1. Introduction
Clownfishes of the family Pomacentridae represent some of the most important and recognizable ornamental marine commodities in the aquarium trade. Additionally, clownfish, namely
Amphiprion ocellaris, have emerged as important model organisms for ocean acidification, climate change, ecotoxicology, and ecology research, as well as developmental, and evolutionary biology studies (eco/evo/devo; [
1,
2]). Clownfish have been cultured in captivity for several decades, with the first record of successful culture dating back to 1974 [
3]. Present day commercial clownfish aquaculture now encompasses over 15 species of clownfish, many of which have several designer varieties that can retail from
$20 USD to
$280 USD [
4]. Designer clownfish are usually distinguished from the wild-type through differences in color and stripe pattern, although differences in body conformation are also considered [
5].
Although clownfish have been commercially cultured for decades, issues associated with larviculture persist. Larviculture is often one of the most costly and labor-intensive stages of marine finfish aquaculture. During early development, larval marine fish culture frequently results in mass mortality events often associated with poor or inappropriate nutritional or environmental factors. Additionally, commercial clownfish producers have raised concerns that the heavy reliance upon live feeds such as rotifers (
Brachionus spp.) and
Artemia spp. nauplii (hereafter referred to as
Artemia) increases the cost of the production process (personal communication). Furthermore, existing larval feeding protocols for clownfish are highly variable, and no “standardized” rearing protocol has been implemented. The larval feeding protocol outlined by Hoff (1996) [
3], which is considered a staple protocol in the aquaculture industry, features over seven different feeds including live feeds, dry feeds, and gelatin-based feeds. This protocol suggests feeding enriched rotifers at 10–15 individuals/mL from 1 to 8 days post-hatch (DPH),
Artemia from 3 to 30 DPH, and supplemental feeds including dry diets, flakes, and gelatin-based diets up to six times daily. A laboratory feeding protocol outlined by Roux et al. (2021) [
6] indicated feeding unenriched rotifers from 1 to 8 DPH,
Artemia from 3 to 30 DPH, and a dry diet from 12 to 30 DPH, with no survival rate reported. Raheem et al. (2021) [
7] used
Acartia southwelli copepod nauplii from 1 to 9 DPH, followed by
Artemia from 9 to 60 DPH and boiled mussel meat from 25 to 60 DPH and achieved approximately 73% survival. These different feeding protocols highlight the absence of a standardized protocol and heavy reliance on multiple types of live feeds. Not only would a standardized larval feeding protocol benefit the aquaculture industry by increasing consistency during larval production, but it would also help standardized laboratory experiments for eco/evo/devo research and minimize the impacts of confounding variables arising from variation in nutritional protocols. Further research on larval feeding protocols is therefore warranted to help streamline clownfish larval production, reduce the overall reliance on live feeds, and optimize larval survival, growth, and quality.
Live feeds, such as rotifers and
Artemia are used in the commercial larval culture of
A. ocellaris because these organisms elicit larval feeding responses via natural movement and are highly digestible by the immature larval digestive system. Historically, rotifers enriched with a lipid emulsion are offered for the first week of
A. ocellaris larval development and
Artemia are frequently offered for an extended period ranging from approximately 5–25 DPH [
3,
8,
9]. These live feeds are costly, labor-intensive, and unreliable due to the necessity of live culture maintenance and unpredictable changes in production [
10]. The use of
Artemia can be precarious due to the volatility of
Artemia wild cyst harvests cyst quality, and price [
11]. In addition, the nutritional profile of rotifers and
Artemia may be insufficient for marine larvae even after enrichment with lipid emulsions due to the specific highly unsaturated fatty acid (HUFAs) requirements of marine fish resulting from their inability to efficiently elongate and desaturate shorter chain fatty acids [
10,
12]. The present study aimed to reduce the use of these live feeds by first determining the earliest timepoint at which
A. ocellaris larvae can be transitioned from rotifers to
Artemia. Because of the size difference between these prey types, larval mouth gape is a key limiting factor in prey selection during early ontogeny. Newly hatched instar I–II
Artemia typically range from 160 to 450 μm in width, whereas
Brachionus plicatilis rotifers measure approximately 90–350 μm depending on life stage [
10,
13]. Additionally, larval fish often use less than 40% of their total gape height when selecting prey [
14,
15]. Our previous work on the digestive ontogeny of
A. ocellaris documented that gape height increases to ~660 μm by 7 DPH [
16], suggesting that larvae at this stage should be able to efficiently capture and ingest
Artemia. Based on these constraints, we selected 3, 5, and 7 DPH to evaluate the earliest timepoint at which
Artemia can be introduced during
A. ocellaris larviculture.
Transitioning larvae to an inert diet, such as a microdiet (MD), can help reduce or eliminate the use of live feeds during larviculture. Microdiets can be a cost-effective replacement for live feeds because they are often available in many sizes to suit the small mouth gape of marine larvae, require no additional maintenance, have reliable availability, and provide dense nutrition to developing larvae [
17,
18]. However, the macronutrients within MDs are more complex and less digestible than those found in live feeds; therefore, larvae often need to have a mature digestive tract to effectively digest MDs [
17,
19]. Weaning larval fish from live feeds to an inert MD therefore is often informed by the timing of the maturation of the digestive tract. Several studies have explored the digestive ontogeny of freshwater and marine fish in aquaculture, where microplate assays were used to quantify the activity of key digestive enzymes throughout the larval period and histology was used concurrently to examine the morphological development of digestive organs [
18,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32]. Digestive enzyme analyses usually include pancreatic enzymes such as trypsin, chymotrypsin, lipase, and amylase and the acid protease, pepsin. The trends in activity of these enzymes throughout development can help identify when the fish transitions from the larval mode of digestion to the adult mode of digestion. Additionally, histology and histochemistry can help identify when gastric glands are formed and become functional, which indicates when larvae should be able to better digest complex MDs [
17,
19,
25]. Two digestive ontogeny studies on
A. ocellaris indicated similar patterns in pancreatic enzymes, where enzymes such as trypsin and lipase were active at first feeding (1 DPH) [
16,
33]. These studies also agreed that stomach functionality occurs between 7 and 9 DPH, supported by patterns in pepsin activity and confirmation of active gastric glands via histochemistry. Considering the timing of a functional stomach in
A. ocellaris, the present study tested the introduction of MD at 1, 5, 7, and 11 DPH to determine the earliest timepoint at which this species could be weaned from live feeds to an inert diet.
