Abstract
Maclura tinctoria is a tree species native from Brazil and rich in phenolic compounds. Since plant antibacterial activity is highly associated with phenolic compound concentration, we aim to evaluate the in vitro antimicrobial activity of different extracts against fish pathogenic bacteria. In addition, some phenolic compounds have central depressant effects and can be useful in aquaculture due to possible sedative and/or anesthetic effects. Four M. tinctoria extracts were extracted separately with ethanol; leaves (LE), bark (BE), heartwood (HE), and the sapwood (SE). In vitro antimicrobial activity was tested against Aeromonas strains at concentrations of 6400 to 3.125 μg/mL. The sedative effect was evaluated for 24 h with 30 and 100 mg/L concentrations. Chemical composition was analyzed by HPLC-DAD-MS. The HE extract had the best MIC (400 µg/mL) and MBC (800 µg/mL) compared to the LE, BE, and SE extracts. LE extract induced deep sedation and the BE, SE, and HE extracts induced light sedation. Additionally, BE, SE, and HE induced a normal behavior without side effects. Polyphenolic compounds with antimicrobial activity and sedative effects were identified mainly in HE. Thus, HE extract is safe and can be used as a sedative for silver catfish.
1. Introduction
Brazil is the 14th largest producer of aquatic animals, with more than 84% of its production coming from continental aquaculture [1]. Silver catfish, Rhamdia quelen, is an endemic species from South America and the main native species raised in southern Brazil [2]. This fish is commonly infected by bacteria of the genus Aeromonas, which is responsible for large economic losses in fish farming [3] and is reported in several parts of the world and is considered the prevalent bacterial disease in freshwater fishes [4]. One of the factors that favor the occurrence of bacterial infections is the weakening of the immune system, which often results from stress in aquaculture [5]. In this way, stress control directly benefits fish health and this effect can be obtained with derivatives of secondary plant metabolism [6].
Virulence factors of the genus Aeromonas are multifactorial and involve the ability of microorganisms to adhere to host cells through protein complexes called adhesins [7]. Other factors include extracellular active products such as aerolysin, lipase, hemolysin and protease; cellular structures such as flagella, which contribute to the formation of biofilm; and the cell signaling quorum sensing [8,9].
The conventional treatment for bacterial infections in aquaculture facilities is usually done with antibiotics [10,11]. However, the increase in antimicrobial misuse, such as their use at sub-therapeutic doses, for inadequate periods, self-medication, or for non-therapeutic purposes has led to the development of resistant strains [12]. Additionally, recent studies have shown that aquatic animals can serve as reservoirs for multidrug-resistant pathogenic bacterial strains [13]. For this reason, the search for new antimicrobial agents, which are safe to the animals and the environment is so important and different natural products have been increasingly researched over the years, aiming at their future application in fish culture practices [13,14]. In this context, plant extractives appear as promising antimicrobial agents against infections caused by bacteria of the genus Aeromonas in fish farming [14,15,16], since plant species antibacterial activity is highly related to the concentration of the phenolic compounds present in their extracts [17]. Phenolic compounds are secondary metabolites essential for plant growth, development, reproduction, and protection against pathogens [18]. On the other hand, phenolic compounds often present γ-aminobutyric acid (GABA) binding affinity [19], which responds to the most abundant inhibitory neurochemical in the adult vertebrate brain [20,21].
Maclura tinctoria (L) D. Don ex Steud. (Moraceae) synonym Chlorophora tinctoria (L) Gaudich. ex B. D. Jackson [22] is a tree species native from Brazil and rich in phenolic compounds [23,24,25]. Extracts obtained from this plant species present wound healing, anti-inflammatory [26], astringent, analgesic [27], chemopreventive [28], antioxidant, and antimicrobial effects [29]. Therefore, the objective of this work was to verify the in vitro antimicrobial potential of extracts obtained from leaves (LE), bark (BE), sapwood (SE) and heartwood (HE) of M. tinctoria. In addition, in order to evaluate the potential of these extracts to control stress, experiments were performed to verify possible central depressant activity and long-term effects.
2. Results
2.1. Maclura Tinctoria Extractive Yield and Chemical Composition
Among the extracts, the M. tinctoria leaves showed the highest extractive yield (19 g%), followed by the heartwood (13 g%), bark (10 g%), and the sapwood (3 g%), respectively. The chemical composition analysis revealed the presence of compounds belonging to the polyphenols in all extracts. The HE contained five identified compounds belonging to the flavonoid class, while one of them is a dimer of flavan-3-ol, classified as proanthocyanidin (Table 1). Similarly, the LE and BE have three flavonoid class compounds and two phenolic acids (Table 2 and Table 3). Additionally, the BE has one compound from the dihydroflavonol class (Table 3). The SE extract has only two flavonoid class compounds, one chalcone, proanthocyanidin, and hydroxycinnamic acid (Table 4).

Table 1.
Major phytochemical compounds identified in the heartwood extract (HE).

Table 2.
Major phytochemical compounds identified in the leaves extract (LE).

Table 3.
Major phytochemical compounds identified in the bark extract (BE).

Table 4.
Major phytochemical compounds identified in the sapwood extract (SE).
2.2. In Vitro Antibacterial Activity
The HE presented the lowest MIC and MBC against the six strains analyzed, compared to the evaluated extracts obtained from the other plant organs (LE, BE, SE). MIC ranged from 400 μg/mL to 1600 μg/mL, and MBC ranged from 800 μg/mL to 6400 μg/mL for HE. The strains 13 (A. hydrophila) and A. veronii were the most susceptible to this extract (MIC of 400 μg/mL and MBC of 800 μg/mL for both), whereas strains of A. hydrophila ATCC 7966, 18, and 169/07 showed intermediate susceptibility (MIC of 800 μg/mL and MBC of 800 and 1600 μg/mL). Leaf extract did not present antimicrobial activity at the tested concentrations against the analyzed strains. The BE and SE samples showed weak antimicrobial activity with MICs and MBCs ranging from 6400 to >6400 μg/mL. The antibiotic florfenicol presented MICs ranging from 0.253 for A. veronii to 0.507 for isolates 13 and 18 of A. hydrophila and ATCC. The MIC of the antibiotic against E. coli ATCC 25922, quality standard, was 8.125 μg/mL (Table 5).

