Integration of Ulva ohnoi in a Recirculating Aquaculture System for Gilthead Seabream (Sparus aurata) and Its Use as Feed for Sea Urchin (Paracentrotus lividus) Production: A Contribution to Circular and Sustainable Aquaculture Practices
Abstract
1. Introduction
2. Material and Methods
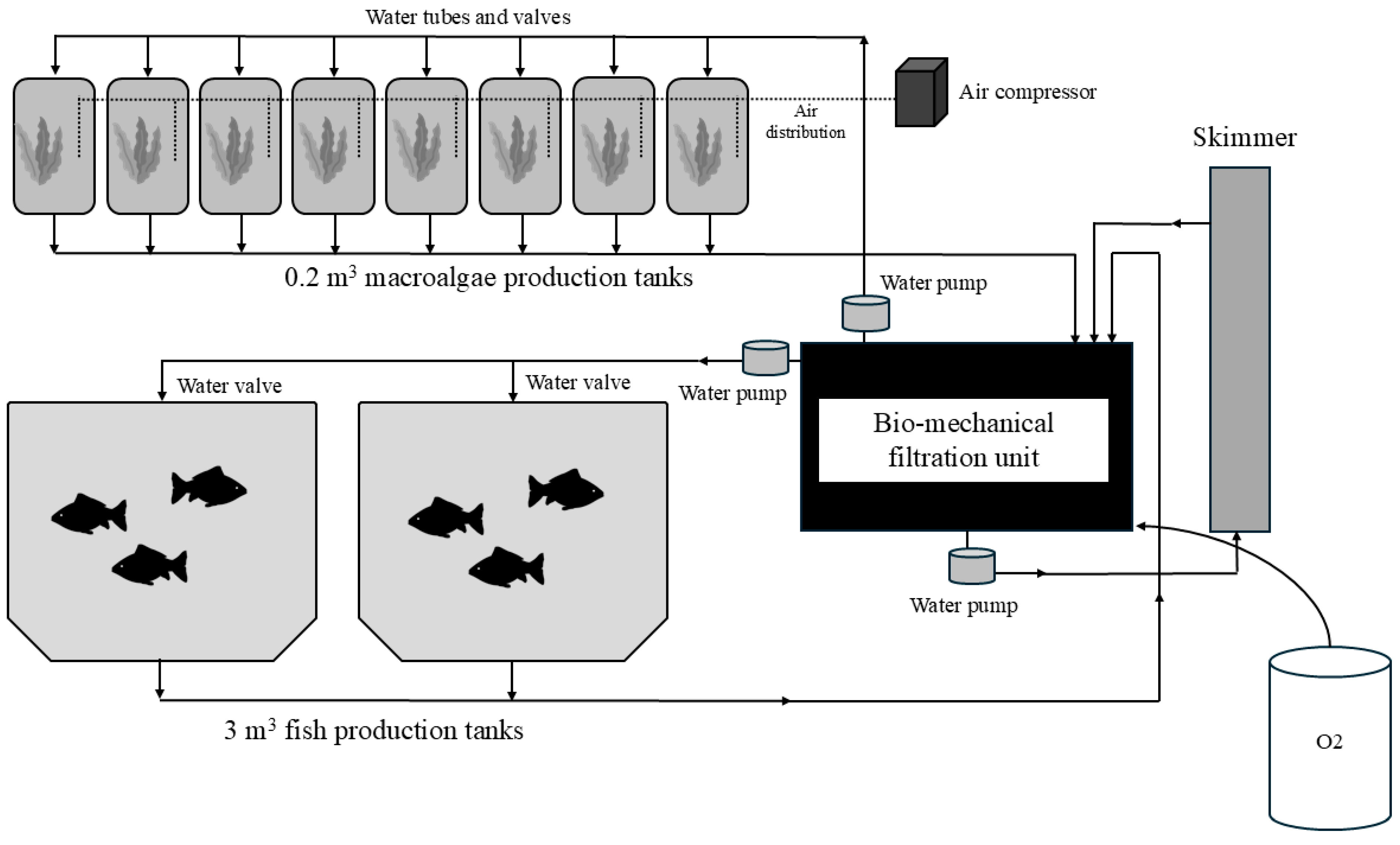
2.1. Integrated Recirculation System (RAS)
2.2. Macroalgae and Sea Urchin Production
2.2.1. Macroalgae
2.2.2. Sea Urchin Rearing and Feeding Protocol
2.2.3. Sea Bream Cultivation Protocol