Fundamental metrics such as larval growth and survival give insight into the overall effectiveness of certain diets and weaning regimes. However, other metrics can also contribute to our understanding of the metabolic effects of these diets and weaning regimes, thus indicating larval quality. Fish nutrition has been linked to the stress physiology of fishes, where deficiencies in certain HUFAs can decrease the ability of fish to cope with stress. Although the cortisol response is an adaptive mechanism used to adjust the metabolism of a fish to a stressor, chronically elevated cortisol levels can be deleterious to the growth, homeostasis, and immune system function [
34,
35]. Burbano et al. (2020) [
36] found that the stress resistance and larval quality of rose snapper (
Lutjanus guttatus) were increased when larvae were fed a HUFA-rich diet of marine copepod nauplii compared to
Artemia spp. nauplii. Additionally, the arachidonic acid (ARA) content of enriched rotifers significantly affected larval gilthead seabream survival after exposure to stressors, where increasing levels of ARA improved larval survival [
37]. In the present study, the effect of different weaning regimes on the stress resistance and cortisol levels of
A. ocellaris larvae exposed to acute stress was examined as a metric for larval quality.
Color is an important factor that determines the economic value of several ornamental species, with brighter coloration or rarer patterns demanding higher retail prices [
38]. Improving culture parameters (i.e., tank color, light intensity, and diet), that can affect coloration are of interest to develop culture protocols that maximize the aesthetic quality of clownfish [
38,
39,
40,
41]. Carotenoids such as astaxanthin have been used as color enhancers to produce clownfish with more red-orange hues and higher color saturation [
42,
43]. A commercially available microdiet, TDO Chroma Boost™ (Reed Mariculture Inc., Campbell, CA, USA), has been developed to produce the “reddest reds and whitest white™” (
reefnutrition.com) through top-dressing the MD with 400 ppm of
Haematococcus pluvialis microalgae.
This study aimed to leverage known digestive development data of
A. ocellaris to design and conduct two weaning trials aimed to streamline and standardize larval feeding practices for
A. ocellaris through reducing the variety of feeds while concomitantly maintaining high larval growth, survival, and quality [
44,
45,
46]. Additionally, a dietetics trial was conducted to evaluate three commercially available MDs and determine which diet yielded the most vibrantly colored juveniles. Lastly, a partial budget analysis (PBA) was conducted to determine the potential economic benefits of using these refined feeding regimes. Together, these results will help refine larval culture protocols of
A. ocellaris to streamline the production process and increase cost-efficiency.
2. Materials and Methods
2.1. Ethics Statement
All methods were approved under the University of Florida’s Institutional Animal Care and Use Committee (IACUC study # 202400000140).
2.2. Broodstock Husbandry and Egg Collection
Clownfish nests from wild-type broodstock were either procured from local marine ornamental fish farms (for the Weaning I and Weaning II trials) or from broodstock housed at the Tropical Aquaculture Laboratory, Ruskin, Florida, USA (for the Dietetics Trial). Wild-type A. ocellaris broodstock were housed in 38–76 L tanks attached to a ~12,000 L recirculating aquaculture system consisting of a moving bed biofilter, UV sterilization, and temperature control. Each broodstock tank contained a sexually mature adult pair of A. ocellaris consisting of one male and one female and ceramic tiles were provided as spawning substrate. This system was housed in a greenhouse, and a natural photoperiod was provided.
Broodstock nutrition included three diets: Otohime C2™ (840–1410 μm; 51% protein, 11% fat, 15% ash, 3.5% fiber, 7% moisture; Marubeni Nisshin Feed Co., Ltd., Tokyo, Japan), LRS Fish Frenzy (LRS Foods, Advance, NC, USA), and a homemade gel diet consisting of mullet roe (Mugil spp.), frozen squid (Doryteuthis opalescens), and INVE Fish Breed-M (INVE Aquaculture, Salt Lake City, UT, USA). Broodstock were fed Otohime C2™ twice daily, LRS Fish Frenzy once daily, and the homemade gel diet once daily. Pairs were monitored for spawning activity twice daily.
Fertilized eggs were collected on the seventh evening of incubation, disinfected in a 1% iodine solution (Ovadine®, Syndel, Ferndale, WA, USA) for 10 min, and placed in a 50 L cylindrical hatching tank described in the following section.
2.3. Live Feeds Culture
Rotifer (
B. plicatilis) cultures were maintained in 60 L static tanks and fed Rotigrow
® Plus (Reed Mariculture, Campbell, CA, USA) daily, as recommended by the manufacturer. Culture tanks were maintained at approximately 30 g/L salinity and 27–28 °C and 100% water changes were performed every two weeks [
47].
Artemia spp. cysts (Brine Shrimp Direct, Elkins Park, PA, USA) were hatched in saltwater every 24 h in 20 L hatching cones at approximately 25 g/L salinity and 25–26 °C, as outlined in Watson and Yanong (2009) [
48].
2.4. Water Quality Monitoring
Water quality parameters were tested weekly with a YSI ProDO (temperature and dissolved oxygen (DO); Xylem, Inc., Yellow Springs, OH, USA), YSI EcoSense pH100A (pH; Xylem, Inc., Yellow Springs, OH, USA), refractometer (salinity), MW700 PRO lux meter (lux; Milwaukee Instruments, Rocky Mount, NC, USA), and HACH® colorimetric test kit (total ammonia-nitrogen (TAN), NO2-N, and alkalinity; Model FF-1A, Hach Company, Loveland, CO, USA) in accordance with manufacturer’s protocols. Water quality parameters were maintained as follows: 27.00 ± 0.50 °C, 8.00 ± 0.20 pH, 6.25 ± 0.25 mg/L DO, 34 ± 1 mg/L salinity, ND (under detectable limit) NH3, ND NO2−, and 171 ± 17 mg/L CaCO3 alkalinity. Using the manufacturer’s protocol, TAN and NO2-N values were converted to NH3− and NO2− where appropriate.
