Abstract
Bisphenol A (BPA) and tetrabromobisphenol A (TBBPA) are widely used industrial chemicals, ubiquitously present in the environment. While BPA is a well-known endocrine disruptor and able to affect all levels of the teleost reproductive axis, information regarding TBBPA on this subject is very limited. Using primary cultures from Atlantic cod (Gadus morhua), the present study was aimed at investigating potential direct effects of acute (72 h) BPA and TBBPA exposure on cell viability and the expression of reproductive-relevant genes in the pituitary. The results revealed that both bisphenols stimulate cell viability in terms of metabolic activity and membrane integrity at environmentally relevant concentrations. BPA had no direct effects on gonadotropin gene expression, but enhanced the expression of gonadotropin-releasing hormone (GnRH) receptor 2a, the main gonadotropin modulator in Atlantic cod. In contrast, TBBPA increased gonadotropin transcript levels but had no effect on GnRH receptor mRNA. In conclusion, both anthropogenic compounds display endocrine disruptive properties and are able to directly interfere with gene expression related to reproductive function in cod pituitary cells at environmentally relevant concentrations in vitro.
1. Introduction
During the last few decades, attention towards the potentially harmful effects of industrial chemicals released into the environment has increased dramatically. Many of these chemicals are endocrine disruptive compounds (EDCs), capable of interfering with normal endocrine function in animals, including fish. One important and susceptible endocrine system is that of reproduction. The physiological compartments of vertebrate reproduction comprise of the brain—pituitary—gonadal (BPG) axis, where neuroendocrine and endocrine substances relay communication between the different axis levels. In teleosts, gonadotropin-releasing hormone (GnRH), released through neuron fibers from the preoptic area and binding to its receptors in the pituitary, stimulates gonadotropin synthesis and release [1]. The gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), in turn, bind to receptors in the gonads, stimulating gametogenesis and steroidogenesis. Once in the bloodstream, sex steroids will provide feedback to the higher levels of the BPG axis [2,3,4].
Bisphenol A (BPA) and its halogenated derivative tetrabromobisphenol A (TBBPA) are widely used industrial and commercial chemicals, the former mainly used in the production of polycarbonate plastics and epoxy resins, and the latter used as a flame retardant in combustible products [5,6]. Both bisphenols are ubiquitously detected in the environment, although at highly variable concentrations dependent on sample matrix and location [7,8,9,10,11,12]. Peak measurements of BPA have been 21 µg/L in surface waters, 56 µg/kg dry weight (DW) in suspended solids and 17.2 mg/L in landfill leachates [7,8,9]. Seawater levels are usually below 1 µg/L, but BPA could potentially be leaching at marine sites of accumulated plastic waste. Further, BPA leaches faster and may withstand degradation longer in saltwater than in freshwater [7,8,13,14,15,16]. Measured peak levels of TBBPA have been 4.87 µg/L in lake waters, 600 µg/kg DW in sewage sludge, 9.8 mg/kg DW in freshwater sediment and 1.8 µg/L in seawater [10,11,12].
BPA is a well-known endocrine disruptor and has been demonstrated to act on all levels of the teleost BPG-axis; brain [17,18,19,20,21], pituitary [22,23,24,25], and gonads [26,27,28,29]. Considerably less information is currently available for TBBPA in this regard, with only a few teleost reproductive studies conducted. These studies indicate that TBBPA can affect teleost reproductive success through decreased egg production, delayed hatching, and decreased hatching rate [28,30,31]. Estrogenic activity from TBBPA exposure has been demonstrated in mammalian in vitro systems [32,33,34], while in teleosts, estrogenic activity has, to our knowledge, not been studied in vitro, and results in vivo are conflicting [30,35,36,37,38,39]. However, the capacity of TBBPA to act as a thyroid-disrupting compound has clearly been demonstrated in both mammalian and teleost assays, e.g., TBBPA can bind transthyretin with higher potency than the natural ligand thyroxine [30,33,40,41,42].
For both BPA and TBBPA, the vast majority of reproductive studies have been on gonads, with fewer studies focusing on the higher BPG-axis levels. For TBBPA, no such studies exist in teleosts, while to our knowledge only two studies exist for other vertebrate taxa [43,44]. While BPA is rather well-studied, most teleost reports are from small, freshwater species. The aim of the present study was therefore to investigate potential direct effects of BPA and TBBPA at the pituitary level in a marine teleost, the Atlantic cod (Gadus morhua). Using pituitary primary cultures from sexually maturing individuals, cell viability and expression of reproductive related genes, i.e., the gonadotropins (FSHb and LHb) and two gonadotropic GnRH receptors (GnRHr1b and GnRHr2a), following acute exposure to a range of concentrations were assessed.
2. Materials and Methods
2.1. Animals
Atlantic cod of both sexes were captured from the southern Norwegian coast (approx. 60° N) on four separate occasions. Specific ethical approval for this study was not needed as the animals themselves were not experimentally treated (Norwegian legislation for use of animals in research, Chapter II, §6). Care was nonetheless taken so that stress and suffering of the animals were minimized. All fish were euthanized by swift decapitation at location prior to immediate dissection of pituitary and gonads. Dissected pituitaries were kept in modified L-15 medium (Life Technologies, Carlsbad, CA, USA, see below for modifications) on ice until culture preparation. After dissection, body and gonads were weighted and the gonadosomatic index (GSI; (Gonadal weight/Total body weight) × 100) calculated. As GSI by itself is not a precise indicator of sexual maturity, gonads were also visually inspected and staged according to von Krogh et al. [3] (Supplementary data). In total, four cultures were prepared using pooled pituitaries from sexually maturing donor fish (n = 48; body weight: 1.48 ± SD 0.56 kg; GSI: 6.35 ± SD 3.28). Sex ratios of donors within cultures were 3M/3F, 3M/1F, 11M/6F, and 10 M/11F (M: males; F: females).
2.2. Dispersed Pituitary Primary Cell Cultures
The present study followed a previously described, optimized protocol for primary cultures of Atlantic cod pituitaries [45]. The optimized conditions included cell density, pCO2, and incubation temperature, as well as pH and osmolality for working solutions. In short, pooled pituitaries were chemically and mechanically dissociated and seeded in culture wells pre-coated with poly-l-lysine (0.1 mg/mL, Sigma-Aldrich, St. Louis, MO, USA) at a density of 1.5 × 105 cells/cm2. Cells were incubated in modified L-15 medium, adjusted to 320 mOsm, at 12 °C in a humidified atmosphere of 0.5% CO2 (pCO2; 3.8 mmHg, which resulted in a medium pH of 7.85). After 24 h, culture medium was replaced to remove damaged and detached cells.