Table 5.
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of florfenicol and Maclura tinctoria extracts from leaves (LE), bark (BE), sapwood (SE), and heartwood (HE) against reference strains and Aeromonas isolated from fish.
2.3. Sedative and Anesthetic Effects
The objective of this assay was to verify the degree of sedation induced by extracts of M. tinctoria. In the sedation stage S2, all samples presented an induction profile similar to the DZP control (induction time 51 s), except HE and BE at 30 and 300 mg/L concentrations, respectively. At 30 mg/L the HE presented significantly longer induction time than LE, BE, and SE, but there was no statistical difference between the extracts at 100 mg/L. At 300 mg/L the induction time of BE was higher than that of SE. The sedation stage S3a was reached by LE at 30, 100, and 300 mg/L and by SE at the concentration of 300 mg/L. The other samples did not reach stage S3a during the total observation time (30 min). The extracts that reached stage S3a presented an induction profile similar to DZP. The SE at the concentration of 300 mg/L presented significantly longer induction time compared eugenol. The S3b stage was reached only by the animals exposed to LE at a concentration of 300 mg/L, with no statistical difference from eugenol. The recovery time of fish exposed to LE at 300 mg/L did not present statistical difference in comparison to DZP and eugenol controls. The groups exposed to the other samples required less time to recover swimming movements compared to DZP and EUG. Animals exposed to vehicle (ethanol 30 mg/L) did not reach any stage of sedation up to the end of observation time (30 min) (Figure 1).

Figure 1.
M. tinctoria crude extracts induce sedation in silver catfish. Sedation levels are described as S2: light sedation; S3a: sedation with partial loss of equilibrium; S3b: sedation with total loss of equilibrium; Recovery: time spend for total sedation recovery, where the animal shows normal behavior similar to those in water control. The abscissa shows the concentrations of the tested samples (in mg/L), while the ordinate represents the latency time (in seconds) until the effect was observed. Different lowercase letters indicate significant differences between samples: Leaves (LE), bark (BE), sapwood (SE), and heartwood extracts (HE) at each evaluated concentration (p < 0.05). * p < 0.05 = statistical difference from diazepam (DZP); # p < 0.05 = statistical difference from eugenol (EUG). Kruskal–Wallis test followed by Dunn’s test. (A) Stage S2—Sedation; (B) Stage S3a—Partial loss of equilibrium; (C) Stage 3b—Total loss of equilibrium; (D) Recovery.
2.4. Prolonged Exposure Experiment
The animals submitted to 30 mg/L of BE, SE, and HE showed behavior similar to the vehicle (control) at all times of observation. Fish of these groups presented high scores, which means that through 24 h the animals reached the stage of light sedation or showed normal swimming behavior. The animals submitted to LE at 30 and 100 mg/L concentrations presented behavior similar to DZP, that is, fish presented a deepening of the sedation as time went by. Through the 24 h-observation, animals reached deeper sedation stages (S3a and S3b) compared to the previous test, with a maximum duration of 30 min. Stage S5 (medullary collapse) was observed in some fish. Mortality was: LE30 mg/L 33%; LE100 mg/L 50%, and HE100 mg/L 67% (Figure 2).
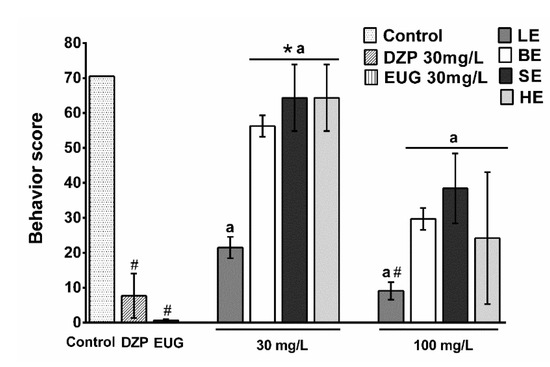
Figure 2.
Fish 24 h-behavior under sedative bath with 30 or 100 mg/L M. tinctoria extracts. Behavior score was adapted from Heldwein et al. Different lowercase letters indicate significant difference between extracts. Leaves (LE), bark (BE), sapwood (SE), and heartwood extracts (HE), Diazepam (DZP) at the evaluated concentrations (p < 0.05). The abscissa shows the concentrations of the tested samples (in mg/L), while the ordinate represents the behavior score. * p < 0.05 = statistical difference from diazepam (DZP); # p < 0.05 = statistical difference from control (Kruskal–Wallis test followed by Dunn’s test).
3. Discussion
The results obtained for M. tinctoria ethanolic extracts in the in vitro tests evidenced antibacterial activity against important pathogens in fish culture. Previous studies had described the antimicrobial activity of extracts of the same plant species, such as Lamounier et al. [29], which described their in vitro activity against oral pathogens that cause tooth decay. There are also antimicrobial activity reports for the extracts obtained from other Moraceae family plants [30,31,32,33]. Furthermore, the in vitro assay showed that HE had the most promising antibacterial activity according to the criteria established by Aligiannis et al. [34].
Studies performed with the isolated compound eugenol and the essential oils (EOs) from leaves of Hesperozigis ringens and Ocimum americanum against strains of A. hydrophila isolated from silver catfish detected equivalent MICs to eugenol and H. ringens EO (800 to 3200 μg/mL), and from 200 to 1600 μg/mL for O. americanum EO [15,16]. The MIC obtained for HE (400 μg/mL for strain 13) is within the range described by Sutili et al. [15,16] for A. hydrophila also isolated from R. quelen. Although both of these studies evaluated effects against A. hydrophila, they used different strains, which frequently present distinct susceptibilities [35]. Another important point to consider is that the EOs from the studies of Sutili et al. [15,16] and the extracts of the present study were obtained from different plant species. Therefore, to confirm the reproducibility of the assay performed, E. coli strain ATCC 25922 was used as the quality standard for the tests, presenting a MIC (8.125 μg/mL) that is within the established reliability standards for the antibiotic florfenicol (MIC between two and 16 μg/mL) [36].