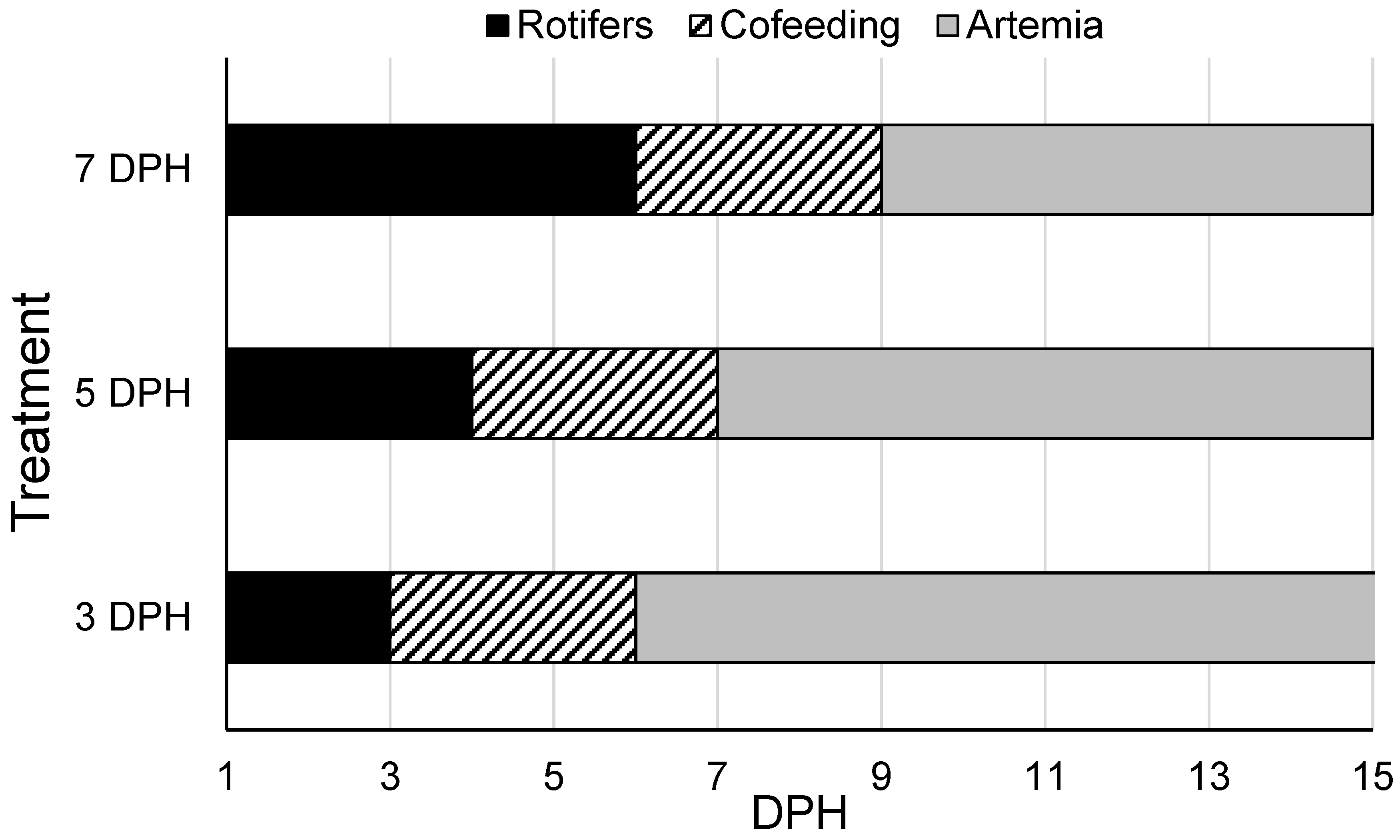
2.5. Weaning I Trial
A 15-day trial was completed to determine the earliest timepoint at which
A. ocellaris larvae can be transitioned from rotifers to
Artemia while maintaining high larval survival, growth, and quality. Newly hatched
A. ocellaris larvae were stocked into 15 L tanks (n = 15) attached to the experimental system, with a total of 25 larvae per tank. Each tank was assigned one of three treatments (n = 5/treatment):
Artemia (instar I-II nauplii) introduction at 3, 5, or 7 DPH (
Figure 1). Prior to
Artemia introduction, larvae were fed
Brachionus plicatilis enriched with Algamac 3050 (Aquafauna Bio-Marine, Hawthorne, CA, USA) according to manufacturer’s recommendations twice daily at a density of 5 rotifers/mL. Live microalgae (
Tisochrysis lutea) were supplied to each tank at a density of 300,000 cells/mL twice daily prior to each experimental feeding. Tank flow was set to three tank turnovers/day (31 mL/min) during daylight hours (18 h) and six tank turnovers/day (62 mL/min) during nighttime hours (6 h). Water quality parameters in each experimental tank were measured at the start (2 DPH) and end of the experiment (15 DPH) and were maintained as follows: 26.40 ± 0.09 °C temperature, 8.18 ± 0.03 pH, 6.28 ± 0.07 mg/L DO, 33 ± 1 mg/L salinity, ND NH
3−, 0.01 ± 0.01 NO
2−, 150 ± 12 mg/L CaCO
3 alkalinity, and 170 ± 6 lux. All water quality parameters except nitrite were similar among treatments. Nitrite levels were higher in tanks fed the 3 DPH weaning regime (0.03 ± 0.01 mg/L) compared to the 5 DPH and 7 DPH regimes (0 mg/L). While these values were statistically different, they are not outside the normal range for nitrite levels in a production scenario and were considered to be biologically insignificant in this case [
49]. At 15 DPH, a subsample of three fish per tank were subjected to a stress test, where larvae were held out of the water on a 500 μm screen for 210 s and immediately placed into a recovery tank. Larval recovery time was measured, where recovery from the stress test was indicated by upright, balanced swimming behavior (no spinning or listing). Additionally, larvae were monitored for survival at 1 h post-stress test, anesthetized in 10 mg/L metomidate HCl (Aquacalm™, Syndel, Ferndale, WA, USA), placed in a 1.5 mL microcentrifuge tube, snap-frozen and preserved at −80.00 °C for whole-body cortisol analysis. The remaining larvae from each tank were harvested, euthanized in 300 mg/L buffered MS-222 (Syncaine
®, Syndel, Ferndale, WA, USA), enumerated, and photographed on a Sedgewick rafter cell. Photomicrographs were analyzed for larval total length (TL; mm) using Image J (v. 1.54e 4-1.54n; U.S. National Institutes of Health, Bethesda, MD, USA).
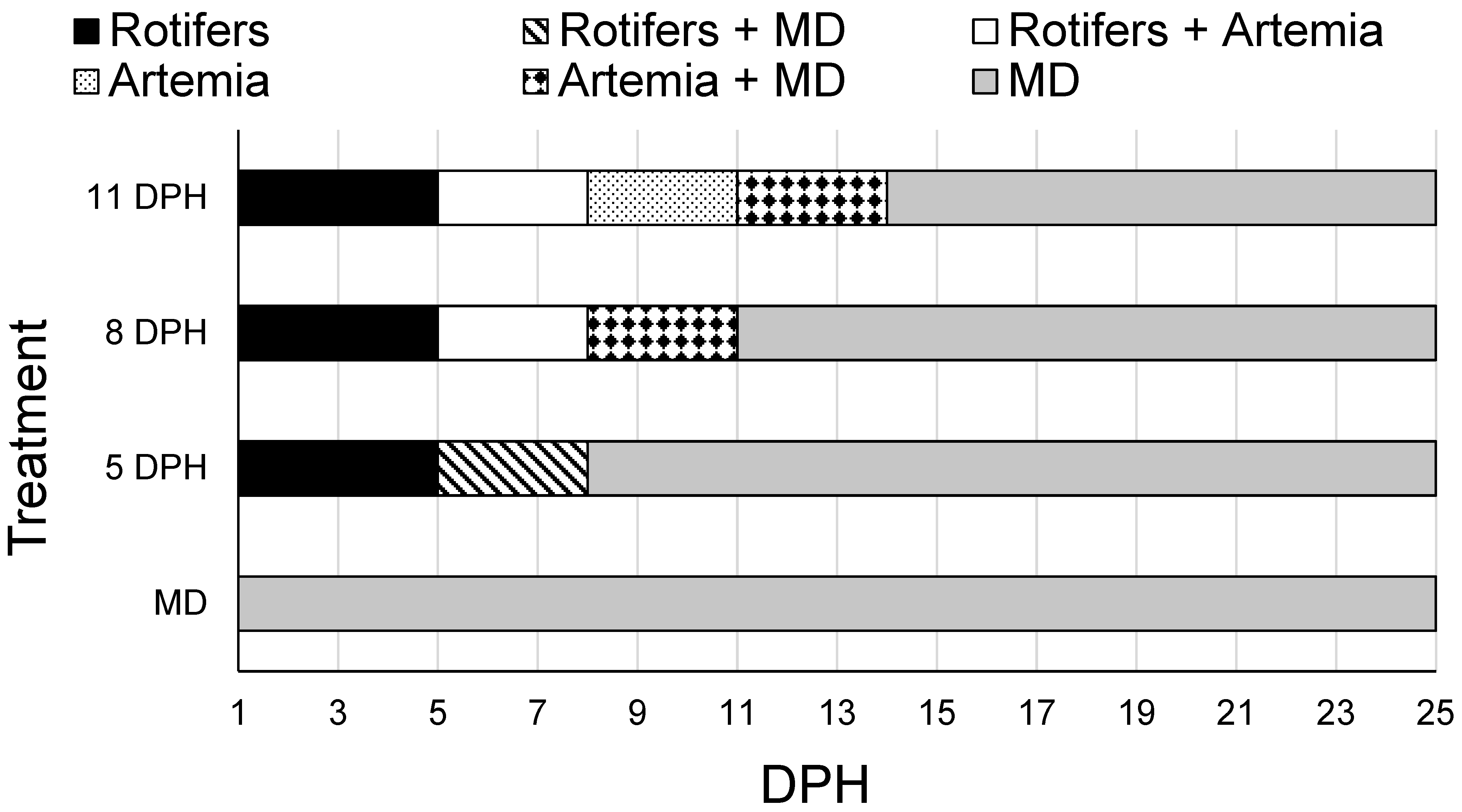
2.6. Weaning II Trial
The appropriate timing of transitioning
A. ocellaris from live feeds to an inert MD was determined in a 25-day trial. First-feeding (1 DPH)
A. ocellaris were stocked into 15 L cylindrical tanks (n = 12) attached to the experimental system, with 15 larvae/tank. Weaning treatments were designed with the results of the previous weaning trial and larval mouth gape data from Murray et al. (2022) [
16] considered. Each tank was assigned one of four weaning treatments (n = 3/treatment): MD from first feeding (1 DPH), or MD introduction at 5, 8, or 11 DPH. Prior to MD introduction, enriched
B. plicatilis and
Artemia were fed to each tank in a similar manner as previously described (
Figure 2). The MD used in this trial was TDO Chroma Boost™ (Reef Nutrition, Reed Mariculture, Inc., Cambell, CA, USA) with A1 (75–250 μm; 50% protein, 13% fat, 3.5% fiber, 15% ash, 2% calcium, 2% phosphorus, 6.5% moisture) used from 1 to 13 DPH and B1 (250–360 μm; 49% protein, 13% fat, 3% fiber, 15% ash, 2.3% calcium, 1.5% phosphorus, 6.5% moisture) used from 14 to 25 DPH to account for changes in larval mouth gape. Each tank was supplied with 300,000 cells/mL of live