2.3. BPA and TBBPA Exposure
Stock solutions of bisphenol A (BPA; 4,4′-(propane-2,2-diyl)diphenol, Sigma-Aldrich, CAS: 80-05-7, ≥99%) and tetrabromobisphenol A (TBBPA; 4,4′-(propane-2,2-diyl)bis(2,6-dibromophenol), Sigma-Aldrich, CAS: 79-94-7, 97%) were prepared in 100% ethanol (EtOH; Kemetyl, Kolbotn, Norway) and stored at −20 °C for up to one week. Prior to cell exposure, stocks were diluted to the desired concentration in modified L-15 medium, with working solutions having a final EtOH concentration of 0.2% (34.25 mM). For each experiment, controls w/wo EtOH (solvent control/control blank) were included. For viability tests, exposure doses were 10−9 to 10−3 M (0.22 to 228,290 µg/L) for BPA and 10−9 to 10−4 M (0.54 to 54,390 µg/L) for TBBPA (n = 6–8 per dose). For gene expression studies, exposure doses were 10−9 to 10−5 M (n = 6 per dose) for both compounds. Note that, due to the limited number of pituitaries, not all doses were included in all four cultures, leading the number of replicates to vary between treatments. Cultures were allowed to settle until day 4, by then the cell count was stable and the cells had started to spurt outgrowths, before being exposed to either BPA or TBBPA. Exposure was given as a single dose and incubation lasted 72 h.
2.4. Viability Assays
Viability testing was carried out using two non-toxic fluorescent indicator dyes, AlamarBlue (AB) and 5-carboxyfluorescein diacetate-actetoxymethyl ester (CFDA-AM) (both from Life Technologies). These assays indicate metabolic activity and plasma membrane integrity, respectively, and measure the conversion of a non-fluorescent dye into a fluorescent dye by enzymes present in intact and viable cells [46]. The test procedures followed the description by Hodne et al. [45]. In short, cells were seeded in 96-well plates (Corning, Amsterdam, The Netherlands) and incubated for 4 days before being exposed to either BPA or TBBPA for 72 h as described above. At day 7, culture medium in all wells was replaced with 100 μL Tris buffer (50 mM, pH 7.5) containing both 5% AB and 4 µM CFDA-AM (from 4 mM stock in dimethyl sulfoxide (DMSO)). After 30 min of incubation, the concentration of fluorescent products was measured simultaneously for both probes using a Bio-Tek FLX 800 fluorescence plate reader (Bio-Tek Instruments Inc., Winooski, VT, USA). Data was collected with Gen5 Data Analysis Software (Bio-Tek Instruments Inc.). As a positive control for cell toxicity, serving as intra/inter assay control, each plate included additional wells that were exposed to copper sulfate (CuSO4; 0.156–2.5 mM, n = 6 per dose) for the last 24 h and analyzed alongside the experimental wells at day 7. The effects from CuSO4 exposure were comparable between individual plates and cultures (data not shown) and similar to previously published data from this cell model (see von Krogh et al. [47], Supporting information), indicating stable cultures.
2.5. Quantification of Gene Expression
2.5.1. RNA Extraction and cDNA Synthesis
Cells used for gene expression analysis were seeded in 24-well plates (Corning), exposed to either BPA or TBBPA at day 4 as described above, and harvested at day 7. Cells were lysed and homogenized by pipetting in Trizol (Life Technologies), from where total RNA was extracted, before being re-suspended in 10 µL RNase-free water (Ambion, Thermo Fisher Scientific, Waltham, MA, USA). DNase treated RNA (TURBO DNase-free (Ambion)) was quantified spectrophotometrically (NanoDrop, Thermo Fisher Scientific), and the quality assessed by electrophoretic validation (Bioanalyzer, Agilent Technologies, Santa Clara, CA, USA) of the RNA Integrity Number (RIN). RNA samples with RIN numbers above 8 were allowed further analysis. Using 500 ng total RNA, first strand cDNA synthesis was performed using random hexamer primers and Super Script III (Life Technologies) according to standard procedures. cDNA was stored at −20 °C until qPCR.
2.5.2. Primers and Reference Genes
qPCR primers (Table 1) were designed using Primer3 shareware (http://frodo.wi.mit.edu/primer3/input.htm) and theoretically tested for possible hairpin loops and primer dimer formations using Vector NTI (Life Technologies). In each primer pair, one primer targeted an exon-exon border to avoid amplification of potential traces of genomic DNA. The genes of interest were LHb (GenBank ID: DQ402374), FSHb (GenBank ID: DQ402373), GnRHr1b (GenBank ID: GU332297) and GnRHr2a (GenBank ID: GU332298.1), all related to pituitary reproductive function. Four reference genes, arp2, bactin, ubiquitin, and ef1a, were tested using Bestkeeper Software [48], giving quantification cycle value (Cq), geometric means, and standard deviations of 27.06 (±0.39), 21.96 (± 0.45), 22.19 (±0.43), and 20.40 (±0.39), respectively. ef1a (GenBank ID: DQ402371.1) was considered most stably expressed and was subsequently used to normalize the qPCR data.

Table 1.
qPCR primers used in the present study.
2.5.3. qPCR Analysis
Using the LightCycler 480 platform (Roche, Basel, Switzerland), qPCR analyses were carried out as previously described [45,50]. Three non-template negative control (NTC) reactions were included for each primer pair by substituting the cDNA template with nuclease-free water (Ambion). To account for plate-to-plate variation, a standard positive calibrator control, prepared by mixing cDNA from all individual samples, was also included on every plate in triplicate. All samples were run in duplicate. Each PCR reaction (10 µL) mixture contained 5 µL of SYBR Green I master mix (Roche), 1 µl (5 µM) of forward primer, 1 µL (5 µM) of reverse primer, and 3 µl of diluted (1:10) cDNA. The qPCR reactions were carried out using an initial step for 10 min at 95 °C to activate the Taq polymerase, followed by 42 cycles consisting of 10 s at 95 °C (denaturation), 10 s at 60 °C (annealing), and elongation at 72 °C for 6 s. After every elongation, the fluorescence was measured and used to determine the Cq. Based on cDNA dilution curves, qPCR efficiencies (E) were determined. Combined with Cq values, E were used to calculate the relative expression [51,52] of each sample:
Relative expression = Etarget∆Cq(calibrator − sample) * Ereference∆Cq(sample − calibrator)
Specificity of the qPCR assay was confirmed by agarose gel electrophoresis and amplicon sequencing at assay set-up, and by melting curve analysis based on slowly heating the reaction mixture from 65 °C to 98 °C directly following each individual PCR reaction.
2.6. Statistical Analysis and Data Presentation
Statistical analysis was performed using the JMP Pro14 software (SAS Institute Inc, Cary, NC, USA). As gene expression and cell viability occur at the level of the cell, and each culture well consisted of their own unique mixture of cells from multiple animals, individual wells were treated statistically as samples. Technical replicates from individual wells were averaged into one observation. Technical replicates had a variation of 0.26% (±0.69), while average variance between wells per treatment was 17.1% (±9.63). Fold changes of exposed samples relative to their respective solvent control mean were calculated and used in the subsequent analysis for both gene expression and viability data. To maintain control variance in the analyses, control samples were calculated in the same manner. All data were tested for normality and equal variances by Shapiro–Wilk W test and Levene’s test, respectively. If the criteria for parametric testing was met, a one-way ANOVA was performed, followed by Dunnett’s method comparing individual groups to the solvent control if these were found to have originated from different populations. However, most data did not meet the assumptions of parametric testing, even after transformation, so non-parametric testing was used. To assess population differences, the Wilcoxon rank-sum test was used to compare solvent control with control blank, whereas the Kruskal–Wallis test was used to compare treatment groups. In the latter case, if the null hypothesis was rejected, statistical differences between treatment groups and the solvent control were assessed by the Steel method. The significance level for rejection of the null-hypothesis was set to 0.05. Scatter plots were made using Prism 8 (GraphPad Software, San Diego, CA, USA). In each graph, the mean ± standard error of the mean (SEM) is indicated. Outliers of more than 2-fold difference to the group mean were excluded from the dataset, while all outliers were included if the statistical test was non-parametric.