2.2.4. Biometric Sampling
2.2.5. Environmental Parameters and Sampling Procedures
2.3. Impact of Integrated Macroalgae Production on Fish Health and Welfare
Fish Welfare: Ectoparasite Analysis
2.4. Data Processing
2.4.1. Macroalgae and Sea Urchin Production
2.4.2. Fish Production and Ectoparasite Analysis
2.5. Use of Artificial Intelligence Assistance
3. Results
3.1. First Phase: Macroalgae and Sea Urchin Production
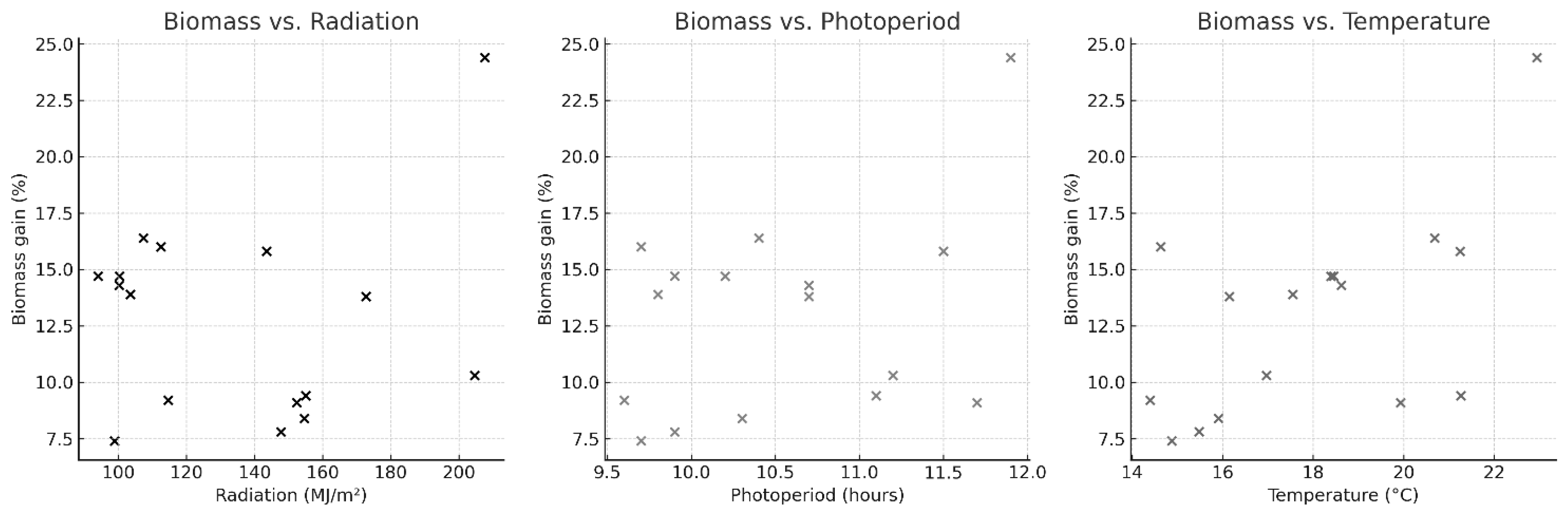
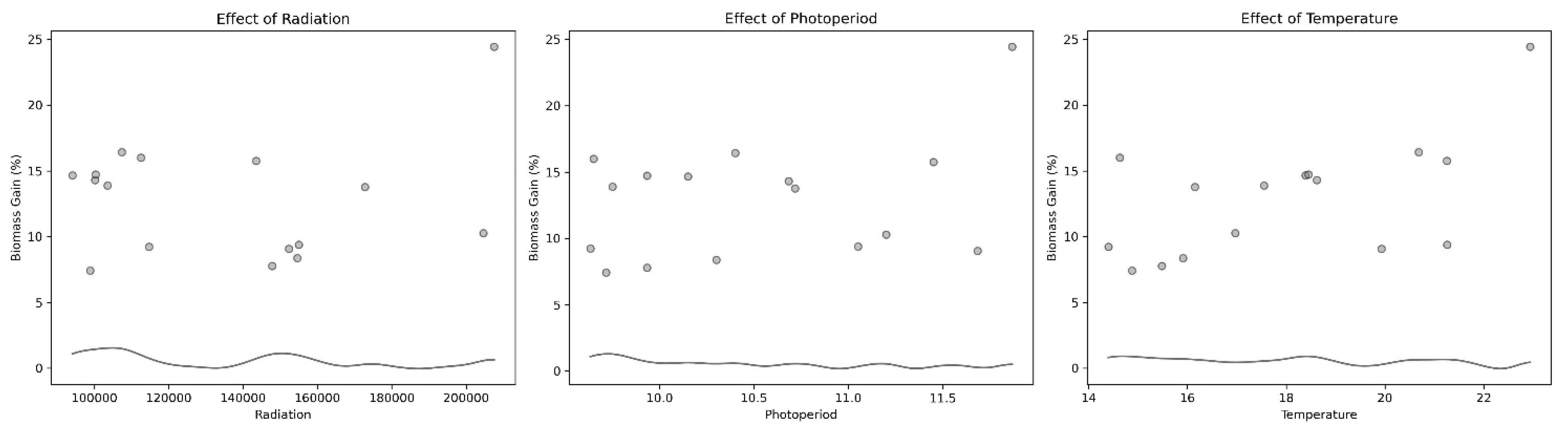
3.1.1. Macroalgae Production