T. lutea twice daily, except when tanks were fed only MD, which is consistent with commercial production protocols. Tank flow was set to three tank turnovers/day (31 mL/min) during daylight hours and six tank turnovers/day (62 mL/min) during nighttime hours from 1 to 13 DPH and 62 mL/min from 14 to 25 DPH. Lastly, water quality parameters were similar among treatments and were maintained as follows: 26.47 ± 0.02 °C temperature, 8.11 ± 0.01 pH, 6.33 ± 0.08 mg/L DO, 34 ± 1 mg/L salinity, ND NH
3−, ND NO
2−, 150 ± 12 mg/L CaCO
3 alkalinity, and 170 ± 6 lux. By 3 DPH, no larvae in the MD from first feed (1 DPH) treatment were alive, therefore these tanks were excluded from further analyses. At 25 DPH, a subsample of three fish from each tank were harvested for a stress test. Methods for the stress test, associated metrics, and larval preservation adhere to those described for weaning I, except the air exposure lasted 4 min. The remainder of the larvae were harvested from each tank, euthanized in 300 mg/L buffered MS-222, enumerated, and photographed on a Sedgewick rafter cell. Image J ver. 1.54e 4-1.54n was used to measure the TL of fish harvested from all tanks.
2.7. Dietetics Trial
A 30-day trial was conducted to determine the commercially available MD that best promoted clownfish survival, growth, and color. The feeding regime used in this trial was designed using the results from the Weaning I and Weaning II trials. Clownfish nests (n = 4) were hatched in 50 L cylindrical tanks attached to the experimental system and larvae were raised in these tanks until 5 DPH. Live microalgae (
T. lutea) was added at a density of 300,000 cells/mL twice daily and larvae were fed a diet of rotifers (
B. plicatilis; enriched as previously described) at a density of 5 rotifers/mL twice daily. On 5 DPH,
A. ocellaris larvae were stocked into 15 L cylindrical tanks (n = 18) with a total of 25 larvae per tank. Larvae were then fed one of three different commercially available MDs (
Table 1) selected because of their wide use in marine ornamental fish hatcheries, and availability to aquaculture producers: MD1—TDO Chroma Boost™ (Reef Nutrition, Reed Mariculture, Inc., Campbell, CA, USA), MD2—Golden Pearl™ (Brine Shrimp Direct, Ogden, UT, USA), or MD 3—GEMMA Micro 300™ (Skretting, Tooele, UT, USA). For the MD introduction, larvae were weaned over three days from rotifers to MD and fed these MDs until 30 DPH. Water quality parameters were maintained 27.09 ± 0.03 °C, 8.45 ± 0.20 pH, 6.38 ± 0.05 mg/L DO, 33.85 ± 0.04 mg/L salinity, ND NH
3, 0.008 ± 0.002 NO
2−, 196 ± 1 mg/L CaCO
3 alkalinity, and 122 ± 2 lux. At 30 DPH, clownfish were harvested from each tank and euthanized in 300 mg/L buffered MS-222. Clownfish were photographed inside of a MyStudio MS20CYC (Pro Cyc, Clackamas, OR, USA) with two Phillips F15T8 natural daylight bulbs (15 watt, 700 lumens; Phillips North America, Cambridge, MA, USA) with a Canon EOS Rebel T3i camera (Canon, U.S.A., Inc., Melville, New York, NY, USA). For each photograph, a DGK color tools WDKK Waterproof Color calibration chart (DGK, B&H Photo Video, New York, NY, USA) was included for color reference (
Figure 3). The camera was oriented as described in Yasir and Qin (2009) [
40] and white balance and focus of the camera were manually set. Fish were then enumerated and photographed again on a Sedgewick rafter cell for length analysis. The resulting photomicrographs were analyzed for standard length (SL) using Image J (v. 1.54e 4-1.54n; U.S. National Institutes of Health, Bethesda, MD, USA).
