal diet. Iran. J. Fish. Sci. 2017, 16, 567–577. [Google Scholar]
- Karapanagiotidis, I.; Daskalopoulou, E.; Vogiatzis, I.; Rumbos, C.; Mente, E.; Athanassiou, C. Substitution of Fishmeal by Fly Hermetia Illucens Prepupae Meal in the Diet of Gilthead Seabream (Sparus Aurata). In Proceedings of the HydroMediT, Volos, Greece, 13–15 November 2014; pp. 110–114. [Google Scholar]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Wang, G.; Peng, K.; Hu, J.; Yi, C.; Chen, X.; Wu, H.; Huang, Y. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture 2019, 507, 144–154. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 1, 57. [Google Scholar] [CrossRef] [PubMed]
- Gasco, L.; Stas, M.; Schiavone, A.; Rotolo, L.; De Marco, M.; Dabbou, S.; Renna, M.; Malfatto, V.; Lussiana, C.; Katz, H.; et al. Use of Black Soldier Fly (Hermetia illucens) meal in rainbow trout (Oncorhynchus mykiss) feeds. In Proceedings of the Aquaculture Europe Meeting, Rotterdam, The Netherlands, 20–23 October 2015. [Google Scholar]
- Cardinaletti, G.; Randazzo, B.; Messina, M.; Zarantoniello, M.; Giorgini, E.; Zimbelli, A.; Bruni, L.; Parisi, G.; Olivotto, I.; Tulli, F. Effects of Graded Dietary Inclusion Level of Full-Fat Hermetia illucens Prepupae Meal in Practical Diets for Rainbow Trout (Oncorhynchus mykiss). Animals 2019, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Bondari, K.; Sheppard, D.C. Soldier fly, Hermetia illucens L.; larvae as feed for channel catfish, Ictalurus punctatus (Rafinesque), and blue tilapia, Oreochromis aureus (Steindachner). Aquac. Fish. Manag. 1987, 18, 209–220. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Rust, M.B. Nutritional physiology. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; The Academic Press: New York, NY, USA, 2002; pp. 367–452. [Google Scholar]
- Vargas, A.; Randazzo, B.; Riolo, P.; Truzzi, C.; Gioacchini, G.; Giorgini, E.; Loreto, N.; Ruschioni, S.; Zarantoniello, M.; Antonucci, M.; et al. Rearing Zebrafish on Black Soldier Fly (Hermetia illucens): Biometric, Histological, Spectroscopic, Biochemical, and Molecular Implications. Zebrafish 2018, 15, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Bruni, L.; Randazzo, B.; Vargas, A.; Gioacchini, G.; Truzzi, C.; Annibaldi, A.; Riolo, P.; Parisi, G.; Cardinaletti, G.; et al. Partial Dietary Inclusion of Hermetia illucens (Black Soldier Fly) Full-Fat Prepupae in Zebrafish Feed: Biometric, Histological, Biochemical, and Molecular Implications. Zebrafish 2018, 15, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Zarantoniello, M.; Randazzo, B.; Truzzi, C.; Giorgini, E.; Marcellucci, C.; Vargas-Abúndez, J.A.; Zimbelli, A.; Annibaldi, A.; Parisi, G.; Tulli, F.; et al. A six-months study on Black Soldier Fly (Hermetia illucens) based diets in zebrafish. Sci. Rep. 2019, 9, 8598. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J.S.; Ratcliffe, B.; Mandir, N.; Carr, K.E.; Goodlad, R.A. Dietary fibre and intestinal microflora: Effects on intestinal morphometry and crypt branching. Gut 1998, 42, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, C.; Couto, A.; Pérez-Jiménez, A.; Serra, C.R.; Díaz-Rosales, P.; Fernandes, R.; Corraze, G.; Panserat, S.; Oliva-Teles, A. Effects of fish oil replacement by vegetable oil blend on digestive enzymes and tissue histomorphology of European sea bass (Dicentrarchus labrax) juveniles. Fish Physiol. Biochem. 2015, 42, 203–217. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Cardenete, G.; Morales, A.E.; García-Alcázar, A.; Abellán, E.; Hidalgo, M.C. Digestive enzymatic profile of Dentex dentex and response to different dietary formulations. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 157–164. [Google Scholar] [CrossRef] [PubMed]
- García-Gasca, A.; Galaviz, M.A.; Gutiérrez, J.N.; García-Ortega, A. Development of the digestive tract, trypsin activity and gene expression in eggs and larvae of the bullseye puffer fish Sphoeroides Annulatus. Aquaculture 2006, 251, 366–376. [Google Scholar] [CrossRef]
- Bates, J.M.; Akerlund, J.; Mittge, E.; Guillemin, K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2007, 2, 371–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefi, S.; Hoseinifar, S.H.; Paknejad, H.; Hajimoradloo, A. The effects of dietary supplement of galactooligosaccharide on innate immunity, immune related genes expression and growth performance in zebrafish (Danio rerio). Fish Shellfish Immunol. 2018, 73, 192–196. [Google Scholar] [CrossRef]
- Carneiro, W.F.; Castro, T.F.D.; Orlando, T.M.; Meurer, F.; de Jesus Paula, D.A.; Virote, B.D.C.R.; Vianna, A.R.D.C.B.; Murgas, L.D.S. Replacing fish meal by Chlorella sp. meal: Effects on zebrafish growth, reproductive performance, biochemical parameters and digestive enzymes. Aquaculture 2020, 528, 735612. [Google Scholar] [CrossRef]
- Fang, L.; Liang, X.F.; Zhou, Y.; Guo, X.Z.; He, Y.; Yi, T.L.; Liu, L.W.; Yuan, X.C.; Tao, Y.X. Programming effects of high-carbohydrate feeding of larvae on adult glucose metabolism in zebrafish, Danio rerio. Br. J. Nutr. 2014, 111, 808–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Diet Ingredients | HI-0 | HI-25 | HI-50 | HI-100 |
|---|---|---|---|---|
| Black soldier fly larvae meal 1 | - | 5 | 10 | 20 |
| Fishmeal 2 | 20 | 15 | 10 | - |
| Predigested fishmeal | 7.7 | 7.7 | 7.7 | 7.7 |
| Squid meal | 14 | 14.5 | 15 | 15.5 |
| Fish gelatin | 2.5 | 2.5 | 2.5 | 2.5 |
| Soy protein concentrate | 5 | 5 | 5 | 5 |
| Pea protein concentrate | 5 | 4.5 | 4.5 | 5 |
| Wheat gluten | 14 | 14.5 | 15.5 | 16 |
| Wheat meal | 15.5 | 15 | 13.5 | 13 |
| Fish oil | 4.5 | 4.5 | 4.5 | 4 |
| Vitamin and mineral premix | 1 | 1 | 1 | 1 |
| Vitamin E | 0.03 | 0.03 | 0.03 | 0.03 |
| Brewer’s yeast | 4 | 4 | 4 | 4 |
| Soy lecithin | 6.5 | 6.5 | 6.5 | 6 |
| Antioxidant powder | 0.2 | 0.2 | 0.2 | 0.2 |
| Sodium propionate | 0.1 | 0.1 | 0.1 | 0.1 |
| Proximate analyses | ||||