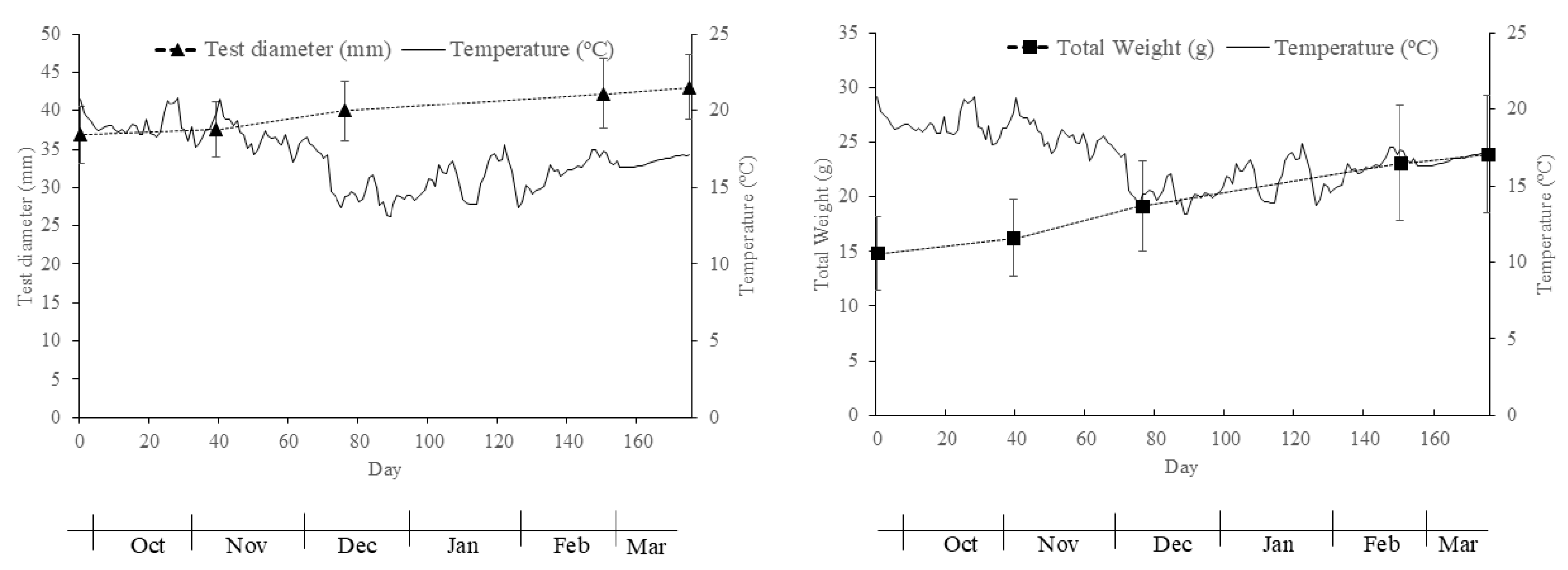
3.1.2. Sea Urchin Production
3.2. Second Phase: Analyzing Fish Welfare
3.2.1. Fish Growth, Condition, and Nutrition Indices
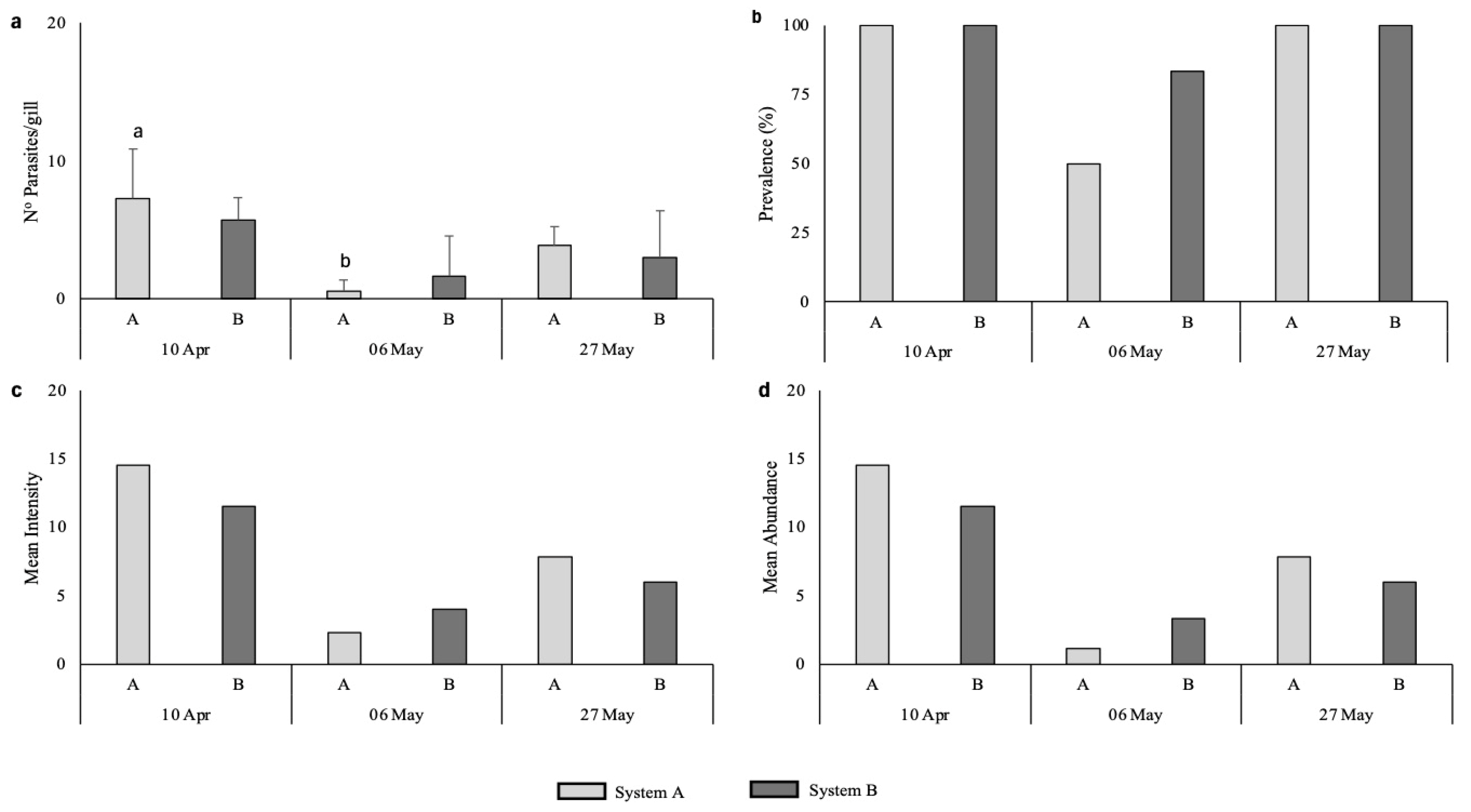
3.2.2. Ectoparasite Analysis
4. Discussion
4.1. Macroalgae Production
4.2. Sea Urchin Production
4.3. Sea Bream Production and Welfare
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. In Brief to The State of World Fisheries and Aquaculture; Blue Transformation in Action; FAO: Rome, Italy, 2024.