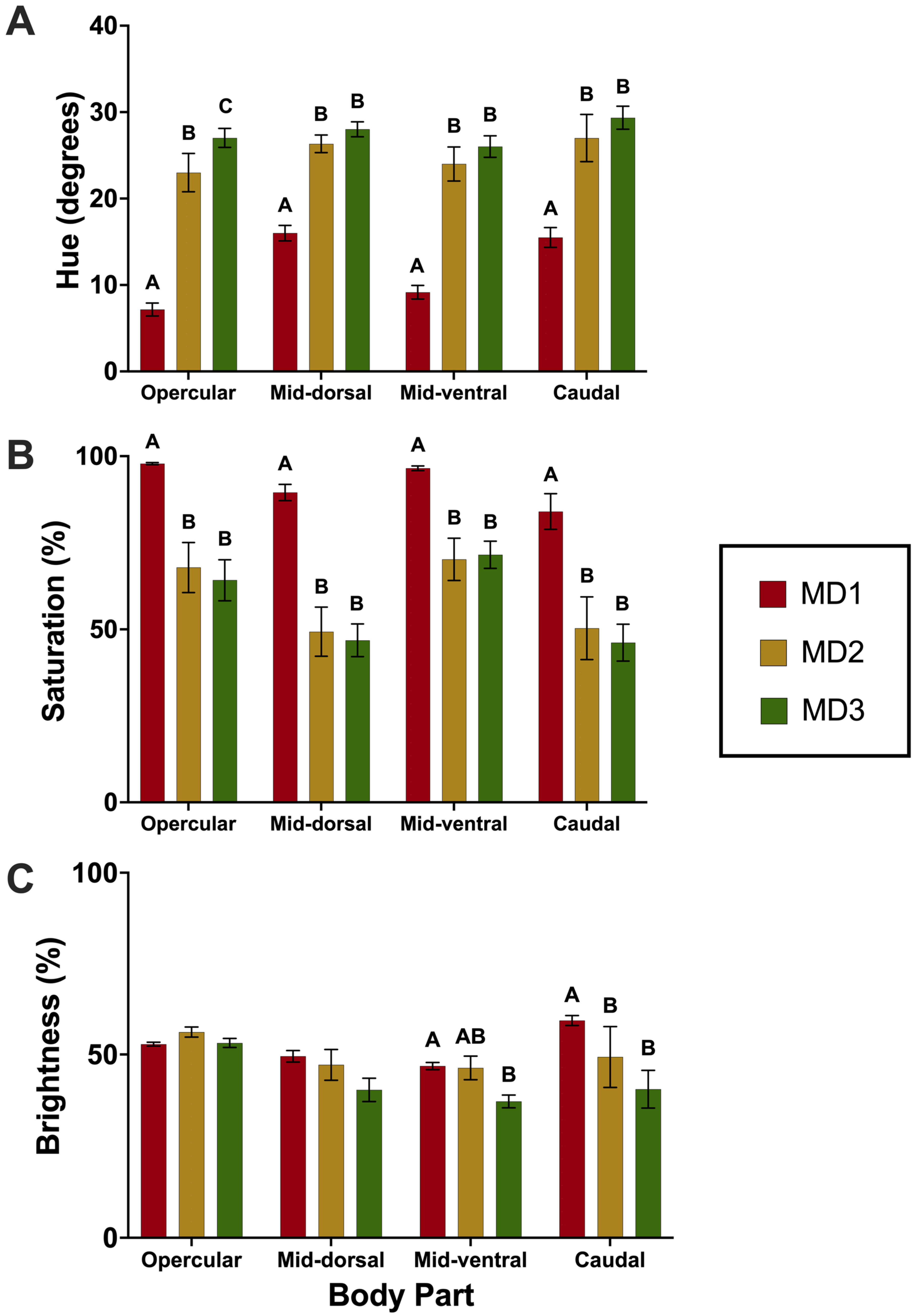
Hue, saturation, and brightness values were measured using Adobe Photoshop (version 25.11.0) with methods adapted from Yasir and Qin (2009) [
40]. The white balance was set for each picture using the “set white point” tool (adjustments, curves) and the white area of the color standard card. The RGB values of the red and yellow area of the color reference cards were then sampled to determine if there was a significant difference between RGB values among pictures. If no significant differences were found between pictures, no further calibration was required. The HSB values were measured for each of four regions per fish: opercular, mid-dorsal, mid-ventral, and caudal (n = 9 ± 3 fish/tank). To measure HSB values for each region, the polygonal lasso tool was used in the respective region, avoiding any glare spots or debris. Then, the color of the region within the polygon was averaged using the blur average tool (filter, blur, average). Next, the eyedropper tool was used to sample the color, and the associated hue, brightness, and saturation values were recorded (window, color, set to HSB sliders). The values for hue, saturation, and brightness for each region were then averaged for each tank (n = 6 per treatment) for statistical analysis.
2.8. Cortisol Quantification
An ELISA (Enzyme-Linked Immunosorbent Assay) was used to quantify whole-body cortisol levels in larval samples from weaning I and weaning II. Methods from Ramee et al. (2020) [
50] were adapted for cortisol extraction and methods from the Smithsonian National Zoological Park Endocrine Manual were adapted to quantify cortisol [
51]. Briefly, 150 μL of deionized water were added to larval sample tubes and samples were homogenized with a motorized micropestle. Samples were then transferred to 12 × 75 mm borosilicate glass tubes, and aliquots of 1.5 mL ethyl acetate were added to each tube to extract cortisol. Tubes were vortexed at 2000 rpm for 1 min and centrifuged for 5 min at 1700 *G. The aqueous portion of the sample was snap frozen in liquid nitrogen and the ethyl acetate portion was decanted into a fresh borosilicate glass tube. The extraction process was repeated a second time for a final volume of 3 mL each. Extracts were evaporated for approximately 30 min at 37.00 °C with nitrogen gas flowing at 5 L/min using a Multivap nitrogen evaporator (Organomation, Berlin, MA, USA). Evaporated samples were stored at 4.00 °C overnight until cortisol quantification.
For each weaning experiment, a separate NUNC 96-well Maxisorp microplate was coated with 50 μL of rabbit anti-cortisol antibody (1:8500 dilution; R4866, University of California, Davis, CA, USA), except for blank wells, sealed, and left to incubate overnight at 4.00 °C. Plates were washed five times) with washing solution (0.15 M NaCl and 0.5 mL/L Tween 20 then blotted dry. Evaporated samples were reconstituted with assay buffer (0.039 M NaH2PO4, 0.061 Na2HPO4, 6.72 M NaCl, 1% BSA) then pipetted in duplicate aliquots of 50 μL into the 96-well plates. Cortisol-horseradish peroxidase (50 μL) was added to each well with a multichannel pipette and plates were sealed and allowed to incubate for 1 h at 23.00 °C.
The plate was subsequently washed five times, blotted dry, and substrate buffer (0.05 M citric acid, 6.0 mM H2O2, 0.4 mM 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), pH 4.00; 100 μL aliquots) was added to all wells and shaken for 5 min. A HTX Multi-Mode Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA) was used to read absorbance at 420 nm and 605 nm (background absorbance). The background absorbance and non-specific absorbance (blank wells), respectively, were subtracted from 420 nm readings. Percent specific binding was plotted against known standard concentrations to generate a standard curve with a 5-order logistic model. Intra-assay coefficients of variation were as follows: 6.90% for weaning I and 6.01% for weaning II.