The extracts’ yields found in the present study were considered satisfactory, due to the extractive process and the separation of the distinct plant organs, which causes loss of material. Radojkov et al. [37] obtained a yield of 23% for the ethanolic extract of the leaves of Morus alba, a species that belongs to the same family of M. tincoria. The lower yield found in the present study may be supported by the processing conditions of the samples and because they are from different plant species [38]. The current study found superior yields than those from Lamounier et al. [29] for the ethanolic extracts of bark (2%) and wood (4%) of M. tinctoria, even with the separation of wood in SE and HE, a result that characterizes the efficiency of the extractive process and the strategy chosen in the present study.
The sedative assay revealed that BE, SE, and HE showed a sedation induction profile similar to each other, even though they were obtained from different plant organs. Only the LE sample at the concentration of 300 mg/L took the animals to the deep sedation stage (S3b). The fish submitted to the extracts present a light sedation induction profile (stage S2) characterized by the absence of reaction to external stimuli [39]. The methanolic extract of Condalia buxifolia (Rhamnaceae) presented an induction profile similar to that found for extracts of M. tinctoria [40]. Previous studies have demonstrated the depressant activity of extracts containing flavonoids on the Central Nervous System of different animals [19,41,42]. Flavone derivatives, such as luteolin-6-C-glucoside, detected in HE, are considered good ligands for the benzodiazepine site of the GABAA receptor [19]. The same authors describe the central depressant activity of a glycoside derived from kaempferol, also present in HE.
Concentrations above 300 mg/L could promote anesthesia in silver catfish submitted to LE. However, in the case of fish undergoing pharmacological treatments involving exposure for a long period, induction of anesthesia is not desired, since it could lead fish to the S5 stage of CNS depression [43]. The concentrations of 30 and 100 mg/L induced light sedation and were therefore chosen for the long exposure experiment. Plant extracts that provide the stage of light sedation are important in fish farming, given the need to control animal stress during simple procedures involving capture and handling [44]. Plant extractives capable of reducing the stress caused by routine procedures in breeding prevent the immune system’s compromise as a result of this stress [13] and, consequently, decrease the number of bacterial infections and mortality in fish farming. According to Das et al. [45], the Ocimum sanctum leaves aqueous extract stimulated the fish immunity and increased the resistance of Labeo rohita fingerlings against A. hydrophila.
Exposure of silver catfish to 30 mg/L M. tinctoria extracts for 24 h mimicked the sedative profile that had been previously detected in the sedative trials. Only the animals exposed to 100 mg/L of LE presented a deepening of the sedation stages, with behavior similar to that observed for DZP. The fish exposed to BE, SE, and HE 30 mg/L presented a behavior similar to the vehicle control after 30 min, while those subjected to BE, SE, and HE 100 mg/L only after 10 h-exposure. Therefore, the results of the long exposure test indicated the greater safety of BE, SE, and HE at the concentration of 30 mg/L. Safety in the use of these extracts is indicated by the behavior of the animals through the 24 h-observation, which varied between normal and light sedation stages, producing no signs of toxicity or mortality. Silver catfish exposed for six hours to the methanolic extract of the leaves of C. buxifolia at 25 and 50 μL/L concentrations developed mild sedation and there was no deepening to the stage of anesthesia through the observation time [40]. This extract was considered safe for use as a sedative by the authors. The same was observed for the isolated compound of Nectandra grandiflora leaves EO, (+)-dehydrofuquinone, at the 10 and 20 mg/L concentrations [43].
The results presented in the in vitro antimicrobial activity assay, sedative, and long exposure experiments showed that HE at the concentration of 30 mg/L was the better extractive considering its biological activities. This sample showed an in vitro promising antimicrobial activity and did not induce anesthesia or any signs of toxicity or mortality in the long exposure test.
Although the MIC and MBC detected for HE in this study are higher than those detected for florfenicol, they can be considered promising. When evaluating this data, we should also consider that sedative activities detected for the extract may contribute to an increase in the resistance to bacterial infections and survival of infected fish, since it may prevent the decline of the immune defenses resulting from stress [46]. In addition, in a review by da Cunha et al. [47], the analyzed literature data indicated that extracts containing complex mixtures of components used to treat bacterial infections in fish are often effective at concentrations lower than the corresponding MIC detected in vitro. This can be explained by the pleiotropic action of most extracts containing mixtures of plant secondary metabolites as phenolics, which can interact with several targets, since they are biosynthesized by plants to protect themselves against a number of harmful agents. Therefore, plant compounds can concomitantly address a large number of different molecular targets present in both animals and procaryotic cells [48].
The identification of the chemical composition of HE revealed the presence of compounds with antimicrobial activity, such as taxifolin [49], kaempferol-3-O-rutinoside [50], and isoflavone genistein [51,52]. Polyphenolic compounds are widely studied because they show promising antimicrobial activity and, among them, flavan-3-ols, flavonols, and proanthocyanidins are the most researched because they present a broad spectrum of action and higher antimicrobial activity when compared with other polyphenols [53]. Flavonols, such as kaempferol-3-O-rutinoside and dihydroflavonols, such as taxifolin, exert their antimicrobial action through the inactivation of cell membrane proteins and also by suppression of virulence factors such as inhibition of biofilm formation, reduction of adhesion of host ligands, and neutralization of bacterial toxins [54].