- FAO. The State of World Fisheries and Aquaculture; Towards Blue Transformation; FAO: Rome, Italy, 2022.
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Hall, S.J.; Delaporte, A.; Phillips, M.; Beveridge, M.; O’Keefe, M.; Center, T. Blue Frontiers: Managing the Environmental Costs of Aquaculture; The WorldFish Center: Penang, Malaysia, 2011. [Google Scholar]
- Boyd, C.; Clay, J. Shrimp Aquaculture and the Environment. Sci. Am. 1998, 278, 58–65. [Google Scholar] [CrossRef]
- Hamilton, S. Assessing the role of commercial aquaculture in displacing mangrove forest. Bull. Mar. Sci. 2013, 89, 585–601. [Google Scholar] [CrossRef]
- Milewski, I. Impacts of Salmon Aquaculture on the Coastal Environment: A Review. In Marine Aquaculture and the Environment: A Meeting for Stakeholders in the Northeast; Cape Cod Press: Falmouth, MA, USA, 2001; 35p. [Google Scholar]
- Taranger, G.L.; Karlsen, Ø.; Bannister, R.J.; Glover, K.A.; Husa, V.; Karlsbakk, E.; Kvamme, B.O.; Boxaspen, K.K.; Bjørn, P.A.; Finstad, B.; et al. Risk assessment of the environmental impact of Norwegian Atlantic salmon farming. ICES J. Mar. Sci. 2015, 72, 997–1021. [Google Scholar] [CrossRef]
- Abbas Dakheel, N.; Al-Saeedi, N.K.; Zainab, D.D. Carp Fish and Effects on the Aquatic Environment. Univ. Thi-Qar J. Agric. Res. 2024, 13, 327–334. [Google Scholar] [CrossRef]
- Aich, N.; Nama, S.; Biswal, A.; Paul, T. A review on recirculating aquaculture systems: Challenges and opportunities for sustainable aquaculture. Innov. Farm. 2020, 5, 17–24. [Google Scholar]
- Roy, S.M.; Choi, H.; Kim, T. Review of state-of-the-art improvements in recirculating aquaculture systems: Insights into design, operation, and statistical modeling approaches. Aquaculture 2025, 605, 742545. [Google Scholar] [CrossRef]
- Neori, A.; Troell, M.; Chopin, T.; Yarish, C.; Critchley, A.; Buschmann, A. The Need for a Balanced Ecosystem Approach to Blue Revolution Aquaculture. Environ. Sci. Policy Sustain. Dev. 2007, 49, 36–43. [Google Scholar] [CrossRef]
- Martins, C.; Eding, E.; Verdegem, M.; Heinsbroek, L.; Schneider, O.; Blancheton, J.; d’Orbcastel, E.; Verreth, A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Chopin, T. Integrated Multi-Trophic Aquaculture. What it is and why you should care... and don’t confuse it with polyculture. North. Aquac. 2006, 12, 4. [Google Scholar]
- Chopin, T.; Buschmann, A.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.; Zertuche, J.; Yarish, C.; Neefus, C. Integrating seaweeds into marine aquaculture systems: A key toward sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Cirino, P.; Ciaravolo, M.; Paglialonga, A.; Toscano, A. Long-term maintenance of the sea urchin Paracentrotus lividus in culture. Aquac. Rep. 2017, 7, 27–33. [Google Scholar] [CrossRef]
- Harris, L.; Eddy, S. Sea urchin ecology and biology. In Echinoderm Aquaculture; Brown, N., Eddy, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 3–24. [Google Scholar]
- Chatzoglou, E.; Kechagia, P.; Tsopelakos, A.; Miliou, H. Co-culture of Ulva sp. and Dicentrarchus labrax in recirculating aquaculture system: Effects on growth, retention of nutrients and fatty acid profile. Aquat. Living Resour. 2020, 33, 19. [Google Scholar] [CrossRef]
- Cardoso, C.; Ripol, A.; Afonso, C.; Freire, M.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Bandarra, N. Fatty acid profiles of the main lipid classes of green seaweeds from fish pond aquaculture. Food Sci. Nutr. 2017, 5, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.; Loureiro, P.; Candeias-Mendes, A.; Gamboa, A.; Bandarra, N.; Cardoso, C.; Soares, F.; Dias, J.; Pousão-Ferreira, P. The effect of a formulated feed on the body growth and gonads quality of purple sea urchin (Paracentrotus lividus) aquaculture produced. J. Aquac. Mar. Biol. 2023, 12, 11–18. [Google Scholar] [CrossRef]
- Ortiz, J. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem. 2006, 5, 998–1006. [Google Scholar]
- Guruge, W.A.H.P. Effects of Ulva lactuca and Sargassum cinereum supplemented diets on haematological parameters and survival of Koi carp (Cyprinus carpio L.) against bacterial pathogen (Aeromonas species). Ceylon J. Sci. 2021, 50, 145–153. [Google Scholar] [CrossRef]