2.9. Partial Budget Analysis
A PBA was conducted to determine the potential costs or benefits of weaning
A. ocellaris to MD at 5 DPH compared to 11 DPH, as was tested in the weaning II trial. The PBA was conducted according to methods outlined in Engle (2010) [
52] and Murray et al. (2025) [
29] and all monetary values are presented as U.S. dollars. The farmgate value of seller-size clownfish was obtained through communication with a local clownfish producer (Personal Communication, Oceans, Reefs, & Aquariums). The costs associated with
Artemia culture included the retail value of cysts at a 90% hatch rate and bleach and labor (at
$20 per hour) associated with the decapsulation process. The costs associated with rotifer culture and enrichment were calculated considering the culture volume, feed price, enrichment price, feeding rates, and labor required (at
$20 per hour). For the PBA, “reduced costs” incorporated the costs associated with feeding rotifers,
Artemia, and MD (TDO™ Chroma Boost;
$110.68/kg) according to the 11 DPH weaning schedule from the weaning II trial. The “added costs” incorporated the costs associated with feeding rotifers and MD according to the 5 DPH weaning schedule from the weaning II trial. The time spent administering MD to experimental tanks was considered to be similar to that of
Artemia, thus was not factored into this PBA. “Additional revenue” was calculated by multiplying the farmgate value of clownfish by the percent survival achieved when weaned to
Artemia prior to MD. “Reduced revenue” was calculated by multiplying the farmgate value with the percent survival achieved when clownfish were weaned to MD at 5 DPH. Because this PBA included inherent assumptions, only covered clownfish production up to 25 DPH, and does not account for fluctuations in material and labor prices, the results of this analysis should be interpreted with caution and producers should use discretion when altering feeding regimes.
2.10. Data Analysis
All analyses were conducted in R (v.4.2.2, © 2022) with graphs produced utilizing GraphPad Prism (v. 9.5.1 © 2023). A Shapiro-Wilks and Bartlett’s test were conducted to check for violations of the assumptions of normality and homoscedasticity, respectively. Additionally, residual plots and qqplots were used to verify data homoscedasticity and normality, respectively. For all statistical tests, significance was determined at α ≤ 0.05. Where applicable, data that met assumptions of normality were analyzed with a one-way ANOVA followed by a Tukey HSD post hoc test if applicable; data that violated these assumptions were analyzed with a Kruskal–Wallis test followed by a Dunn’s post hoc. All error values reported for these data are the standard error of the mean proportion (SEMp).
Hue, saturation, and brightness data were analyzed using linear mixed-effects models (LMMS). Models included diet treatment, body region, and their interaction as fixed effects and replicate as the random effect (R package lme4, “lmer” function). Significant fixed effects were assessed using type III ANOVA using the lmerTest R package. When the models detected significant effects, Tukey’s post hoc tests were conducted for pairwise comparisons (R package emmeans).
A generalized linear mixed model (GLMM) with a Bernoulli response (R package lme4, “glmer” function, family = binomial) was used to analyze survival data from the endpoint of each experiment and stress tests. ‘Tank’ and ‘nest’ were set as random factors, where applicable, to account for these potential effects. Pairwise differences were determined using a likelihood ratio test followed by a Tukey HSD on the log odds of survival where necessary (R package emmeans).
4. Discussion
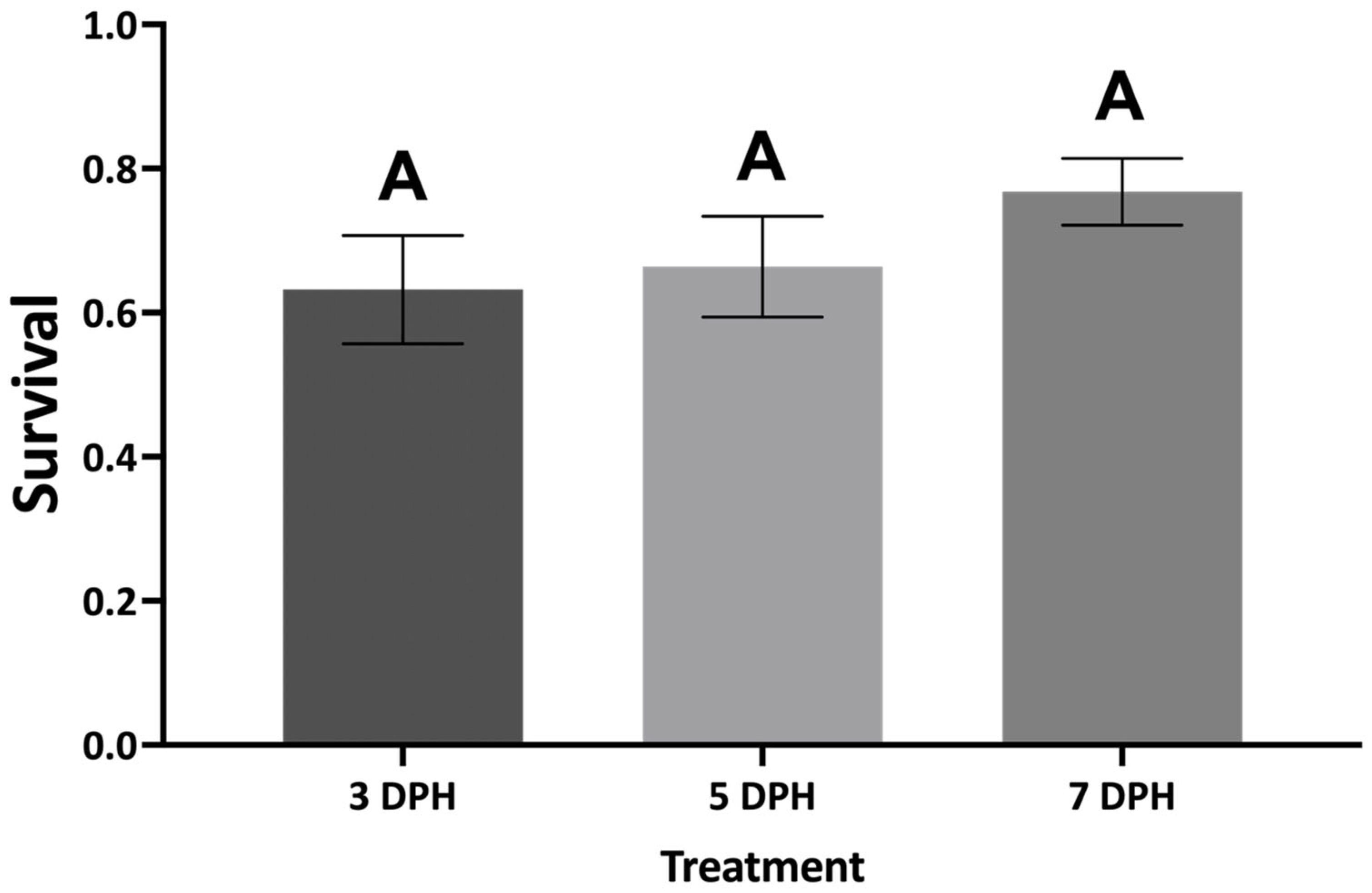
Larval
A. ocellaris can be transitioned from enriched rotifers to
Artemia as early as 5 DPH without affecting survival, growth, or larval quality. Statistically significant differences were not found for stress resistance and cortisol levels among treatments, suggesting that the timing of
Artemia introduction may not have impacted larval quality and the nutrition provided may have been adequate across weaning regimes. Although no significant pairwise differences were detected for survival in weaning I, potential differences in survival across weaning regimes may have been influenced by differing ingestion rates of rotifers and
Artemia due to inherent differences in prey size and movement. Instar 1–2
Artemia measure approximately 160–450 μm wide while
B. plicatilis can measure 90–350 μm wide depending on life stage [
10,
13]. Studies have found that larval fish often only utilize approximately 20–36% of their mouth gape when consuming prey [
14,
15,
53,
54]. At 3 DPH, larval
A. ocellaris have a gape height of approximately 430 μm [
16], suggesting that larvae of this age will more likely pursue prey items that range from 85 to 154 μm in width. This size range is more consistent with the size of rotifers compared to
Artemia, which suggests that 3 DPH larvae may be pursuing rotifers over
Artemia and that
Artemia introduction should occur no earlier than 5 DPH when larvae can more efficiently ingest larger prey items.