3. Results
3.1. Solvent Control vs Control Blank
For every culture prepared, control wells with and without 0.2% EtOH (solvent control and control blank, respectively) added to the media were included. The AB and CFDA-AM viability assays performed on these cells revealed that the solvent had statistically significant negative effects on both metabolic activity and membrane integrity (Supplementary data, Figure S1). No influence on gene expression was detected from the solvent (Supplementary data, Figure S2). Note that data from exposed cells in the following sections are compared to the effects seen in solvent control cells.
3.2. BPA Exposure
BPA at all experimental doses (10−9 M to 10−3 M) affected cell metabolic activity (Figure 1A), while all except 10−9 M affected membrane integrity (Figure 1B). A hormesis effect was detected in both assays, with low doses stimulating and high doses inhibiting cell viability. At 10−3 M, no membrane integrity was detected. In the AB assay, lower BPA concentrations increased data variance compared to higher concentrations. At 10−4 and 10−3 M, the cellular outgrowths visible at lower concentrations and control had disappeared completely (see example in Supplementary data, Figure S3).

Figure 1.
Viability in terms of metabolic activity (AlamarBlue (AB) assay) (A) and membrane integrity (5-carboxyfluorescein diacetate-actetoxymethyl ester (CFDA-AM) assay) (B) in Atlantic cod pituitary cells after 7 days of primary culture w/wo Bisphenol A (BPA) added to the culture media for the last 72 h. Each data point represents the sample fold change relative to mean solvent control. For each column, the group mean ± SEM is indicated (n = 13–30). Statistical significance (* p < 0.05, *** p < 000.1) was assessed using the Kruskal–Wallis test followed by the Steel method.
Gene expression levels of gonadotropin subunits FSHb and LHb (Figure 2A,B, respectively), and of GnRH receptor GnRHr1b (Figure 3A), were unaffected by BPA exposure at all doses tested (10−9 M to 10−5 M). In contrast, doses from 10−7 M to 10−5 M increased GnRHr2a transcription levels (Figure 3B).
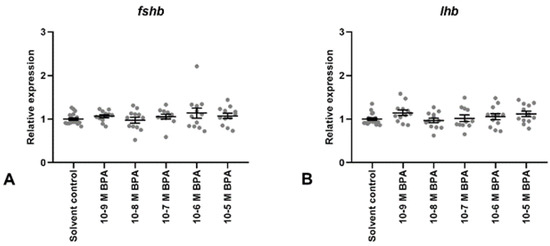
Figure 2.
Gene expression of gonadotropin subunits (FSHb; (A), LHb; (B)) in Atlantic cod pituitary cells after 7 days of primary culture w/wo BPA added to the culture media for the last 72 h. Each data point represents the sample fold change relative to mean solvent control. For each column, the group mean ± SEM is indicated (n = 11–18). No statistical differences between groups were detected (tested by Kruskal–Wallis; (A), or one-way ANOVA; (B)).
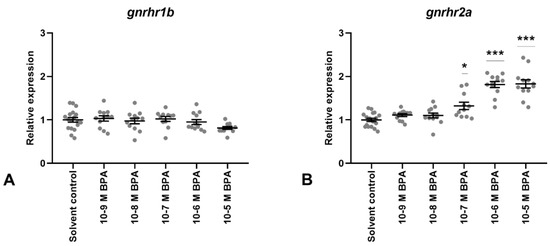
Figure 3.
Gene expression of two gonadotropin-releasing hormone receptors (GnRHr1b; (A), GnRHr2a; (B)) in Atlantic cod pituitary cells after 7 days of primary culture w/wo BPA added to the culture media for the last 72 h. Each data point represents the sample fold change relative to mean solvent control. For each column, the group mean ± SEM is indicated (n = 11–18). Statistical significance (* p < 0.05, *** p < 000.1) was assessed using a one-way ANOVA followed by Dunnett’s method.
3.3. TBBPA Exposure
All experimental doses of TBBPA (10−9 M to 10−4 M) significantly affected cell viability, both in terms of metabolic activity and membrane integrity (Figure 4A,B, respectively). As with BPA, a hormesis effect was detected for both parameters. TBBPA cytotoxicity appeared ten times more potent than that of BPA on these cells, as negative effects were detected from 10−5 M TBBPA. At 10−4 M, no membrane integrity was detected. At 10−5 and 10−4 M, no cellular outgrowths were visible through the microscope.
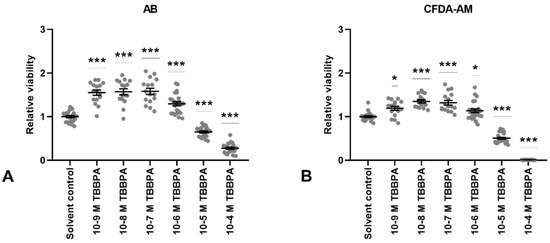
Figure 4.
Viability in terms of metabolic activity (AB assay; (A)) and membrane integrity (CFDA-AM assay; (B)) in Atlantic cod pituitary cells after 7 days of primary culture w/wo tetrabromobisphenol A (TBBPA) added to the culture media for the last 72 h. Each data point represents the sample fold change relative to mean solvent control. For each column, the group mean ± SEM is indicated (n = 16–24). Statistical significance (* p < 0.05, *** p < 000.1) was assessed using the Kruskal–Wallis test followed by the Steel method.
Small, but statistically significant, increases in FSHb expression levels (Figure 5A) were observed in samples treated with 10−9 M to 10−7 M TBBPA. Similarly, a non-significant tendency was seen after 10−6 M exposure (p = 0.11). Levels of LHb mRNA (Figure 5B) increased significantly after 10−9, 10−8, and 10−6 M treatment, while a small, non-significant, increase was observed in 10−7 M (p = 0.17) samples. No changes were detected after 10−5 M TBBPA exposure.
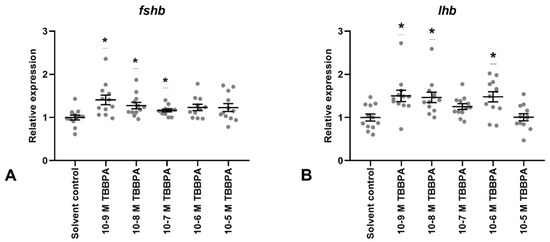
Figure 5.
Gene expression of gonadotropin subunits (FSHb; (A), LHb; (B)) in Atlantic cod pituitary cells after 7 days of primary culture w/wo TBBPA added to the culture media for the last 72 h. Each data point represents the sample fold change relative to mean solvent control. For each column, the group mean ± SEM is indicated (n = 11–12). Statistical significance (* p < 0.05) was assessed using the Kruskal–Wallis test followed by the Steel method.
Gene expression levels of both GnRH receptors (GnRHr1b and grnrh2a; Figure 6A,B, respectively) was unaffected by TBBPA concentrations of 10−9 M to 10−6 M, but decreased significantly after 10−5 M TBBPA treatment. In terms of gene expression, TBBPA exposed samples displayed larger in-group variation than BPA exposed samples.