- Amin, M.A.; Chondra, U.; Mostafa, E.; Alam, M.M. Green seaweed Ulva lactuca, a potential source of bioactive peptides revealed by in silico analysis. Inform. Med. Unlocked 2022, 33, 101099. [Google Scholar] [CrossRef]
- Afonso, C.; Cardoso, C.; Ripol, A.; Varela, J.; Quental-Ferreira, H.; Pousão-Ferreira, P.; Ventura, M.S.; Delgado, I.M.; Coelho, I.; Castanheira, I.; et al. Composition and bioaccessibility of elements in green seaweeds from fish pond aquaculture. Food Res. Int. 2018, 105, 271–277. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Lourenço-Marques, C.; Baptista, T.; Pousão-Ferreira, P.; Soares, F. First reporto f Calceostoma glandulosum (Monogenea) in Argyrosomus regius: Morphological and molecular characterization and themperature effects on life cycle. Aquac. Res. 2025, 2025, 9397751. [Google Scholar] [CrossRef]
- Andree, K.B.; Roque, A.; Duncan, N.; Gisbert, E.; Estevez, A.; Tsertou, M.I.; Katharios, P. Diplectanum sciaenae (Van Beneden & Hesse, 1863) (Monogenea) infecting meagre, Argyrosomus regius (Asso, 1801) broodstock in Catalonia, Spain. A case report. Vet. Parasitol. Reg. Stud. Rep. 2015, 1–2, 75–79. [Google Scholar]
- Doan, H.V.; Soltani, E.; Ingelbrecht, J.; Soltani, M. Medicinal Herbs and Plants: Potential Treatment of Monogenean Infections in Fish. Rev. Fish. Sci. Aquac. 2020, 28, 260–282. [Google Scholar] [CrossRef]
- Hutson, K.S.; Mata, L.; Paul, N.A.; de Nys, R. Seaweed extracts as a natural control against the monogenean ectoparasite, Neobenedenia sp., infecting farmed barramundi (Lates calcarifer). Int. J. Parasitol. 2012, 42, 1135–1141. [Google Scholar] [CrossRef]
- Riera-Ferrer, E.; Del Pozo, R.; Piazzon, M.C.; Sitjà-Bobadilla, A.; Estensoro, I.; Palenzuela, O. Sparicotyle chrysophrii experimental infection of gilthead seabream (Sparus aurata): Establishment of an in vivo model reproducing the pathological outcomes of sparicotylosis. Aquaculture 2023, 573, 739588. [Google Scholar] [CrossRef]
- Shinn, A.P.; Pratoomyot, J.; Bron, J.E.; Paladini, G.; Brooker, E.E.; Brooker, A.J. Economic costs of protistan and metazoan parasites to global mariculture. Parasitology 2015, 142, 196–270. [Google Scholar] [CrossRef]
- Kearn, G.C.; Ogawa, K.; Maeno, Y. Hatching Patterns of the Monogenean Parasites Benedenia seriolae and Heteraxine heterocerca from the Skin and Gills, Respectively, of the Same Host Fish, Seriola quinqueradiata. Zool. Sci. 1992, 9, 451–455. [Google Scholar]
- Cheng, T.C. Monogenea: The Monogenetic Trematodes. In General Parasitology; Academic Press: Orlando, FL, USA, 1986; pp. 273–298. [Google Scholar]
- Repullés-Albelda, A.; Holzer, A.S.; Raga, J.A.; Montero, F.E. Oncomiracidial development, survival and swimming behaviour of the monogenean Sparicotyle chrysophrii (Van Beneden and Hesse, 1863). Aquaculture 2012, 338–341, 47–55. [Google Scholar] [CrossRef]
- Jahangiri, L.; MacKinnon, B.; St-Hilaire, S. Infectious diseases reported in warm-water marine fish cage culture in East and Southeast Asia—A systematic review. Aquac. Res. 2022, 53, 2081–2108. [Google Scholar] [CrossRef]
- Sitjà-Bobadilla, A.; Redondo, M.J.; Alvarez-Pellitero, P. Occurrence of Sparicotyle chrysophrii (Monogenea: Polyopisthocotylea) in gilthead sea bream (Sparus aurata L.) from different mariculture systems in Spain. Aquac. Res. 2010, 41, 939–944. [Google Scholar] [CrossRef]
- Villar-Torres, M.; Montero, F.E.; Raga, J.A.; Repullés-Albelda, A. Come rain or come shine: Environmental effects on the infective stages of Sparicotyle chrysophrii, a key pathogen in Mediterranean aquaculture. Parasites Vectors 2018, 11, 558. [Google Scholar] [CrossRef]
- Antonelli, L.; Quilichini, Y.; Marchand, B. Sparicotyle chrysophrii (Van Beneden and Hesse 1863) (Monogenea: Polyopisthocotylea) parasite of cultured Gilthead sea bream Sparus aurata (Linnaeus 1758) (Pisces: Teleostei) from Corsica: Ecological and morphological study. Parasitol. Res. 2010, 107, 389–398. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Communication. Circular Economy Action Plan—For a Cleaner and More Competitive Europe; Publications Office of the European Union: Luxembourg, 2020.