Flavonols are compounds with a hydrophobic characteristic and are therefore capable of overcoming the phospholipid membranes of bacteria exerting their activity within the bacterial cell [55]. Proanthocyanidins, in turn, promote plasma membrane destabilization, enzyme inhibition, and may also block the substrates necessary for microbial growth [56]. On the other hand, the HE extract may also be related to the immunostimulatory capacity reported for genistein [57]. Additionally, M. tinctoria chemical composition (Table 1, Table 2, Table 3 and Table 4) revealed many compounds never before described for the species. Thus, the predominance of flavonoid class compounds may possibly be correlated with the M. tinctoria species functional application variety. Similarity between all plant organs is evident when we observe the presence of compounds such as caffeic acid or its derivatives in all the organs under study. Moreover, the same occurs with the compound lutein, a flavone, identified in all parts of the plant.
The HE presented the most promising results of the extracts of M. tinctoria tested in the biological assays of the present study. A promising in vitro antibacterial activity, low sedative activity, and absence of signs of toxicity in animals after 24 h-exposure, characterized this extract in the preliminary tests. Thus, HE can be considered a safe and efficient agent for use as a sedative in silver catfish, since this extract reduces fish stress during minor veterinary procedures that do not require anesthesia. However, additional studies are needed to determine its mechanisms of action as well as the concentrations to be used in other fish species.
4. Materials and Methods
4.1. Plant Material
The different plant organs (leaves, bark, sapwood, and heartwood) were collected in the State of Rio Grande do Sul, Brazil at 27°35′26″ S and 54°40′46″ W. The licensing of the collected material was processed by the Province Department of Environment, Department of Forests and Protected Areas, National Agency of Santa Rosa/RS, under registry 0030671D. The voucher was identified by the forest engineer Rodrigo Coldebella and deposited in the Herbarium of the Department of Biology, UFSM, Brazil (SMDB 17.258). Our research group obtained authorization to perform scientific activities involving the plant species through the Biodiversity Information and Authorization System (SISBIO, number 60108-1). Access to the national genetic patrimony was given by the Genetic Heritage Management Council (SisGen A9350BA).
4.2. Drugs and Reagents
To obtain the crude extracts, 95% ethanol was used (Química Moderna, Barueri, Brazil). In the in vivo tests of central depressant activity and long exposure, the benzodiazepine receptor agonist GABAa Diazepam (DPZ, Germed Pharma®, Campinas, Brazil) and Eugenol (SS White®, Rio de Janeiro, Brazil) with recognized sedative/anesthetic activity were used as positive controls [58,59]. For the in vitro antimicrobial assays the antibiotic florfenicol (Sigma Aldrich®, São Paulo, Brazil) was used. The solvents used for the chromatographic analysis were methanol (Dynamica®, Indaiatuba, Brazil), acetic acid (Sigma Aldrich®) both HPLC grade, and Ultrapure water (Milli-Q).
4.3. Plant Material Extraction
Leaves, bark, sapwood, and heartwood were separately pulverized in Willey mill (Model TE-680, TECNAL) and subjected to hot extraction with Soxhlet [60], using ethanol until exhaustion of the plant material. The extraction occurred in triplicate. Then, the crude ethanolic extracts were concentrated in a rotary evaporator (BUCHI Rotavapor, Model R-144) at 50 °C and kept in a desiccator until a constant weight for yield determination (m/m%). The extracts were lyophilized (Model L101 Liotop®, São Carlos, Brazil) for all solvent residue removal.
4.4. Fish Pathogens
Reference strains (A. hydrophila ATCC 7966 and Escherichia coli ATCC 25922) were used as control [36]. Three strains of A. hydrophila identified as 13 (MF372509), 18 (MF372510) [61], and 169/17 (MH397689) and one strain of Aeromonas veronii (MH397688), isolated from naturally infected fish (R. quelen) obtained from local fish farms, were used in the assays. Strains were isolated from skin lesions and kidney of the animals and plated on blood agar 5% and MacConkey (Kasvi®, São José dos Pinhais, Brazil). After 48 h of incubation at 28 °C, bacterial growth was observed and the colonies were submitted to biochemical and Gram staining tests [62] for identification. Molecular identification was performed by extracting DNA from colonies by the boiling method and subsequent partial sequence analysis (~1400 base pairs) of the 16S rRNA gene amplified with universal pairs according to the method developed by Fredricks and Relaman [63] and previously described by Bandeira Jr. et al. [64]. PCRs were sequenced by ACTGene Molecular Analysis (Biotechnology Center UFRGS, Porto Alegre, Brazil) with ABIPRISM 3100 automatic sequencer. Consensus sequences were assembled using four programs from the Staden package [65]. The consensus sequence was compared to GenBank available sequences using the BLASTN tool. The closest relative 16S rRNA sequences were used for identification.
4.5. Animals
For the in vivo experiments, silver catfish (R. quelen) obtained from local fish farms were used. For the evaluation of sedative and anesthetic effects, as well as for long-term exposure, silver catfish juveniles were used, with the following characteristics: 5.30 ± 0.37 g (7.74 ± 0.01 cm). The fish were transferred to the laboratory and kept in continuously aerated 250 L tanks with controlled water parameters for acclimation during seven days before the experiments. The temperature and dissolved oxygen level (19.9 ± 2.6 °C, 8.0 ± 0.7 mg/L) were determined using the YSI oxygen meter (Model Y5512, Colombus, OH, USA). For pH control (7.7 ± 0.05), the DMPH-2 device was used (Digimed, São Paulo, Brazil). Total ammonia levels (0.9 ± 0.3 mg/L) were determined using the salicylate method [66]. The animals were fed daily with commercial feed (28% crude protein) and fasted for 24 h before the experiments. The water was changed daily just after feeding to remove waste. The protocol was approved by the Ethics and Animal Welfare Committee of the Federal University of Santa Maria (Process 5307210617).