| Crude protein | 60.4 | 60 | 60.2 | 59.8 |
| Crude lipid | 15.2 | 15.5 | 15.9 | 15.5 |
| Fiber | 0.8 | 1.4 | 2 | 3.3 |
| Starch | 12.1 | 11.8 | 10.9 | 10.6 |
| Ash | 5 | 4.7 | 4.4 | 3.8 |
| Gross energy (kJ/g) | 21.9 | 21.9 | 21.8 | 21.6 |
| Diets | HI-0 | HI-25 | HI-50 | HI-100 |
|---|---|---|---|---|
| Amino Acids (g 100/g of protein) | ||||
| Arginine | 4.4 | 5.5 | 6.5 | 8.6 |
| Histidine | 1.2 | 2.2 | 3.1 | 5.0 |
| Isoleucine | 2.5 | 3.6 | 4.7 | 7.0 |
| Leucine | 4.4 | 6.2 | 8.0 | 11.6 |
| Lysine | 4.0 | 5.4 | 6.8 | 9.7 |
| Threonine | 2.3 | 3.3 | 4.3 | 6.3 |
| Tryptophan | 0.4 | 0.9 | 1.3 | 2.2 |
| Valine | 2.6 | 4.4 | 6.2 | 9.8 |
| Methionine + Cysteine | 1.9 | 2.5 | 3.1 | 4.2 |
| Phenylalanine + Tyrosine | 4.8 | 5 | 5.3 | 5.8 |
| Taurine | 0.2 | 0.1 | 0.1 | 0.1 |
| Minerals and Vitamins | ||||
| Total P (%) | 0.8 | 0.8 | 0.7 | 0.6 |
| Available P (%) | 0.6 | 0.6 | 0.5 | 0.4 |
| Phytate P (%) | 0.2 | 0.1 | 0.1 | 0.1 |
| Ca (%) | 1.0 | 0.9 | 0.8 | 0.6 |
| Ca/P | 1.2 | 1.2 | 1.1 | 1.0 |
| Na (%) | 0.3 | 0.2 | 0.2 | 0.0 |
| K (%) | 0.2 | 0.2 | 0.1 | 0.0 |
| Mn (mg/kg) | 11.3 | 11 | 10.7 | 10.1 |
| Zn (mg/kg) | 23.1 | 19.7 | 16.2 | 9.4 |
| Fe (mg/kg) | 26.3 | 21.2 | 16.2 | 6.1 |
| Cu (mg/kg) | 10 | 9.8 | 9.6 | 9.2 |
| Mg (mg/kg) | 515 | 386.3 | 257.5 | 0.0 |
| Vit C (mg/kg) | 1020.4 | 1020.4 | 1020.4 | 1020.4 |
| Vit E (mg/kg) | 255.1 | 255.1 | 255.1 | 255.1 |
| Vit D (IU/kg) | 2347.3 | 2293.6 | 2240 | 2122.5 |
| Fatty Acids (%) | ||||
| C14 | 0.6 | 0.5 | 0.5 | 0.4 |
| C16 | 1.2 | 1.1 | 1.0 | 0.7 |
| C18:1n9 | 0.9 | 0.9 | 0.8 | 0.6 |
| C18:2n6 (LNA) | 0.2 | 0.2 | 0.2 | 0.2 |
| C18:3n3 (ALA) | 0.1 | 0.1 | 0.1 | 0.1 |
| C20:4n6 (ARA) | 0.02 | 0.02 | 0.01 | 0.01 |
| C20:5n3 (EPA) | 0.7 | 0.6 | 0.6 | 0.5 |
| C22:6n3 (DHA) | 0.7 | 0.7 | 0.6 | 0.5 |
| DHA/EPA | 1.1 | 1.1 | 1.0 | 1.0 |
| SFA | 2.2 | 2.0 | 1.8 | 1.3 |
| MUFA | 3.1 | 2.9 | 2.8 | 2.3 |
| PUFA | 2.0 | 1.9 | 1.7 | 1.3 |
| HI-0 | HI-25 | HI-50 | HI-100 | SEM 1 | p-Value | |
|---|---|---|---|---|---|---|
| Body weight (mg) | ||||||
| Day 1 | 84.9 | 85.0 | 85.4 | 86.0 | 1.44 | 0.9932 |
| Day 7 | 109.4 | 114.3 | 114.2 | 113.8 | 2.04 | 0.8008 |
| Day 14 | 136.6 | 143.5 | 142.8 | 139.2 | 2.76 | 0.7903 |
| Day 21 | 152.2 | 164.3 | 163.5 | 158.0 | 3.33 | 0.5459 |
| Day 35 | 178.8 | 191.0 | 189.4 | 182.1 | 3.94 | 0.6512 |
| Day 49 | 228.8 | 236.8 | 240.6 | 229.3 | 4.68 | 0.7687 |
| Body weight gain (mg) | ||||||
| Day 1–day 7 | 24.5 | 29.3 | 28.8 | 27.8 | 1.25 | 0.5335 |
| Day 8–day 14 | 27.2 | 29.2 | 28.6 | 25.4 | 1.55 | 0.7617 |
| Day 15–day 21 | 15.6 | 20.8 | 20.7 | 18.8 | 1.23 | 0.3767 |
| Day 22–day 35 | 26.6 | 26.7 | 25.9 | 24.1 | 1.63 | 0.7798 |
| Day 36–day 49 | 50.0 | 45.8 | 51.2 | 47.2 | 1.54 | 0.1956 |
| Cumulative BWg | 143.9 | 151.8 | 155.2 | 143.3 | 4.38 | 0.6921 |
| Feed intake (mg) | ||||||
| Day 1–day 7 | 35.3 | 38.7 | 36.6 | 34.7 | 1.02 | 0.8650 |
| Day 8–day 14 | 50.0 | 55.8 | 53.5 | 50.5 | 1.22 | 0.3273 |