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575. [Google Scholar] [CrossRef]
- Servén, D.; Brummitt, C. pyGAM: Generalized Additive Models in Python. Zenodo 2018. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blonder, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Schneider, O.; Sereti, V.; Eding, E.H.; Verreth, J.A.J. Analysis of nutrient flows in integrated intensive aquaculture systems. Aquacult. Eng. 2005, 32, 379–401. [Google Scholar] [CrossRef]
- Lupatsch, I.; Kissil, G.W.; Sklan, D. Comparison of energy and protein efficiency among three fish species: Gilthead sea bream (Sparus aurata), European sea bass (Dicentrarchus labrax) and white grouper (Epinephelus aeneus). Aquaculture 2003, 196, 87–94. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Li, J.; Ma, Z.; Yu, G.; Qin, C. Effects of light color on the growth, feeding, digestion, and antioxidant enzymes of Tripneustes gratilla. Biology 2024, 13, 65. [Google Scholar] [CrossRef]
- Lourenço, S.; José, R.; Andrade, C.; Valente, L.M.P. Growth performance and gonad yield of sea urchin Paracentrotus lividus (Lamarck, 1816) fed with diets of increasing protein: Energy ratios. Anim. Feed Sci. Technol. 2020, 270, 114690. [Google Scholar] [CrossRef]
- Araújo, J.; Gamboa, M.; Choulis, I.; Candeias-Mendes, A.; Cabrita, E.; Pousão-Ferreira, P.; Soares, F. Histological evaluation of purple sea urchin (Paracentrotus lividus) gonads: Influence of temperature, photoperiod regimes, and diets. Fishes 2024, 9, 207. [Google Scholar] [CrossRef]
- Bonaldo, A.; Isani, G.; Fontanillas, R.; Parma, L.; Grilli, E.; Gatta, P.P. Growth and feed utilization of gilthead sea bream (Sparus aurata, L.) fed to satiation and restrictively at increasing dietary energy levels. Aquacult. Int. 2010, 18, 909–919. [Google Scholar] [CrossRef]
- Favot, G.; Engelen, A.E.; Cunha, M.E.; Serrão, M.E.A. Identification of Ulva sp. grown in multitrophic aquaculture systems. J. Aquac. Fish. 2019, 3, 024. [Google Scholar] [CrossRef]
- Carl, C.; de Nys, R.; Paul, N.A. The seeding and cultivation of a tropical species of filamentous Ulva for algal biomass production. PLoS ONE 2014, 9, e98700. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, N.; Solidoro, C. The influence of environmental variables on Ulva rigida C. Ag. growth and production. Bot. Mar. 1996, 39, 27–32. [Google Scholar] [CrossRef]
- Taylor, R.; Fletcher, R.L.; Raven, J.A. Preliminary studies on the growth of selected ‘Green tide’ algae in laboratory culture: Effects of irradiance, temperature, salinity and nutrients on growth rate. Bot. Mar. 2001, 44, 327–336. [Google Scholar] [CrossRef]
- Olischläger, M.; Bartsch, I.; Gutow, L.; Wiencke, C. Effects of ocean acidification on growth and physiology of Ulva lactuca (Chlorophyta) in a rockpool-scenario. Phycol. Res. 2013, 61, 180–190. [Google Scholar] [CrossRef]
- Toth, G.B.; Harrysson, H.; Wahlström, N.; Olsson, J.; Oerbekke, A.; Steinhagen, S.; Kinnby, A.; White, J.; Albers, E.; Edlund, U.; et al. Effects of irradiance, temperature, nutrients, and pCO2 on the growth and biochemical composition of cultivated Ulva fenestrata. J. Appl. Phycol. 2020, 32, 3243–3254. [Google Scholar] [CrossRef]
- Nakamura, M.; Kumagai, N.; Tamaoki, M.; Arita, K.; Ishii, Y.; Nakajima, N.; Tohru, Y. Photosynthesis and growth of Ulva ohnoi and Ulva pertusa (Ulvophyceae) under high light and high temperature conditions, and implications for green tide in Japan. Phycol. Res. 2019, 68, 152–160. [Google Scholar] [CrossRef]
- NASA. Prediction of Worldwide Energy Resources (POWER) Data Access Viewer. Available online: https://power.larc.nasa.gov/ (accessed on 23 June 2025).