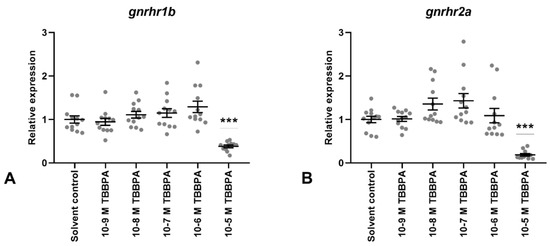
Figure 6.
Gene expression of two gonadotropin-releasing hormone receptors (GnRHr1b; (A), GnRHr2a; (B)) in Atlantic cod pituitary cells after 7 days of primary culture w/wo TBBPA added to the culture media for the last 72 h. Each data point represents the sample fold change relative to mean solvent control. For each column, the group mean ± SEM is indicated (n = 11–12). Statistical significance (*** p < 000.1) was assessed using the Kruskal–Wallis test followed by the Steel method.
4. Discussion
All wildlife is potentially susceptible to the impacts of anthropogenic activity. The substantial use and subsequent environmental release of synthetic compounds are examples of such activity and a source of increasing concern. This study demonstrates that single, acute exposure to either BPA or TBBPA at environmentally relevant concentrations can affect cell viability and reproduction-related gene expression in Atlantic cod pituitary cells, potentially affecting the reproductive capacities of this species.
4.1. Methodological Considerations
To obtain physiologically relevant results using in vitro systems for toxicological testing, it is crucial that cells are kept under physiologically relevant conditions [45,53]. Nevertheless, it is common practice to use mammalian protocols on fish tissue cultures, with only the incubation temperature adjusted to accommodate differences between mammalian and fish physiology. The present study followed protocols optimized for Atlantic cod physiology, regarding not only temperature, but also cell density, pCO2, and pH and osmolality of the working solutions and incubation media [45]. These optimized conditions allow cultured pituitary cells to maintain stable membrane potentials, fire action potentials, and to exhibit steady GnRH responses for at least two weeks. Moreover, cell viability is significantly improved compared to traditional cell culture conditions [45].
To be able to expose the pituitary cells to high concentrations of BPA and TBBPA, the bisphenols were initially dissolved in EtOH. The working concentration of 0.2% did not affect gene expression of any gene analyzed, but did negatively affect cell viability compared to those incubated in EtOH free media (control blank). Consequently, all exposed cells were compared to that of the solvent control. Most studies use either EtOH or dimethyl sulfoxide (DMSO) as the solvent for BPA and TBBPA, both of which are able to induce cytotoxicity [54,55]. Despite this, effects from the solvent itself are rarely reported, making comparison between studies difficult in this regard. For future studies, we would recommend lower EtOH working concentrations, as negative effects were detected here. The water solubility of BPA and TBBPA is 120–300 mg/L and 4.16 mg/L at 25 °C, respectively, with decreasing solubility at lower temperatures [5,15]. The cell medium used for the cod cultures was prepared and incubated at 12 °C. The highest doses administered to the cells thus exceed the water solubility threshold for these compounds and are unlikely to be environmentally relevant for Atlantic cod. They may still, however, be mechanistically informative. It should also be noted that high concentrations, similar to the higher doses administered in this study, have been detected in landfill leachates (17.2 mg/L for BPA) [9] and freshwater sediment (9.8 mg/kg DW for TBBPA) [10], and could potentially reach other wildlife. In marine waters, levels are usually below 1 µg/L (4.39 nM) for BPA and below 1.8 µg/L (3.3 nM) for TBBPA [7,12,16], which corresponds roughly to the two lowest doses, 10−9 and 10−8 M (0.23 and 2.28 µg/L for BPA, and 0.54 and 5.44 µg/L for TBBPA, respectively), used in this study. In Atlantic cod liver, detected levels of BPA and TBBPA have been 107.2 and 9 ng/g lipid weight (LW), respectively [56].
Cells were exposed once (at 4 day post plating) and incubated for 72 h prior to harvest and analysis. While the half-life of BPA and TBBPA in fish is rather short, <1 day [5,15], in a pituitary cell culture, the metabolism is expected to be slower than in the intact body, as the main site of metabolism is in the liver. The final concentrations of the compounds or possible metabolites formed during the incubation were not measured. Metabolites may have differential properties than the parent compound, and we cannot rule out the possibility that any observed effect described here is a result of such.
4.2. BPA
BPA, often referred to as a xenoestrogen because of its ability to act as an estrogen agonist, binds to estrogen receptors (ERS) and promotes endogenous 17β-estradiol (E2) effects in vertebrates [9]. Not surprisingly, the vast majority of reproductive studies on BPA in fish has been on ovaries or hepatic vitellogenin production, which are important sites of estrogenic activity. The studies reveal that in many teleosts, including Atlantic cod, BPA is able to induce vitellogenin production and act as an estrogen [35,37,57,58]. In addition to the gonads and liver, estrogen acts on the higher levels of the reproductive axis. Correspondingly, in the brain of developing zebrafish (Danio rerio) larvae, BPA has been shown to activate estrogenic markers, such as enhanced transcription of brain aromatase (cyp19a1b), through Er-dependent transcription [19,21]. In a recent study using Atlantic cod pituitary cultures, 72 h of 25 ng/mL E2 exposure had no effect on gene expression levels (FSHb, LHb, GnRHr1b, GnRHr2a) in cells from sexually maturing donors [3]. In the current study, BPA had no effect on FSHb, LHb, or GnRHr1b at any concentration tested but was able to stimulate GnRHr2a expression at 10−7 to 10−5 M (22.8–2282.9 µg/L), the lowest dose being similar to the E2 dose previously used by von Krogh et al. [3]. This indicates that BPA is able to induce GnRHr2a expression through some Er-independent pathway. While E2 did not induce gene expression in maturing Atlantic cod pituitary cells, it stimulated the expression of both FSHb and GnRHr2a in cells from sexually mature fish [3]. Keeping this is mind, it is possible that BPA exposure at different stages of the reproductive cycle could, like E2, induce differential effects.
Another physiological aspect of E2 is the ability to stimulate cell proliferation, including at the pituitary level [59]. In sexually maturing Atlantic cod pituitary cultures, 25 ng/mL, but not 2.5 ng/mL, E2 stimulated cell viability [3]. Here, however, BPA was able to stimulate viability at lower concentrations than did E2, increasing metabolic activity from 10−9 M and membrane integrity from 10−8 M. Similar proliferative potency has been seen in the offspring of female mice, with increased pituitary proliferation and gonadotrope numbers when exposed prenatally to just 0.5 µg/kg/day of BPA [60]. In concurrence with concentrations considered safe by authorities (Predicted no effect concentration (PNEC) for marine waters; 0.15 µg/L) [6], no adverse effects were detected after BPA exposure at this level. However, metabolic activity was stimulated by concentrations close to the safe limit (i.e., 10−9 M/0.228 µg/L), indicating that cellular mechanisms may be affected even at this level. Both through visual inspection and cell viability testing, doses of ≥10−4 M were found to induce cytotoxicity, and at 10−3 M, no membrane integrity was detected. Similar results have been measured in a zebrafish hepatocyte cell line (ZFL), with 24h LC50 and 96h LC50 for BPA of 367.1 and 357.6 µM, respectively [61]. In chicken embryonic hepatocytes, the 36h LC50 for BPA was somewhat lower, calculated at 61.7 µM [62].