Larvae weaned to MD at first feeding (1 DPH) exhibited 0% survival, indicating this species may still require live feeds and/or greenwater during early ontogeny. This complete lack of survival may have been influenced by several factors, including environmental variations due to the absence of turbidity provided by microalgae, difficulties in feed recognition, and/or limitations in digestive capacity. The use of live or concentrated microalgae to darken culture water has been shown to improve larval fish survival and feeding incidence. Sowaske et al. (2025) [
55] reported a ~35% increase in melanurus wrasse (
Halichoeres melanurus) survival when
Tisochrysis lutea was used as greenwater compared to controls, highlighting benefits such as a favorable light environment, supplemental nutrition through passive ingestion, and contributions to a stable microbiome [
55]. Similar positive effects of microalgae use have been found in other marine ornamental species including yellow tang (
Zebrasoma flavescens) and Pacific blue tang (
Paracanthurus hepatus) [
56,
57]. Additionally, marine fish larvae are primarily visual predators, and the natural movement of live feeds is thought to stimulate their feeding response. In the absence of such movement, clownfish larvae may not have initially recognized the MD as prey, suggesting that co-feeding MDs alongside live feeds could provide the necessary visual and behavioral cues to promote acceptance at MD introduction [
58]. Lastly, even if larvae ingested MD at this early stage, they likely could not have efficiently digested the complex polypeptides due to the immaturity of their digestive tract and low acid protease activity [
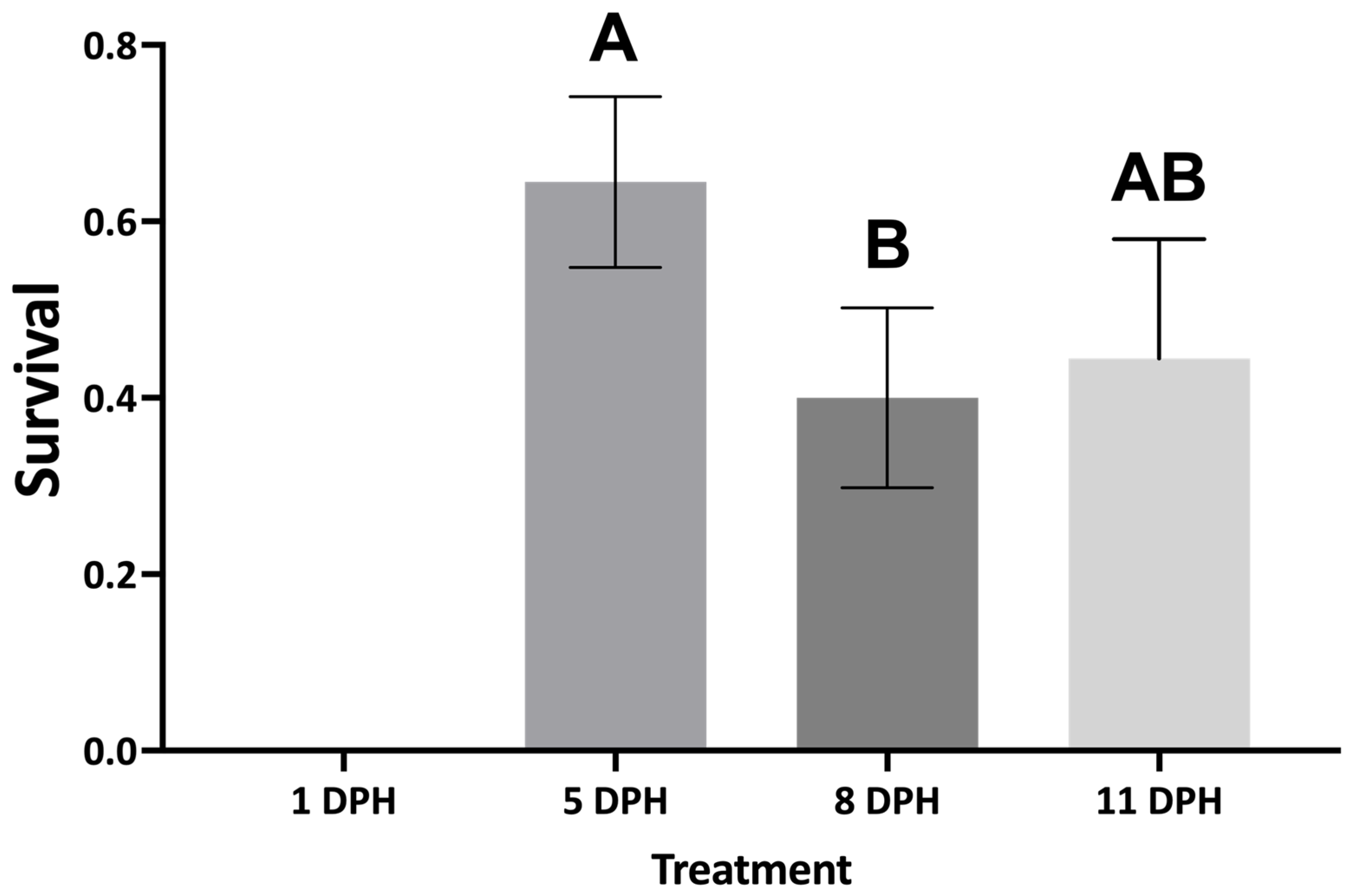
16].
However, the second weaning trial indicated that
A. ocellaris larvae can be transitioned to MD as early as 5 DPH, thus avoiding the use of
Artemia altogether. Removing
Artemia from larviculture protocols of
A. ocellaris is promising because this live feed has experienced frequent fluctuations in quality, availability, and price due to unpredictable weather patterns experienced by the Great Salt Lake, UT, USA, where the majority of
Artemia cysts originate [
11]. Additionally, weaning
A. ocellaris directly from rotifers to MD can potentially save producers up to
$1.60 per fish, when compared to weaning to MD at 11 DPH, which involves feeding
Artemia for several days. Decreased survival experienced by larvae weaned to MD later in the larval period may be due to the inclusion of
Artemia in the larval diet for a longer period.