- Lawton, R.J.; Mata, L.; de Nys, R.; Paul, N.A. Algal bioremediation of waste waters from land-based aquaculture using Ulva: Selecting target species and strains. PLoS ONE 2013, 8, e77344. [Google Scholar] [CrossRef]
- Pérez-Mayorga, D.M.; Ladah, L.; Zertuche, J.; Leichter, J.J.; Filonov, A.; Lavín, M.F. Nitrogen uptake and growth by the opportunistic macroalga Ulva lactuca (Linnaeus) during the internal tide. J. Exp. Mar. Biol. Ecol. 2011, 406, 108–115. [Google Scholar] [CrossRef]
- Fujita, R.M. The role of nitrogen status in regulating transient ammonium uptake and nitrogen storage by macroalgae. J. Exp. Mar. Biol. Ecol. 1985, 92, 283–301. [Google Scholar] [CrossRef]
- Gómez-Pinchetti, J.L.G.; Fernandez, E.C.; Diez, P.M.; Reina, G.G. Nitrogen availability influences the biochemical composition and photosynthesis of tank-cultivated Ulva rigida (Chlorophyta). J. Appl. Phycol. 1998, 10, 383–389. [Google Scholar] [CrossRef]
- Hernández, I.; Peralta, G.; Pérez-Lloréns, J.; Vergara, J.J.; Niell, F.X. Biomass and dynamics of growth of Ulva species in Palmones River Estuary. J. Phycol. 1997, 33, 764–772. [Google Scholar] [CrossRef]
- Oca, J.; Cremades, J.; Jiménez, P.; Pintado, J.; Masaló, I. Culture of the seaweed Ulva ohnoi integrated in a Solea senegalensis recirculating system: Influence of light and biomass stocking density on macroalgae productivity. J. Appl. Phycol. 2019, 31, 2461–2467. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Photosynthetic suspended-growth systems in aquaculture. Aquac. Eng. 2006, 34, 344–363. [Google Scholar] [CrossRef]
- Candeias-Mendes, A.; Araújo, J.; Santos, M.; Namora, M.; Soares, F.; Gomes, R.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Pousão-Ferreira, P. Growth, survival and fatty acids profile of sea urchins, Paracentrotus lividus juveniles fed with Ulva spp. and maize in aquaculture production. First results using G1 generation in Portugal. J. Aquac. Mar. Biol. 2020, 9, 208–214. [Google Scholar] [CrossRef]
- Cyrus, M.; Bolton, J.; De Wet, L.; Macey, B.M. The development of a formulated feed containing Ulva (Chlorophyta) to promote rapid growth and enhanced production of high-quality roe in the sea urchin Tripneustes gratilla (Linnaeus). Aquac. Res. 2012, 45, 159–176. [Google Scholar] [CrossRef]
- Prato, E.; Fanelli, G.; Angioni, A.; Biandolino, F.; Parlapiano, I.; Papa, L.; Denti, G.; Secci, M.; Chiantore, M.; Kelly, M.; et al. Influence of a prepared diet and a macroalga (Ulva sp.) on the growth, nutritional and sensory qualities of gonads of the sea urchin Paracentrotus lividus. Aquaculture 2018, 493, 240–250. [Google Scholar] [CrossRef]
- Turon, X.; Giribet, G.; López, S.; Palacín, C. Growth and population structure of Paracentrotus lividus (Echinodermata: Echinoidea) in two contrasting habitats. Mar. Ecol. Prog. Ser. 1995, 122, 193–204. [Google Scholar] [CrossRef]
- Loureiro, P. The Effect of Two Inert Diets on Purple Sea Urchin, Paracentrotus lividus (Lamarck, 1816) Growth and Gonadal Development, in Aquaculture. Master’s Thesis, Faculdade de Ciências e Tecnologia, Universidade do Algarve, Faro, Portugal, 2021. [Google Scholar]
- Fernandez, C.; Pergent, G. Effect of different formulated diets and rearing conditions on growth parameters in the sea urchin Paracentrotus lividus. J. Shellfish Res. 1998, 17, 1571–1581. [Google Scholar]
- Shpigel, M.; Shauli, L.; Odintsov, V.; Ben, D.; Neori, A.; Guttman, L. The sea urchin, Paracentrotus lividus, in an Integrated Multi-Trophic Aquaculture (IMTA) system with fish (Sparus aurata) and seaweed (Ulva lactuca): Nitrogen partitioning and proportional configurations. Aquaculture 2018, 490, 260–269. [Google Scholar] [CrossRef]
- Parma, L.; Pelusio, N.F.; Gisbert, E.; Esteban, M.A.; D’Amico, F.; Soverini, M.; Candela, M.; Dondi, F.; Gatta, P.P.; Bonaldo, A. Effects of rearing density on growth, digestive conditions, welfare indicators and gut bacterial community of gilthead sea bream (Sparus aurata, L. 1758) fed different fishmeal and fish oil dietary levels. Aquaculture 2020, 518, 734854. [Google Scholar] [CrossRef]