4.6. Antibacterial Activity In Vitro Evaluation
The extract activities were evaluated by the Müller–Hinton broth (MHB) microdilution method (Sigma Aldrich®). Minimal inhibitory concentration (MIC) and minimal bactericidal (MBC) were determined according to the guidelines of the Clinical Laboratory Standards Institute [67]. The extracts were diluted in ethanol and incorporated into Müller–Hinton cation adjusted broth (MHCAB, Sigma Aldrich®) to obtain the concentrations of 6400, 3200, 1600, 800, 400, 200, 100, 50, 25, 12.5, 6.25, and 3.125 μg/mL (in triplicate) [63]. The controls used were: positive control (Florfenicol®, Sigma Aldrich®, São Paulo, Brazil) at concentrations of 0.063 to 130 μg/mL (in triplicate), growth control (MHCAB, inoculum, and ethanol 70%), and negative control (MHCAB). The inoculum was prepared in 0.9% saline solution with turbidity equivalent to the McFarland 0.5 scale (≈2 × 108 CFU/mL), optical density 600 nm (OD600) 0.13 ± 0.01. From the inoculum solution, one mL was diluted in nine mL of MHCAB (≈1 × 107 CFU/mL). From this suspension, 10 μL were inoculated into each well, for a total volume of 100 μL/well (≈1 × 106 CFU/mL). MIC was defined as the lowest concentration of the sample capable of inhibiting the visible growth of the isolates after incubation at 28 °C for 24 h under aerobiosis conditions by the addition of 10 μL of the 0.1% resazurin dye solution (Sigma Aldrich®). For MBC verification, before the addition of the dye in the wells, 10 μL of each well was plated on MHCAB and incubated under the same conditions used for MIC determination. MBC was defined as the lowest concentration capable of causing strain death without visible colony growth [63].
4.7. In Vivo Experiments
The sedative and anesthetic effects, as well as the effects of long exposure to the extracts, were evaluated in order to define the most effective and safest extract and also the optimal concentration range.
4.7.1. Sedative and Anesthetic Effects
The sedative and anesthetic effects of the extracts were evaluated at 30, 100, and 300 mg/L concentrations. Positive controls diazepam (DZP) and eugenol were tested at 30 mg/L. As a negative control, ethanol was used at the highest concentration used for the previous solubilization of the samples before their addition to the water. In this experiment, 144 animals (n = 8 each treatment and concentration) were used. The behavioral protocol was adapted to evaluate induction and recovery of sedation/anesthesia [39], through the observation of five stages of induction described in Table 6. The animals were individually maintained in one-liter aquaria until they reached S4 stage or for a maximum time of 30 min. After the induction time, animals were transferred to a treatment-free aquarium to monitor the recovery time, which was considered complete when fish had normal swimming and response to external stimulus. In the recovery, animals were observed for a maximum time of 30 min. The induction and recovery time was set in the digital timer (ZSD-808, Hong Kong). After recovery, the animals were grouped according to the experimental protocol and placed in 40 L aquaria to observe any abnormal behavior, diseases, or mortality, for 48 h. This protocol follows the same patterns of observation described in other studies [14,43,58,59,68,69].

Table 6.
Fish sedation and anesthesia stages.
4.7.2. Prolonged Exposure Experiments
The long exposure and survival tests were performed to analyze the possible side effects of the extracts for 24 h. For these experiments, the best sedative concentrations were selected after the first experiment (Section 4.7.1). The extracts prolonged exposure effect in silver catfish (96 animals, n = 8) was used. Juveniles were evaluated to observe possible adverse/toxic effects induced by the extracts in the fish, at 30 mg/L and 100 mg/L sedative concentrations as described in 2.7.1. Water and vehicle (95% ethanol at the highest concentration used for sample dilution) were used as negative controls, DZP (30 mg/L) and eugenol (30 mg/L) as positive controls. The juveniles were transferred individually to one-liter aquaria and exposed to 30 mg/L or 100 mg/L of extract and observed at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, and 24 h [68]. The sedation degree of the animals was evaluated as described in the previous in vivo experiment. For each sedation or anesthesia stage, a score was assigned to the animal: normal behavior (three; stage S2) (two; stage S3a) (one; stage S3b) (0.5; stage S4) 0. Therefore, the lower the score, the deeper the stage of sedation the animal was in. After 24 h, the animals were reassembled in treatment-free aquaria to verify possible signs of toxicity.
4.8. Chromatographic Analysis
The extracts samples were analyzed by HPLC-DAD-MS. The instrument consists of a Shimadzu model Prominence UFLC (Shimatzu, Kyoto, Japan) sequentially coupled to an SPD-M20A diode-array UV-VIS detector and a QTOF Compact mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) controlled by OtofControl software. The optimized ESI-MS parameters were: capillary tension, 4500 V; drying gas temperature 215 °C; drying gas flow 10.0 L/min; fogging gas pressure 5.0 bar; RF collision, 150 Vpp; transfer time, 70 Is; pre-pulse storage, 5 Is; collision energy m/z 100; 20 eV; m/z 500; 30 eV; m/z 1000; and 35 eV using nitrogen as collision gas. Data processing was performed with Data Analysis software 4.0 (Bruker Daltonics, Bremen, Germany). The analyses were performed on a C-18 column (4.6 × 250 mm) with a particle size of 5 μM diameter and pre-column C-18. The mobile phase consisted of A: 2% acetic acid (pH 4.2) and B: methanol: acetic acid: ultrapure water at a ratio of 18:1:1, following the gradient elution: 0 min: 20% B; 0–25 min: 50% B; 25–30 min: 20% B at a flow rate of 0.8 mL/min. Peaks were identified by comparing their retention time and mass spectrum with the apparatus database, external standards, and data published in the literature. The analyzes were performed in triplicate.
4.9. Statistical Analysis
Data are reported as mean ± SEM. Data from the central depressant activity and long exposure were analyzed by Kruskal–Wallis followed by Dunn’s test. Statistical analysis was performed using the program GraphPad Prism© version 6.01 with a minimum significance level of p < 0.05.