In the present study, BPA exposure did not affect transcript levels of FSHb, LHb, or GnRHr1b at any dose but increased GnRHr2a expression at ≥10−7 M. To our knowledge, there are no other studies looking at this in vitro, making direct comparison difficult. There are, however, some studies in vivo. The first teleost study looking at the effect of BPA exposure on gonadotropins was conducted by Rhee et al. [22], using the mangrove killifish (Kryptolebias marmoratus) as the model. Waterborne BPA exposure increased transcript levels of the gonadotropin β-subunits in both juvenile (300 µg/L for 24 h) and adult fish (600 µg/L for 96 h). Similar results have been reported from zebrafish. Qiu et al. [23] investigated BPA exposure during development (from 2 to 120 hpf) in zebrafish larva and found increased mRNA levels of FSHb after 1000 µg/L and LHb after 10, 100, and 1000 µg/L treatments. In juvenile zebrafish, exposure to 10−5 M BPA for 20 days increased LHb, but decreased FSHb pituitary expression [24]. In contrast, the recent work of Wang et al. [25], showed that female goldfish (Carassius auratus) exposed to 1, 50, and 500 µg/L BPA for 30 days had reduced levels of FSHb and LHb transcripts, whereas no effect was detected in males. The discrepancies between the studies described above and also our own results could be due to species-specific differences, for instance in BPA Er affinity, uptake, or metabolism. Furthermore, experimental design (e.g., in vivo vs in vitro), age of the experimental animals, and exposure time could all affect the outcome. Conflicting results have also been reported on the effects of BPA in mammalian studies. Results are conflicting, also in mammalian reports. For instance, rats treated postnatally from day 21–35 with 2.4. µg/kg/day had decreased serum LH and pituitary LHb levels [63], while adult male rats exposed to 5 and 25 mg/kg/d for 40 days, had reduced serum FSH and LH, but increased FSHb and LHb transcript levels [64]. Moreover, female mice treated with BPA at 0.5 µg/kg/day had increased gonadotropin mRNA levels, whereas mice treated with 50 µg/kg/day had decreased levels [60].
The GnRH receptors mediate hypothalamic GnRH responses on the pituitary cells, and in Atlantic cod, GnRHr2a is thought to be the main modulator of gonadotropin regulation [65]. In Atlantic cod, expression of GnRHr1b and GnRHr2a are susceptible to glucocorticoid regulation, and the latter also to sex steroid regulation in Atlantic cod [3,47], making them both likely targets for endocrine disruption. Presently, GnRHr1b expression was unaffected by BPA, while GnRHr2a transcription increased following exposure to 10−7, 10−6, and 10−5 M BPA. Acute exposure to ≥0.1 µM BPA could thereby, assuming translation into protein, increase GnRHr2a receptor levels, enhancing GnRH sensitivity and subsequent LH production. Also in this regard, there are no in vitro studies for comparison. However, in the killifish (K. marmoratus), in vivo exposure to 600 µg/L BPA for 96 h increased brain/pituitary transcript levels of GnRHr, an orthologue of Atlantic cod GnRHr2a, in secondary males, similarly to our findings [66]. In contrast, no effect was seen in hermaphrodite killifish. Li et al. [67] found no effect on brain GnRHr (also a GnRHr2a orthologue) expression from 1, 10, 50, 125, and 250 mg/kg BPA exposure in tongue sole (Cynoglossus semilaevis) females, whereas a decrease was seen in males exposed to 250 mg/kg BPA. While the present study did not detect any effects from BPA on GnRHr1b levels, the orthologue GnRHr1a, was significantly upregulated in brains of adult female rare minnows (Gobiocypris rarus) following 15 µg/L BPA exposure for 35 days [17]. No effect was detected in males. The above mentioned studies describe brain levels, or in one case a mixture between brain and pituitary levels, of GnRHr, and we have not managed to find a teleost study looking solely at pituitary GnRHr levels. In mammals, however, pituitary GnRHr, the paralogue to teleost GnRHr, showed increased transcript levels after 40 days of 5 and 25 mg BPA/kg/d in adult male Wistar rats [64]. In female mice pituitaries, GnRHr transcripts increased after treatment with 0.5 µg/kg/day BPA, but decreased after 50 µg/kg/day treatment [60]. Although little information is available, it appears as if there are both species and gender differences regarding the effect of BPA on GnRHr gene expression. In the present study, due to a limited number of available fish and lack of external dimorphic traits in Atlantic cod, the pituitary cultures were prepared as a mixture between male and female donors. For future mechanistic studies, it seems to be worth pursuing cultures separated by sex.
4.3. TBBPA
The effects of TBBPA on fish reproduction is far less studied than that of BPA. Though studies have indicated that TBBPA can affect teleost reproductive success [28,30,31], there are no previous studies, known to us, looking at the higher levels of the BPG-axis in this animal group. Here, we demonstrate that TBBPA can stimulate pituitary cell viability and gene expression of gonadotropin β-subunits at acute, environmentally relevant concentrations.
In the present study, 10−9 to 10−6 M TBBPA exposure stimulated both metabolic activity and membrane integrity in cod pituitary cells. At some doses, the cell viability levels of TBBPA exposed cells exceeded the level of not only the solvent control, but also the control blank samples, most likely reflecting cell proliferation. Similar findings, albeit at higher concentrations, were previously demonstrated in a rat pituitary tumor cell line, GH3, where 10−6 to 10−4 M TBBPA induced cell growth and increased growth hormone (GH) production [41]. While E2 may be a stimulator of proliferation, as mentioned above, the estrogenic properties of TBBPA is still debated. Nevertheless, in the human breast adenocarcinoma cell line, MCF-7, estrogenic proliferation was seen following 24 h TBBPA exposure up to 500 µM [34]. This is a much higher concentration than that which induced cytotoxicity in cod pituitary cells, ≥10−5 M (10 µM), indicating differential sensitivity between cell types. However, our findings are in concert with other teleost studies. For instance, in ZFL cells, the 24 h LC50 and 96 h LC50 was 4.0 and 4.2 µM, respectively [61]. The zebrafish standard embryo assay demonstrated that TBBPA ≥ 0.75 mg/L (1.38 µM) caused lethality or malformation [68]. Similar results are reported for zebrafish larvae, where the 96 h LC50 for TBBPA was 5.27 mg/L (9.7 µM) [37]. Many mammalian studies also report similar findings. In GH3 cells, measuring the reduction of blue resazurin dye to red fluorescent resorufin, the same cytochrome as used in the AlamarBlue assay, cytotoxicity was detected at doses >1 µM [33]. Moreover, in primary cultures of rat cerebellar granule cells, trypan blue measurements, indicating loss of membrane integrity in stained cells, demonstrated a 24 h LC50 value of 7 µM TBBPA [69]. The same study demonstrated that all membrane integrity was lost at 20 µM TBBPA, in concert with the present results.
The cytotoxicity of EtOH was apparently counteracted by both bisphenols tested in the present study. However, the EtOH reduced cell viability was not reflected in reduced gene expression compared to control blank cells. Similarly, the cytotoxicity induced by 10−5 M TBBPA exposure did not affect FSHb and LHb expression, which both remained at the level of the solvent control. The lowered cell viability did, however, correlate with reduced gene expression of the GnRHr receptors that were otherwise unaffected by TBBPA treatment. It is not clear to us what might cause this discrepancy and further studies are needed for elucidation.