| Day 15–day 21 | 49.0 | 48.7 | 45.5 | 46.6 | 0.55 | 0.1112 |
| Day 22–day 35 | 88.3 | 86.5 | 95.3 | 89.9 | 1.31 | 0.7563 |
| Day 36–day 49 | 90.5 | 85.6 | 80.4 | 79.8 | 1.13 | 0.8747 |
| Cumulative FI | 313.1 | 315.3 | 311.3 | 301.5 | 2.94 | 0.5016 |
| Feed Conversion Rate | ||||||
| Day 1–day 7 | 1.44 | 1.32 | 1.27 | 1.25 | 0.0287 | 0.1468 |
| Day 8–day 14 | 1.84 | 1.91 | 1.87 | 1.99 | 0.0385 | 0.5403 |
| Day 15–day 21 | 3.14 | 2.34 | 2.20 | 2.48 | 0.1124 | 0.0526 |
| Day 22–day 35 | 3.32 | 3.24 | 3.68 | 3.73 | 0.1975 | 0.7594 |
| Day 36–day 49 | 1.81 | 1.87 | 1.57 | 1.69 | 0.0502 | 0.2168 |
| Cumulative FCR | 2.18 | 2.08 | 2.01 | 2.10 | 0.0317 | 0.1791 |
| HI-0 | HI-100 | SEM 1 | p-Value | |
|---|---|---|---|---|
| Wall height to tunica serosa, dorsal side | 402 | 393 | 28.0 | 0.0798 |
| Wall height to tunica muscularis, dorsal side | 358 | 353 | 26.0 | 0.0863 |
| Wall height to tunica serosa, ventral side | 301 | 338 | 19.6 | 0.0494 * |
| Wall height to tunica muscularis, ventral side | 270 | 287 | 18.1 | 0.0271 * |
| Villi width, dorsal side | 140 | 132 | 8.2 | 0.3248 |
| Villi width, ventral side | 142 | 138 | 7.3 | 0.1221 |
| Villi height to tunica serosa, dorsal side | 355 | 366 | 24.4 | 0.1188 |
| Villi height to tunica muscularis, dorsal side | 317 | 321 | 23.9 | 0.2991 |
| Villi height to tunica serosa, ventral side | 275 | 307 | 18.4 | 0.1155 |
| Villi height to tunica muscularis, ventral side | 248 | 268 | 17.8 | 0.2457 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fronte, B.; Licitra, R.; Bibbiani, C.; Casini, L.; De Zoysa, M.; Miragliotta, V.; Sagona, S.; Coppola, F.; Brogi, L.; Abramo, F. Fishmeal Replacement with Hermetia illucens Meal in Aquafeeds: Effects on Zebrafish Growth Performances, Intestinal Morphometry, and Enzymology. Fishes 2021, 6, 28. https://doi.org/10.3390/fishes6030028
Fronte B, Licitra R, Bibbiani C, Casini L, De Zoysa M, Miragliotta V, Sagona S, Coppola F, Brogi L, Abramo F. Fishmeal Replacement with Hermetia illucens Meal in Aquafeeds: Effects on Zebrafish Growth Performances, Intestinal Morphometry, and Enzymology. Fishes. 2021; 6(3):28. https://doi.org/10.3390/fishes6030028
Chicago/Turabian StyleFronte, Baldassare, Rosario Licitra, Carlo Bibbiani, Lucia Casini, Mahanama De Zoysa, Vincenzo Miragliotta, Simona Sagona, Francesca Coppola, Letizia Brogi, and Francesca Abramo. 2021. "Fishmeal Replacement with Hermetia illucens Meal in Aquafeeds: Effects on Zebrafish Growth Performances, Intestinal Morphometry, and Enzymology" Fishes 6, no. 3: 28. https://doi.org/10.3390/fishes6030028
APA StyleFronte, B., Licitra, R., Bibbiani, C., Casini, L., De Zoysa, M., Miragliotta, V., Sagona, S., Coppola, F., Brogi, L., & Abramo, F. (2021). Fishmeal Replacement with Hermetia illucens Meal in Aquafeeds: Effects on Zebrafish Growth Performances, Intestinal Morphometry, and Enzymology. Fishes, 6(3), 28. https://doi.org/10.3390/fishes6030028