5. Conclusions
From the four Maclura tinctoria extracts evaluated, HE presented the better results considering in vitro antibacterial activity, sedation and safety. Therefore, HE can be considering a promisor sedative for silver catfish. However, additional research is recommended, such as experiments of transport simulation, with verification of stress indicators, such as glucose and cortisol. On the other hand, considering the bioactive constituents detected in HE, experiments to assess its in vivo antibacterial and immunomodulatory activity are also recommended.
Author Contributions
Design of the experiments: L.d.C.P., B.M.H., B.B. and A.P.C.d.V.; plant collection, extraction, and analysis: L.d.C.P., P.R., R.C., L.B.B., C.P., A.G. and Q.I.G.; performing of in vivo and in vitro experiments: L.d.C.P., P.R., B.P.d.S., A.P.C.d.V., G.B.J., L.d.L.S., Q.I.G. and L.B.B.; writing or contributing to the writing of the paper: L.d.C.P., P.R., G.B.J., B.M.H. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES (Finance Code 001), PIBITI-CNPq, and FIT-UFSM.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics and Animal Welfare Committee of the Federal University of Santa Maria (Process 5307210617).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of the United Nations. The State of the World Fisheries and Aquaculture; FAO: Rome, Italy, 2014. [Google Scholar]
- Baldisserotto, B. Piscicultura continental no Rio Grande do Sul: Situação atual, problemas e perspectivas para o futuro. Cienc. Rural 2009, 39, 291–299. [Google Scholar] [CrossRef]
- Rather, M.A.; Willayat, M.M.; Wani, S.A.; Hussain, S.A.; Shah, S.A. Enterotoxin gene profile and molecular epidemiology of Aeromonas species from fish and diverse water sources. J. Appl. Microbiol. 2019, 127, 921–931. [Google Scholar] [CrossRef]
- Chenia, H.Y. Prevalence and characterization of plasmid-mediated quinolone resistance genes in Aeromonas spp. isolated from South African freshwater fish. Int. J. Food Microbiol. 2016, 231, 26–32. [Google Scholar] [CrossRef]
- de Freitas Souza, C.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential Oils as Stress-Reducing Agents for Fish Aquaculture: A Review. Front. Physiol. 2019, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Sutili, F.J.; Gatlin, D.M.; Heinzmann, B.M.; Baldisserotto, B. Plant essential oils as fish diet additives: Benefits on fish health and stability in feed. Rev. Aquacult. 2017, 10, 716–726. [Google Scholar] [CrossRef]
- Kozinska, A.; Pekala, A. Characteristics of disease spectrum in relation to species, serogroups, and adhesion ability of motile aeromonads in fish. Sci. World J. 2012, 949358. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.E.F.; Magalhães, T.C.; Souza, R.C.; Oliveira, S.T.L.; Ibelli, A.M.G.; Demarqui, F.N.; Gouveia, G.V. Environmental factors on virulence of Aeromonas hydrophila. Aquac. Int. 2017, 26, 495–507. [Google Scholar] [CrossRef]
- Jahid, I.; Mizan, M.; Ha, A.; Ha, S. Effect of salinity and incubation time of planktonic cells on biofilm formation, motility, exoprotease production, and quorum sensing of Aeromonas hydrophila. Food Microbiol. 2015, 49, 142–151. [Google Scholar] [CrossRef]
- Bebak, J.; Wagner, B.; Burnes, B.; Hanson, T. Farm size, seining practices, and salt use: Risk factors for Aeromonas hydrophila outbreaks in farm-raised catfish, Alabama, USA. Prev. Vet. Med. 2015, 118, 161–168. [Google Scholar] [CrossRef]
- Serrano, P.H. Responsible use of antibiotics in aquaculture. In FAO Fisheries Technical Paper 469 (Rome; United Nations); Food and Agriculture Organization of the United Nations: Rome, Italy, 2005. [Google Scholar]
- Adegoke, A.A.; Faleye, A.C.; Singh, G.; Stenstrom, T.A. Antibiotic Resistant Superbugs: Assessment of the Interrelationship of Occurrence in Clinical Settings and Environmental Niches. Molecules 2016, 22, 29. [Google Scholar] [CrossRef]
- Citarasu, T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2009, 18, 403–414. [Google Scholar] [CrossRef]
- Silva, L.L.; Balconi, L.S.; Gressler, L.T.; Garlet, Q.I.; Sutili, F.J.; Vargas, A.P.C.; Heinzmann, B.M. S-(+)- and R-(-)-linalool: A comparison of the in vitro anti-Aeromonas hydrophila activity and anesthetic properties in fish. Acad. Bras. Cienc. 2017, 89, 203–212. [Google Scholar] [CrossRef]
- Sutili, F.J.; Kreutz, L.C.; Noro, M.; Gressler, L.T.; Heinzmann, B.M.; de Vargas, A.C.; Baldisserotto, B. The use of eugenol against Aeromonas hydrophila and its effect on hematological and immunological parameters in silver catfish (Rhamdia quelen). Vet. Immunol. Immunopathol. 2014, 157, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Sutili, F.J.; Silva, L.L.; Gressler, L.T.; Gressler, L.T.; Battisti, E.K.; Heinzmann, B.M.; Baldisserotto, B. Plant essential oils against Aeromonas hydrophila: In vitro activity and their use in experimentally infected fish. J. Appl. Microbiol. 2015, 119, 47–54. [Google Scholar] [CrossRef]
- Rodríguez Vaquero, M.J.; Tomassini Serravalle, L.R.; Manca de Nadra, M.C.; Strasser de Saad, A.M. Antioxidant capacity and antibacterial activity of phenolic compounds from argentinean herbs infusions. Food Contr. 2010, 21, 779–785. [Google Scholar] [CrossRef]