As mentioned above, studies on the potential effects of TBBPA at the higher levels of the BPG axis are currently very limited. To our knowledge, there are only two vertebrate studies, only one of which concerns gonadotropin levels. Further, to our knowledge, no study has investigated the effect of TBBPA exposure on GnRH receptor expression or possible direct effects at the pituitary level. van der Ven et al. [44] found no obvious changes in pituitary, nor gonadotrope, histology after TBBPA exposure in a one-generation study using Wistar rats. However, male pituitary weight dose-dependently increased. The authors could not conclude the mechanisms behind this, but suggested that it might be caused by feedback stimulation by the observed decreased levels of circulating thyroxine (T4). In a recent study by Zhang et al. [43], adult male frogs (Rana nigromaculata) were exposed to 0.001–1 mg/L TBBPA for 14 days. Doses 0.001–0.1 mg/L decreased serum LH, while 0.1 and 1 mg/L decreased serum FSH. This is in the opposite direction of our findings, where TBBPA stimulated gene expression of both gonadotropins, even at environmentally relevant concentrations. On the other hand, TBBPA treatment also led to increased serum testosterone and E2 levels in the male frogs. It is therefore possible that the reduced FSH and LH levels were a result of negative feedback from the sex steroids at the brain and pituitary level, rather than direct effects from TBBPA. It is also possible that the steroid action masked potential direct effects from TBBPA at the pituitary level. While direct effects on gonadotropin expression are evident from the present findings, it remains to be seen if this could lead to physiological alterations in vivo for Atlantic cod.
The mechanisms at which TBBPA affects gene expression and other reproductive endpoints remains to be assessed. However, TBBPA has the potential to act as a thyroid hormone agonist [41], and disruption of the thyroid axis can lead to sexual dysfunction [70]. Furthermore, thyroid hormones are thought to be involved in the seasonal regulation of reproduction [71]. It would therefore be interesting to investigate possible TBBPA effects throughout the reproductive cycle. Not only for the connection to thyroid hormones and because available information on the reproductive effects of TBBPA is limited, but also because the sensitivity of potential endocrine targets might differ through the different stages of sexual maturity [3,47].
5. Conclusions
The present study demonstrates that single, acute, and environmentally relevant doses of BPA or TBBPA are able to directly affect both cell viability and gene expression in Atlantic cod dispersed pituitary cells in vitro. Cell viability was stimulated by low dose exposure to both phenols, while cytotoxicity was evident only at high, non-environmentally relevant doses. Bromination seemingly increased cytotoxicity, as TBBPA exposure decreased pituitary cell viability at 10-fold lower doses than BPA. BPA stimulated gene expression of the GnRH receptor 2a, the main gonadotropin modulator in Atlantic cod, while TBBPA stimulated gene expression of both gonadotropins. The differential effects seen from BPA and TBBPA exposure at the transcriptional level indicate that they act on different receptors or activate different pathways.
Supplementary Materials
The following are available online at https://www.mdpi.com/2410-3888/4/3/48/s1, Figure S1: Viability assays, controls, Figure S2: Gene expression, controls, Figure S3: Morphological changes, control cells vs high BPA exposure cells.
Author Contributions
Conceptualization, K.v.K., E.R., T.M.H., and F.-A.W.; data curation, K.v.K. and R.N.-L.; formal analysis, K.v.K.; funding acquisition, T.M.H. and F.-A.W.; investigation, K.v.K.; methodology, K.v.K., R.N.-L., and T.M.H.; project administration, F.-A.W.; resources, E.R. and F.-A.W.; supervision, E.R., T.M.H., and F.-A.W.; validation, K.v.K., E.R., R.N.-L., T.M.H., and F.-A.W.; visualization, K.v.K.; writing—original draft, K.v.K.; writing—review and editing, E.R., R.N.-L., T.M.H., and F.-A.W.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Ketil Hylland, Ørjan Karlsen, and the crew at R/V Trygve Braarud for help aquiring cod. We kindly thank Ian Mayer for proofreading the manuscript. Our appreciation goes also to Gersende Maugars for phylogeny assistance. This research was fundedby the Research Council of Norway (Grant Nos. 184851 and 191825) and by the Norwegian University of Life Sciences.
Conflicts of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Yaron, Z.; Gur, G.; Melamed, P.; Rosenfeld, H.; Elizur, A.; Levavi-Sivan, B. Regulation of fish gonadotropins. In International Review or Cytology—A Survey of Cell Biology; Jeon, K.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 225, pp. 131–185. [Google Scholar]
- Levavi-Sivan, B.; Bogerd, J.; Mananos, E.L.; Gomez, A.; Lareyre, J.J. Perspectives on fish gonadotropins and their receptors. Gen. Comp. Endocrinol. 2010, 165, 412–437. [Google Scholar] [CrossRef] [PubMed]
- von Krogh, K.; Bjørndal, G.T.; Nourizadeh-Lillabadi, R.; Hodne, K.; Ropstad, E.; Haug, T.M.; Weltzien, F.A. Sex steroids differentially regulate FSHb, LHb and GnRHr expression in Atlantic cod (Gadus morhua) pituitary. Reproduction 2017, 154, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Weltzien, F.A.; Andersson, E.; Andersen, Ø.; Shalchian-Tabrizi, K.; Norberg, B. The brain-pituitary-gonad axis in male teleosts, with special emphasis on flatfish (pleuronectiformes). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 447–477. [Google Scholar] [CrossRef] [PubMed]
- WHO/ICPS. Environmental Health Criteria 172: Tetrabromobisphenol A and Derivatives; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- EU. European Union Risk Assessment Report for 4,40-isopropylidenediphenol (bisphenol-A); Office for Official Publications of the European Communities Communities: Luxembourg, 2008. [Google Scholar]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Wong, C.K.C.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlstrom, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Allchin, C.R.; Zegers, B.N.; Haftka, J.J.; Boon, J.P.; Belpaire, C.; Leonards, P.E.; Van Leeuwen, S.P.; De Boer, J. Distribution and fate of HBCD and TBBPA brominated flame retardants in North Sea estuaries and aquatic food webs. Environ. Sci. Technol. 2004, 38, 5497–5504. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, S.; Liu, H.; Yan, Z. Tetrabromobisphenol A: Tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ. Sci. Pollut. Res. Int. 2012, 19, 4090–4096. [Google Scholar] [CrossRef]
- Gong, W.-J.; Zhu, L.-Y.; Jiang, T.-T.; Han, C. The occurrence and spatial-temporal distribution of tetrabromobisphenol A in the coastal intertidal zone of Qingdao in China, with a focus on toxicity assessment by biological monitoring. Chemosphere 2017, 185, 462–467. [Google Scholar] [CrossRef]