- Zohar, Y.; Tal, Y.; Schrier, H.; Steven, C.; Stubblefield, J.; Place, A. Commercially feasible urban recirculated aquaculture: Addressing the marine sector. In Urban Aquaculture; Costa-Pierce, B., DesBonnet, A., Edwards, P., Baker, D., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 159–171. [Google Scholar]
- Petridis, D.; Rogdakis, I. The development of growth and feeding equations for sea bream, Sparus aurata L., culture. Aquac. Res. 1996, 27, 413–419. [Google Scholar] [CrossRef]




| Pair of Samples | Q Value | p Value |
|---|---|---|
| 4 October/30 December | 3.854 | 0.014 |
| 4 October/9 January | 4.589 | <0.001 |
| 4 October/23 January | 4.480 | <0.001 |
| 4 October/5 February | 4.240 | 0.003 |
| 4 October/14 March | 3.900 | 0.012 |
| 12 November/9 January | 4.149 | 0.004 |
| 12 November/23 January | 4.016 | 0.007 |
| 12 November/5 February | 3.721 | 0.024 |
| 20 December/9 January | 4.028 | 0.007 |
| 20 December/23 January | 3.895 | 0.012 |
| 20 December/5 February | 3.600 | 0.038 |
| Sea Urchins | At 0 Days T (°C) = 20.8 ± 0.99 | At 76 Days T (°C) = 17.7 ± 1.59 | At 150 Days T (°C) = 15.3 ± 1.16 | At 175 Days T (°C) = 16.4 ± 0.61 |
|---|---|---|---|---|
| Biomass (kg) | 3.11 ± 0.35 | 4.01 ± 0.81 | 4.82 ± 1.05 | 4.99 ± 1.23 |
| DIR (%) | - | 2.04 ± 0.06 | 1.83 ± 0.02 | 1.77 ± 0.02 |
| FCR | - | 5.97 ± 0.27 | 6.36 ± 0.10 | 6.60 ± 0.06 |
| SGR (% day−1) | - | 0.33 ± 0.23 | 0.29 ± 0.29 | 0.27 ± 0.34 |
| GSI (%) | 7.34 ± 1.72 | - | - | 2.88 ± 1.23 |
| At 0 Days T (°C) = 17.2 ± 0.17 | At 43 Days T (°C) = 20.0 ± 1.41 | At 65 Days T (°C) = 21.8 ± 1.98 | |||
|---|---|---|---|---|---|
| Gilthead Seabream (S. aurata) Production | In the presence of algae | Number of Fish | 220 | 213 | 207 |
| Biomass (kg) | 50.66 ± 8.46 | 56.58 ± 8.69 | 57.30 ± 9.33 | ||
| Density (kg m3 −1) | 16.89 ± 2.82 | 18.86 ± 2.90 | 19.10 ± 3.11 | ||
| DIR (%) | - | 0.70 ± 0.02 | 0.71 ± 0.01 | ||
| FCR | - | 2.13 ± 0.13 | 2.58 ± 0.05 | ||
| SGR (% day−1) | - | 0.33 ± 0.14 | 0.28 ± 0.24 | ||
| CF (g cm−3) | 1.66 ± 0.13 | 1.72 ± 0.25 | 1.65 ± 0.10 | ||
| In the absence of algae | Number of Fish | 220 | 214 | 208 | |
| Biomass (kg) | 51.47 ± 8.36 | 57.16 ± 8.67 | 58.68 ± 10.78 | ||
| Density (kg m3 −1) | 17.16 ± 2.79 | 19.05 ± 2.89 | 19.56 ± 3.59 | ||
| DIR (%) | - | 0.71 ± 0.02 | 0.71 ± 0.01 | ||
| FCR | - | 2.50 ± 0.12 | 2.53 ± 0.03 | ||
| SGR (% day−1) | - | 0.31 ± 0.15 | 0.29 ± 0.48 | ||
| CF (g cm−3) | 1.64 ± 0.14 | 1.78 ± 0.21 | 1.64 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, J.; Carvalho, A.C.; Matias, A.C.; Ribeiro, M.C.; Soares, F.; Pousão-Ferreira, P. Integration of Ulva ohnoi in a Recirculating Aquaculture System for Gilthead Seabream (Sparus aurata) and Its Use as Feed for Sea Urchin (Paracentrotus lividus) Production: A Contribution to Circular and Sustainable Aquaculture Practices. Fishes 2025, 10, 447. https://doi.org/10.3390/fishes10090447
Araújo J, Carvalho AC, Matias AC, Ribeiro MC, Soares F, Pousão-Ferreira P. Integration of Ulva ohnoi in a Recirculating Aquaculture System for Gilthead Seabream (Sparus aurata) and Its Use as Feed for Sea Urchin (Paracentrotus lividus) Production: A Contribution to Circular and Sustainable Aquaculture Practices. Fishes. 2025; 10(9):447. https://doi.org/10.3390/fishes10090447
Chicago/Turabian StyleAraújo, João, Ana Catarina Carvalho, Ana Carolina Matias, Maria Carolina Ribeiro, Florbela Soares, and Pedro Pousão-Ferreira. 2025. "Integration of Ulva ohnoi in a Recirculating Aquaculture System for Gilthead Seabream (Sparus aurata) and Its Use as Feed for Sea Urchin (Paracentrotus lividus) Production: A Contribution to Circular and Sustainable Aquaculture Practices" Fishes 10, no. 9: 447. https://doi.org/10.3390/fishes10090447
APA StyleAraújo, J., Carvalho, A. C., Matias, A. C., Ribeiro, M. C., Soares, F., & Pousão-Ferreira, P. (2025). Integration of Ulva ohnoi in a Recirculating Aquaculture System for Gilthead Seabream (Sparus aurata) and Its Use as Feed for Sea Urchin (Paracentrotus lividus) Production: A Contribution to Circular and Sustainable Aquaculture Practices. Fishes, 10(9), 447. https://doi.org/10.3390/fishes10090447