Artemia are known to be deficient in certain HUFAs, especially for marine species, and can supply larvae with an imbalance of neutral lipids in the form of triacylglycerols even after enrichment [
59]. Commercially available MDs, such as TDO Chroma boost™, are often formulated with fish-based protein and lipid sources, which can help provide a more complete nutritional profile to larval marine fish because they contain advantageous concentrations and ratios of HUFAs and essential amino acids [
60]. Additionally, marine-sourced ingredients can also enhance the palatability, digestibility, and absorption of feeds [
60]. The MD used in this study lists the protein sources as krill meal, fish meal, and squid meal while the lipid source is fish oil (Reef Nutrition
®, Reed Mariculture, Inc., CA, USA), indicating that this MD may have provided a more complete nutritional profile to
A. ocellaris larvae compared to
Artemia, thus increasing the survival of larvae fed MD earlier in the larval period. The base diet of TDO Chroma boost™, Otohime (Marubeni Nisshin Feed Co., Ltd.; Tokyo, Japan), was designed to meet the general nutritional requirements of larval marine fish, although this diet is not specifically formulated for clownfish. Generally, there is a lack of species-specific feed formulations in the ornamental aquaculture industry due to the breadth of species produced. Overall, TDO Chroma boost™ performed well in
A. ocellaris in this study; however, additional research on species-specific nutritional requirements is warranted to further optimize clownfish larviculture.
Previous studies that have investigated the digestive ontogeny of
A. ocellaris found gastric gland structures at approximately 6 to 7 DPH, with both studies suggesting that complete stomach functionality occurs at approximately 8 DPH [
16,
33]. Both studies indicated that 7 DPH may be the earliest at which MD should be introduced to
A. ocellaris, which was two days later than the successful weaning of
A. ocellaris in the current study, suggesting that
A. ocellaris can efficiently digest MD prior to complete stomach functionality. Most studies that follow similar weaning methods found that weaning marine larvae to MD prior to stomach functionality resulted in reduced larval growth or survival such as in pigfish (
Orthopristis chrysoptera [
18]), southern flounder (
Paralichthys lethostigma [
22]), and cobia (
Rachycentron canadum [
61]), which was usually attributed to the immaturity of the larval digestive systems. Other ornamental species have been able to transition to an inert MD prior to the onset of a functional stomach such as black skirt tetra (
G. ternetzi [
25]), neon tetra (
Paracheirodon innesi [
27]), and orchid dottyback (
P. fridmani [
21]) without affecting larval growth or survival. The high survival of larvae fed MD starting at 5 DPH indicates that
A. ocellaris can be transitioned to MD early in the larval period and
Artemia are not essential in the larval production of this species. Overall, these results will help develop larval culture protocols that minimize the use of live feeds in the production of
A. ocellaris and potentially other species of clownfish.
Results from this study suggest that larval feeding protocols for
A. ocellaris can be simplified down to just two feeds, one live feed and MD, compared to several other published feeding protocols that often feature at least three feeds. Roux et al. (2021) [
6] outlined a larval rearing protocol that used unenriched
B. plicatilis (from 1 to 8 DPH),
Artemia (from 3 to 30 DPH), and a dry diet (from 12 to 30 DPH). While this study aimed to set a standard protocol for larval feeding of
A. ocellaris under laboratory conditions, it was unclear whether favorable larval survival was achieved under this feeding regime. Raheem et al. (2021) [
7] conducted larval rearing experiments where rotifers were replaced with
A. southwelli copepod nauplii from 1 to 9 DPH and
Artemia spp. nauplii were fed from 9 to 60 DPH. While larval survival appeared to increase from 55% in fish fed enriched rotifers to 73% in fish fed
A. southwelli nauplii, it is unclear whether these data were statistically significant. Although an increase in survival could result from using copepod nauplii in place of rotifers, the increased cost of maintaining copepod cultures must also be considered. The larval feeding protocol outlined by Hoff (1996) [
3] suggests that in addition to rotifers,
Artemia, and MD, feedings should also include fine flake food, krill meal, and a gelatin-based diet throughout larval production up to six times daily.
Clownfish, survival and growth was not affected by MD brand in the dietetics trial, suggesting that each MD satisfied the nutritional requirements of clownfish larvae. However, clownfish fed a diet of TDO ChromaBoost™ exhibited more red-shifted hue values and significantly higher color saturation; therefore, TDO ChromaBoost™ produced
A. ocellaris with a more desirable color hue and saturation, likely due to the inclusion of astaxanthin in this diet. Astaxanthin supplementation has been known to stimulate color development in the skin in a variety of ornamental species including
A. ocellaris, common goldfish (
Carassius auratus), kissing gourami (
Helostoma temminckii), koi carp (
Cyprinus carpio), and red flame dwarf gourami (
Trichogaster lalius) [
42,
62,
63,
64,
65,
66,
67].
Dietary astaxanthin inclusion has been shown to enhance coloration in
Amphiprion spp. although optimal concentrations vary across studies. Hoff (1996) [
3] visually ranked coloration and found that
A. ocellaris fed an experimental diet containing 200 ppm astaxanthin exhibited “very good, bright orange” coloration with 178 ppm astaxanthin and 111 ppm zeaxanthin deposited in the skin, compared to 0 and 58 ppm, respectively, in controls. Similarly, Tanaka et al. (1992) [
68] reported a coloration shift from “yellowish orange” to “pinkish orange” hue when
A. ocellaris were fed varying amounts of astaxanthin (0 ppm astaxanthin, 2.15 ppm zeaxanthin vs. 17.8 ppm astaxanthin, 11.16 ppm zeaxanthin, respectively). Yasir and Qin (2010) [
43] also observed red-shifted hue values in astaxanthin-fed fish, though no differences were detected among the 20, 50, and 100 ppm treatments. In
A. percula, higher red values were measured in groups fed 75–150 ppm astaxanthin compared to controls and lower doses [
69]. Despite these findings, no single concentration has consistently yielded optimal coloration, likely due to differences in diet formulation and astaxanthin source, which influence absorption and bioactivity [
70].
Due to its importance as a dietary additive, industry growth in aquaculture is one factor that has led to increased demand and market value of astaxanthin. The market for astaxanthin exceeded 447 million USD in 2014, and has been growing consistently, with some projections estimating the market size to reach 3.4 billion USD by 2027 [
67,
71]. Astaxanthin is extracted from several sources including petroleum distillate, yeast, green algae, or from the shells of marine arthropods; however, the market is largely dominated by the synthetically derived form (>95%) due to lower production costs [
67,
72]. Inclusion of color-enhancing compounds can lead to increased price of aquaculture feeds. For example, Otohime size A is priced at
$74/kg through Reed Mariculture; however, TDO ChromaBoost size A (the same pellet top-dressed with astaxanthin containing algal powder) is
$110/kg (prices are accurate at time of publication). Despite a higher price associated with TDO, it remains the industry standard for clownfish rearing (personal communication). Moderate pricing combined with the more desirable color development demonstrated that TDO was the most effective larval microdiet of those investigated in this study.