- Sajiki, J.; Yonekubo, J. Leaching of bisphenol A (BPA) to seawater from polycarbonate plastic and its degradation by reactive oxygen species. Chemosphere 2003, 51, 55–62. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F. Bisphenol A degradation in seawater is different from that in river water. Chemosphere 2005, 60, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Koniecko, I.; Falkowska, L.; Krzymyk, E. Occurrence and distribution of bisphenol A and alkylphenols in the water of the gulf of Gdansk (Southern Baltic). Mar. Pollut. Bull. 2015, 91, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Wang, L.H.; Wang, X.Q.; Liu, S.Z.; Xu, P.; Wang, H.P.; Wu, T.T.; Zhang, Y.Y.; Zheng, Y.; Li, M.; et al. Bisphenol A affects gene expression of gonadotropin-releasing hormones and type I GnRH receptors in brains of adult rare minnow Gobiocypris rarus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 157, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Jahan, N.; Khaliq, S.; Lone, K.P. Modulation of brain kisspeptin expression after bisphenol-A exposure in a teleost fish, Catla catla. Fish. Physiol. Biochem. 2019, 45, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Genco, M.C.; Megrelis, L.; Ruderman, J.V. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc. Natl. Acad. Sci. USA 2011, 108, 17732–17737. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Abril, N.; Morales-Prieto, N.; Monterde, J.; Ayala, N.; Lora, A.; Moyano, R. Hypothalamic-pituitary-ovarian axis perturbation in the basis of bisphenol A (BPA) reproductive toxicity in female zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 156, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Cano-Nicolau, J.; Vaillant, C.; Pellegrini, E.; Charlier, T.D.; Kah, O.; Coumailleau, P. Estrogenic Effects of Several BPA Analogs in the Developing Zebrafish Brain. Front. Neurosci. 2016, 10, 112. [Google Scholar] [CrossRef]
- Rhee, J.-S.; Kim, R.-O.; Seo, J.S.; Kang, H.S.; Park, C.-B.; Soyano, K.; Lee, J.; Lee, Y.-M.; Lee, J.-S. Bisphenol A modulates expression of gonadotropin subunit genes in the hermaphroditic fish, Kryptolebias marmoratus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 456–466. [Google Scholar] [CrossRef]
- Qiu, W.H.; Zhao, Y.L.; Yang, M.; Farajzadeh, M.; Pan, C.Y.; Wayne, N.L. Actions of Bisphenol A and Bisphenol S on the Reproductive Neuroendocrine System During Early Development in Zebrafish. Endocrinology 2016, 157, 636–647. [Google Scholar] [CrossRef]
- Chen, W.T.; Lau, S.W.; Fan, Y.Q.; Wu, R.S.S.; Ge, W. Juvenile exposure to bisphenol A promotes ovarian differentiation but suppresses its growth—Potential involvement of pituitary follicle stimulating hormone. Aquat. Toxicol. 2017, 193, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, H.; Yang, M.; Yu, Y.; Yan, M.; Zhou, L.; Liu, X.; Xiao, S.; Yang, Y.; Wang, Y.; et al. Toxic effects of bisphenol A on goldfish gonad development and the possible pathway of BPA disturbance in female and male fish reproduction. Chemosphere 2019, 221, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Mandich, A.; Bottero, S.; Benfenati, E.; Cevasco, A.; Erratico, C.; Maggioni, S.; Massari, A.; Pedemonte, F.; Vigano, L. In vivo exposure of carp to graded concentrations of bisphenol A. Gen. Comp. Endocrinol. 2007, 153, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Khaliq, S.; Lone, K.P. Disruption of the Reproductive Axis in Freshwater Fish, Catla catla, After Bisphenol-A Exposure. Zool. Sci. 2017, 34, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, Y.; Lin, L.; Liu, Y.; Chi, Y.; Lin, Y.; Ye, G.; Zhu, H.; Dong, S. Different effects of bisphenol a and its halogenated derivatives on the reproduction and development of Oryzias melastigma under environmentally relevant doses. Sci. Total Environ. 2017, 595, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Hatef, A.; Alavi, S.M.H.; Abdulfatah, A.; Fontaine, P.; Rodina, M.; Linhart, O. Adverse effects of bisphenol A on reproductive physiology in male goldfish at environmentally relevant concentrations. Ecotoxicol. Environ. Saf. 2012, 76, 56–62. [Google Scholar] [CrossRef]
- Kuiper, R.V.; Canton, R.F.; Leonards, P.E.G.; Jenssen, B.M.; Dubbeldam, M.; Wester, P.W.; Van den Berg, M.; Vos, J.G.; Vethaak, A.D. Long-term exposure of European flounder (Platichthys flesus) to the flame-retardants tetrabromobisphenol A (TBBPA) and hexabromocyclododecane (HBCD). Ecotoxicol. Environ. Saf. 2007, 67, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, R.V.; van den Brandhof, E.J.; Leonards, P.E.G.; Van der Ven, L.T.M.; Wester, P.W.; Vos, J.G. Toxicity of tetrabromobisphenol A (TBBPA) in zebrafish (Danio rerio) in a partial life-cycle test. Arch. Toxicol. 2007, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Kester, M.H.A.; Andersson, P.L.; Legler, J.; Brouwer, A. In Vitro Profiling of the Endocrine-Disrupting Potency of Brominated Flame Retardants. Toxicol. Sci. 2006, 92, 157–173. [Google Scholar] [CrossRef]
- Krivoshiev, B.V.; Dardenne, F.; Covaci, A.; Blust, R.; Husson, S.J. Assessing in-vitro estrogenic effects of currently-used flame retardants. Toxicol. In Vitro 2016, 33, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, L.B.; Pedersen, K.L.; Pedersen, S.N.; Korsgaard, B.; Bjerregaard, P. In vivo comparison of xenoestrogens using rainbow trout vitellogenin induction as a screening system. Environ. Toxicol. Chem. 2000, 19, 1867–1874. [Google Scholar] [CrossRef]
- Song, M.; Liang, D.; Liang, Y.; Chen, M.; Wang, F.; Wang, H.; Jiang, G. Assessing developmental toxicity and estrogenic activity of halogenated bisphenol A on zebrafish (Danio rerio). Chemosphere 2014, 112, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.S.; Chan, W.K.-L.; Chan, K.M. Toxicity assessment and vitellogenin expression in zebrafish (Danio rerio) embryos and larvae acutely exposed to bisphenol A, endosulfan, heptachlor, methoxychlor and tetrabromobisphenol A. J. Appl. Toxicol. 2013, 33, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-Y.; Ying, G.-G.; Liang, Y.-Q.; Zhao, J.-L.; Yang, B.; Liu, S.; Liu, Y.-S. Hormonal effects of tetrabromobisphenol A using a combination of in vitro and in vivo assays. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 157, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Ronisz, D.; Farmen Finne, E.; Karlsson, H.; Förlin, L. Effects of the brominated flame retardants hexabromocyclododecane (HBCDD), and tetrabromobisphenol A (TBBPA), on hepatic enzymes and other biomarkers in juvenile rainbow trout and feral eelpout. Aquat. Toxicol. 2004, 69, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.; van Zanden, J.J.; Luijks, E.A.C.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, A.; Brouwer, A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000, 56, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, S.; Jinno, N.; Ohta, S.; Kuroki, H.; Fujimoto, N. Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem. Biophys. Res. Commun. 2002, 293, 554–559. [Google Scholar] [CrossRef]