- Tanase, C.; Cosarca, S.; Muntean, D.L. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Loscalzo, L.M.; Wasowski, C.; Marder, M. Neuroactive flavonoid glycosides from Tilia petiolaris DC. extracts. Phytother. Res. 2009, 23, 1453–1457. [Google Scholar] [CrossRef]
- Mueller, T.; Vernier, P.; Wullimann, M.F. A phylotypic stage in vertebrate brain development: GABA cell patterns in zebrafish compared to mouse. J. Comp. Neurol. 2006, 494, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.S.; Rosah, T.W.; Cirone, J.; O’Meara, G.F.; Haythornthwaite, A.; Newman, R.J.; Myers, J.; Sur, C.; Howell, O.; Rutter, A.R.; et al. Sedation and anesthesia mediated by distinct GABAA receptor isoforms. J. Neurosci. 2003, 23, 8608–8617. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.E.R. Espécies Arbóreas Brasileiras; Embrapa Florestas: Curitiba, Brazil, 2003. [Google Scholar]
- Cioffi, G.; Morales, E.L.; Braca, A.; Tommasi, N. Antioxidant chalcone glycosides and flavanones from Maclura (Chlophora) tinctoria. J. Nat. Prod. 2003, 66, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Elsohly, H.N.; Joshi, A.S.; Nimrod, A.C.; Walker, L.A.; Clark, A.M. Antifungal chalcones from Maclura tinctorial. Planta Med. 2001, 67, 87–89. [Google Scholar] [CrossRef]
- Groweiss, A.; Cardellina, J.H.; Boyd, M.R. HIV-Inhibitory prenylated xanthones and flavones from Maclura tinctoria. J. Nat. Prod. 2000, 63, 1537–1539. [Google Scholar] [CrossRef]
- Pott, A.; Pott, V.J. Plantas do Pantanal; Embrapa: Brasília, Brazil, 1994. [Google Scholar]
- Duarte, M.R.; Gomes, J.B.; Santos, R.H.; Yano, M. Leaf microscopic characters of Maclura tinctoria (L.) D. DON EX STEUD., Moraceae. Visão Acadêmica 2012, 13, 4–15. [Google Scholar] [CrossRef]
- Calderon, A.I.; Angerhof, C.K.; Pezzuto, J.M.; Farnsworth, N.R.; Foster, R.; Condit, R.; Soejarto, D.D. Forest plot as a tool to demonstrate the pharmaceutical potential of plants in a tropical forest of Panamá. Econ. Bot. 2000, 54, 278–294. [Google Scholar] [CrossRef]
- Lamounier, K.C.; Cunha, L.C.; de Morais, S.A.; de Aquino, F.J.; Chang, R.; do Nascimento, E.A.; Cunha, W.R. Chemical analysis and study of phenolics, antioxidant activity, and antibacterial effect of the wood and bark of Maclura tinctoria (L.) D. Don ex Steud. Evid. Based Complement Alternat. Med. 2012, 2012, 451039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hussain, F.; Rana, Z.; Shafique, H.; Malik, A.; Hussain, Z. Phytopharmacological potential of different species of Morus alba and their bioactive phytochemicals: A review. Asian Pac. J. Trop. Biomed. 2017, 7, 950–956. [Google Scholar] [CrossRef]
- Mawa, S.; Husain, K.; Jantan, I. Ficus carica L. (Moraceae): Phytochemistry, Traditional Uses and Biological Activities. Evid. Based Complement Alternat. Med. 2013, 2013, 974256. [Google Scholar] [CrossRef] [PubMed]
- Pethakamsetty, L.; Ganapaty, S.; Bharathi, K.M. Phytochemical and antimicrobial examination of the root extracts of Morus indica. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 75–80. [Google Scholar]
- Trinetta, V.; Morgan, M.T.; Coupland, J.N.; Yucel, U. Essential oils against pathogen and spoilage microorganisms of fruit juices: Use of versatile antimicrobial delivery systems. J. Food Sci. 2017, 82, 471–476. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef]
- Radlinski, L.; Conlon, B.P. Antibiotic efficacy in the complex infection environment. Curr. Opin. Microbiol. 2018, 42, 19–24. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated from Aquatic Animals; Second Informational Supplement. Document VET03/VET04-S2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Radojkovic, M.; Zekovic, Z.; Vidovic, S.; Kocar, D.; Maskovic, P. Free radical scavenging activity, total phenolic and flavonoid contents of mulberry (Morus spp. L., Moraceae) extracts. Hem. Ind. 2012, 66, 547–552. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Atanasov, A.G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trends Analyt. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Gomes, D.P.; Chaves, B.W.; Becker, A.G.; Baldisserotto, B. Water parameters affect anaesthesia induced by eugenol in silver catfish, Rhamdia quelen. Aquacult. Res. 2011, 42, 878–886. [Google Scholar] [CrossRef]
- Becker, A.G.; Cunha, M.A.; Garcia, L.O.; Zeppenfeld, C.C.; Parodi, T.V.; Maldaner, G.; Baldisserotto, B. Efficacy of engenol and methanolic extract of Condalia buxifolia during the transport of the silver catfish Rhandia quelen. Neotrop. Ichthyol. 2013, 11, 675–681. [Google Scholar] [CrossRef]
- Gazola, A.C.; Costa, G.M.; Castellanos, L.; Ramos, F.A.; Reginatto, F.H.; Lima, T.C.M.d.; Schenkel, E.P. Involvement of GABAergic pathway in the sedative activity of apigenin, the main flavonoid from Passiflora quadrangularis pericarp. Rev. Bras. Farmacog. 2015, 25, 158–163. [Google Scholar] [CrossRef]
- Jager, A.K.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471–1485. [Google Scholar] [CrossRef]