- Chan, W.K.; Chan, K.M. Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat. Toxicol. 2012, 108, 106–111. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Chen, B.; He, J.; Chen, F.; Shan, X.; Du, Q.; Li, N.; Jia, X.; Tang, J. Differences in reproductive toxicity of TBBPA and TCBPA exposure in male Rana nigromaculata. Environ. Pollut. 2018, 243, 394–403. [Google Scholar] [CrossRef]
- van der Ven, L.T.M.; van de Kuil, T.V.; Verhoef, A.; Verwer, C.M.; Lilienthal, H.; Leonards, P.E.G.; Schauer, U.M.D.; Canton, R.F.; Litens, S.; De Jong, F.H.; et al. Endocrine effects of tetrabromobisphenol-A (TBBPA) in Wistar rats as tested in a one-generation reproduction study and a subacute toxicity study. Toxicology 2008, 245, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Hodne, K.; von Krogh, K.; Weltzien, F.A.; Sand, O.; Haug, T.M. Optimized conditions for primary culture of pituitary cells from the Atlantic cod (Gadus morhua). The importance of osmolality, pCO(2), and pH. Gen. Comp. Endocrinol. 2012, 178, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Lettieri, T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- von Krogh, K.; Bjørndal, G.T.; Nourizadeh-Lillabadi, R.; Ropstad, E.; Haug, T.M.; Weltzien, F.-A. Cortisol differentially affects cell viability and reproduction-related gene expression in Atlantic cod pituitary cultures dependent on stage of sexual maturation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 236, 110517. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Hodne, K.; Haug, T.M.; Weltzien, F.A. Single-cell qPCR on dispersed primary pituitary cells—An optimized protocol. BMC Mol. Biol. 2010, 11, 82. [Google Scholar] [CrossRef]
- Weltzien, F.A.; Pasqualini, C.; Vernier, P.; Dufour, S. A quantitative real-time RT-PCR assay for European eel tyrosine hydroxylase. Gen. Comp. Endocrinol. 2005, 142, 134–142. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Roche. Roche Applied Science; Technical note No. LC 13; Roche Diagnostics GmbH: Mannheim, Germany, 2001. [Google Scholar]
- Ager-Wick, E.; Hodne, K.; Fontaine, R.; von Krogh, K.; Haug, T.M.; Weltzien, F.A. Preparation of a High-quality Primary Cell Culture from Fish Pituitaries. J. Vis. Exp. 2018, e58159. [Google Scholar] [CrossRef]
- Baker, R.C.; Kramer, R.E. Cytotoxicity of short-chain alcohols. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 127–150. [Google Scholar] [CrossRef]
- Pal, R.; Mamidi, M.K.; Das, A.K.; Bhonde, R. Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation potential of human embryonic stem cells. Arch. Toxicol. 2012, 86, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Fjeld, E.; Schlabach, M.; Berge, J.A.; Eggen, T.; Snilsberg, P.; Källberg, G.; Rognerud, S.; Enge, E.K.; Borgen, A.; Gundersen, H. Kartlegging av Utvalgte nye Organiske Miljøgifter—Bromerte Flammehemmere, Klorerte Parafiner, Bisfenol A og Triclosan; NIVA Rapport 4809-2004; Norsk institutt for vannforskning (NIVA): Oslo, Norway, 2004. (In Norwegian) [Google Scholar]
- Lv, X.; Zhou, Q.; Song, M.; Jiang, G.; Shao, J. Vitellogenic responses of 17β-estradiol and bisphenol A in male Chinese loach (Misgurnus anguillicaudatus). Environ. Toxicol. Pharmacol. 2007, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.K.; Bjornstad, A.; Sundt, R.C.; Taban, I.C.; Pampanin, D.M.; Andersen, O.K. Comparison of protein expression in plasma from nonylphenol and bisphenol A-exposed Atlantic cod (Gadus morhua) and turbot (Scophthalmus maximus) by use of SELDI-TOF. Aquat. Toxicol. 2006, 78, S25–S33. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Chen, D.W.; Liu, J.L.; Zhang, J.H.; Luo, H.S.; Cui, S. Estradiol promotes pituitary cell proliferation and gonadotroph differentiation at different doses and with different mechanisms in chick embryo. Steroids 2009, 74, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Brannick, K.E.; Craig, Z.R.; Himes, A.D.; Peretz, J.R.; Wang, W.; Flaws, J.A.; Raetzman, L.T. Prenatal Exposure to Low Doses of Bisphenol A Increases Pituitary Proliferation and Gonadotroph Number in Female Mice Offspring at Birth. Biol. Reprod. 2012, 87, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chan, K.M. Evaluation of the toxic effects of brominated compounds (BDE-47, 99, 209, TBBPA) and bisphenol A (BPA) using a zebrafish liver cell line, ZFL. Aquat. Toxicol. 2015, 159, 138–147. [Google Scholar] [CrossRef]
- Ma, M.; Crump, D.; Farmahin, R.; Kennedy, S.W. Comparing the effects of tetrabromobisphenol-A, bisphenol A, and their potential replacement alternatives, TBBPA-bis(2,3-dibromopropyl ether) and bisphenol S, on cell viability and messenger ribonucleic acid expression in chicken embryonic hepatocytes. Environ. Toxicol. Chem. 2015, 34, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Akingbemi, B.T.; Sottas, C.M.; Koulova, A.I.; Klinefelter, G.R.; Hardy, M.P. Inhibition of Testicular Steroidogenesis by the Xenoestrogen Bisphenol A Is Associated with Reduced Pituitary Luteinizing Hormone Secretion and Decreased Steroidogenic Enzyme Gene Expression in Rat Leydig Cells. Endocrinology 2004, 145, 592–603. [Google Scholar] [CrossRef]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.L.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-Da-Silva, M.R.; Romano, M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef]
- Hildahl, J.; Sandvik, G.K.; Edvardsen, R.B.; Norberg, B.; Haug, T.M.; Weltzien, F.-A. Four gonadotropin releasing hormone receptor genes in Atlantic cod are differentially expressed in the brain and pituitary during puberty. Gen. Comp. Endocrinol. 2011, 173, 333–345. [Google Scholar] [CrossRef]
- Rhee, J.-S.; Seo, J.S.; Raisuddin, S.; Ki, J.-S.; Lee, K.-W.; Kim, I.-C.; Yoon, Y.-D.; Lee, J.-S. Gonadotropin-releasing hormone receptor (GnRHR) gene expression is differently modulated in gender types of the hermaphroditic fish Kryptolebias marmoratus by endocrine disrupting chemicals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Z.; Wang, Q.; Zhai, Y. Effects of BPA and E2 on expression profiles of genes related to hypothalamic-pituitary-gonadal axis of half-smooth tongue sole Cynoglossus semilaevis. Chin. J. Oceanol. Limnol. 2013, 31, 598–608. [Google Scholar] [CrossRef]
- Hu, J.; Liang, Y.; Chen, M.; Wang, X. Assessing the toxicity of TBBPA and HBCD by zebrafish embryo toxicity assay and biomarker analysis. Environ. Toxicol. 2009, 24, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Reistad, T.; Mariussen, E.; Ring, A.; Fonnum, F. In vitro toxicity of tetrabromobisphenol-A on cerebellar granule cells: Cell death, free radical formation, calcium influx and extracellular glutamate. Toxicol. Sci. 2007, 96, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, R.; Beckmann, M.W.; Oppelt, P.G.; Hoffmann, I.; Lotz, L.; Kuwert, T.; Mueller, A. Thyroid hormone receptors and reproduction. J. Reprod. Immunol. 2011, 90, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Hazlerigg, D.G.; Ebling, F.J.P. Thyroid hormone and seasonal rhythmicity. Front. Endocrinol. 2014, 5, 19. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).