- Garlet, Q.I.; Pires, L.C.; Silva, D.T.; Spall, S.; Gressler, L.T.; Burger, M.E.; Heinzmann, B.M. Effect of (+)-dehydrofukinone on GABAA receptors and stress response in fish model. Braz. J. Med. Biol. Res. 2016, 49, e4872. [Google Scholar] [CrossRef]
- Ross, L.G.; Ross, B. Anaesthetic and Sedative Techniques for Aquatic Animals; Blackwell Science: Oxford, UK, 2008; Volume 3. [Google Scholar]
- Das, R.; Raman, R.P.; Saha, H.; Singh, R. Effect of Ocimum sanctum Linn. (Tulsi) extract on the immunity and survival of Labeo rohita (Hamilton) infected with Aeromonas hydrophila. Aquacult. Res. 2015, 46, 1111–1121. [Google Scholar] [CrossRef]
- da Cunha, J.A.; Sutili, F.J.; Oliveira, A.M.; Gressler, L.T.; Scheeren, C.A.; Silva, L.L.; Vaucher, R.A.; Baldisserotto, B.; Heinzmann, B.M. The essential oil of Hyptis mutabilis in Ichthyophthirius multifiliis infection and its effect on hematological, biochemical, and immunological parameters in silver catfish, Rhamdia quelen. J. Parasitol. 2017, 103, 778–785. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, J.A.; Heinzmann, B.M.; Baldisserotto, B. The effects of essential oils and their major compounds on fish bacterial pathogens—A review. J. Appl. Microbiol. 2018, 125, 328–344. [Google Scholar] [CrossRef]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef]
- Fongang, Y.S.F.; Bankeu, J.J.K.; Ali, M.S.; Awantu, A.F.; Zeeshan, A.; Assob, C.N.; Tsamo, E. Flavonoids and other bioactive constituents from Ficus thonningii Blume (Moraceae). Phytochem. Lett. 2015, 11, 139–145. [Google Scholar] [CrossRef]
- Bisignano, G.; Sanogo, R.; Marino, A.; Aquino, R.; D’Angelo, V.; Germano, M.P.; Pizza, C. Antimicrobial activity of Mitracarpus scaber extract and isolated constituents. Lett. Appl. Microbiol. 2000, 30, 105–108. [Google Scholar] [CrossRef]
- Choi, H.; Park, J.-S.; Kim, K.-M.; Kim, M.; Ko, K.-W.; Hyun, C.-G.; Kim, S.-Y. Enhancing the antimicrobial effect of genistein by biotransformation in microbial system. J. Ind. Eng. Chem. 2018, 63, 255–261. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Li, X.; Xu, Q.; Feng, Y.; Yang, S. Isoflavones from green vegetable soya beans and their antimicrobial and antioxidant activities. J. Sci. Food Agric. 2018, 98, 2043–2047. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.; Bhatia, A. Genistein: A multipurpose isoflavone. Int. J. Green Pharm. 2009, 3, 176. [Google Scholar] [CrossRef]
- Benovit, S.C.; Silva, L.L.; Salbego, J.; Loro, V.L.; Mallmann, C.A.; Baldisserotto, B.; Heinzmann, B.M. Anesthetic activity and bio-guided fractionation of the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. in silver catfish Rhamdia quelen. Acad. Bras. Cienc. 2015, 87, 1675–1689. [Google Scholar]
- Cunha, M.A.d.; Zeppenfeld, C.C.; Garcia, L.d.O.; Loro, V.L.; Fonseca, M.B.d.; Emanuelli, T.; Baldisserotto, B. Anesthesia of silver catfish with eugenol: Time of induction, cortisol response and sensory analysis of fillet. Cienc. Rural 2010, 40, 2107–2114. [Google Scholar] [CrossRef]
- European Pharmacopoeia. European Directorate for the Quality of Medicines; Pharmacopoeia, E., Ed.; European Pharmacopoeia: Strassbourg, France, 2010. [Google Scholar]
- Bandeira, G., Jr.; Pês, T.S.; Saccola, E.M.H.; Sutili, F.J.; Rossi, W., Jr.; Murari, A.L.; Var, A.C. Potential uses of Ocimum gratissimum and Hesperozygis ringens essential oils in aquaculture. Ind. Crops Prod. 2017, 97, 484–491. [Google Scholar] [CrossRef]
- Quin, P.J.; Carter, M.E.; Markey, B.K.; Carter, G.R. Aeromonas, Plesiomonas and Vibrio species. Clin. Vet. Microbiol. 1994, 1, 243–253. [Google Scholar]
- Fredricks, D.N.; Relaman, D.A. Improved amplification of microbial DNA from blood cultures by removal of the PCR inhibitor sodium polyanetholesulfonate. J. Clin. Microbiol. 1996, 36, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, G., Jr.; Sutili, F.J.; Gressler, L.T.; Ely, V.L.; Silveira, B.P.; Tasca, C.; Baldisserotto, B. Antibacterial potential of phytochemicals alone or in combination with antimicrobials against fish pathogenic bacteria. J. Appl. Microbiol. 2018, 125, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Staden, R.; Beal, K.F.; Bonfield, J.K. The staden package. Met. Mol. Biol. 1998, 132, 115–130. [Google Scholar]
- Verdouw, H.; Van Echteld, C.J.A.; Dekkers, E.M.J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- CLSI. Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated from Aquatic Animals; Approved Guideline-Second Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Heldwein, C.G.; Silva, L.L.; Reckziegel, P.; Barros, F.M.C.; BUrger, M.E.; Baldisserotto, B.; Heinzmann, B.M. Participation of the GABAergic system in the anesthetic effect of Lippia alba (Mill.) N.E. Brown essential oil. Braz. J. Med. Biol. Res. 2012, 45, 436–443. [Google Scholar] [CrossRef]
- Silva, L.L.; Garlet, Q.I.; Benovit, S.C.; Dolci, G.; Mallmann, C.A.; Burger, M.E.; Heinzmann, B.M. Sedative and anesthetic activities of the essential oils of Hyptis mutabilis (Rich.) Briq. and their isolated components in silver catfish (Rhamdia quelen). Braz. J. Med. Biol. Res. 2013, 46, 771–779. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).