A Complicated History of Mitogenome Introgression Among Luxilus Species (Teleostei, Family Leuciscidae) in the Ozark Highlands
Abstract
1. Introduction
2. Materials and Methods
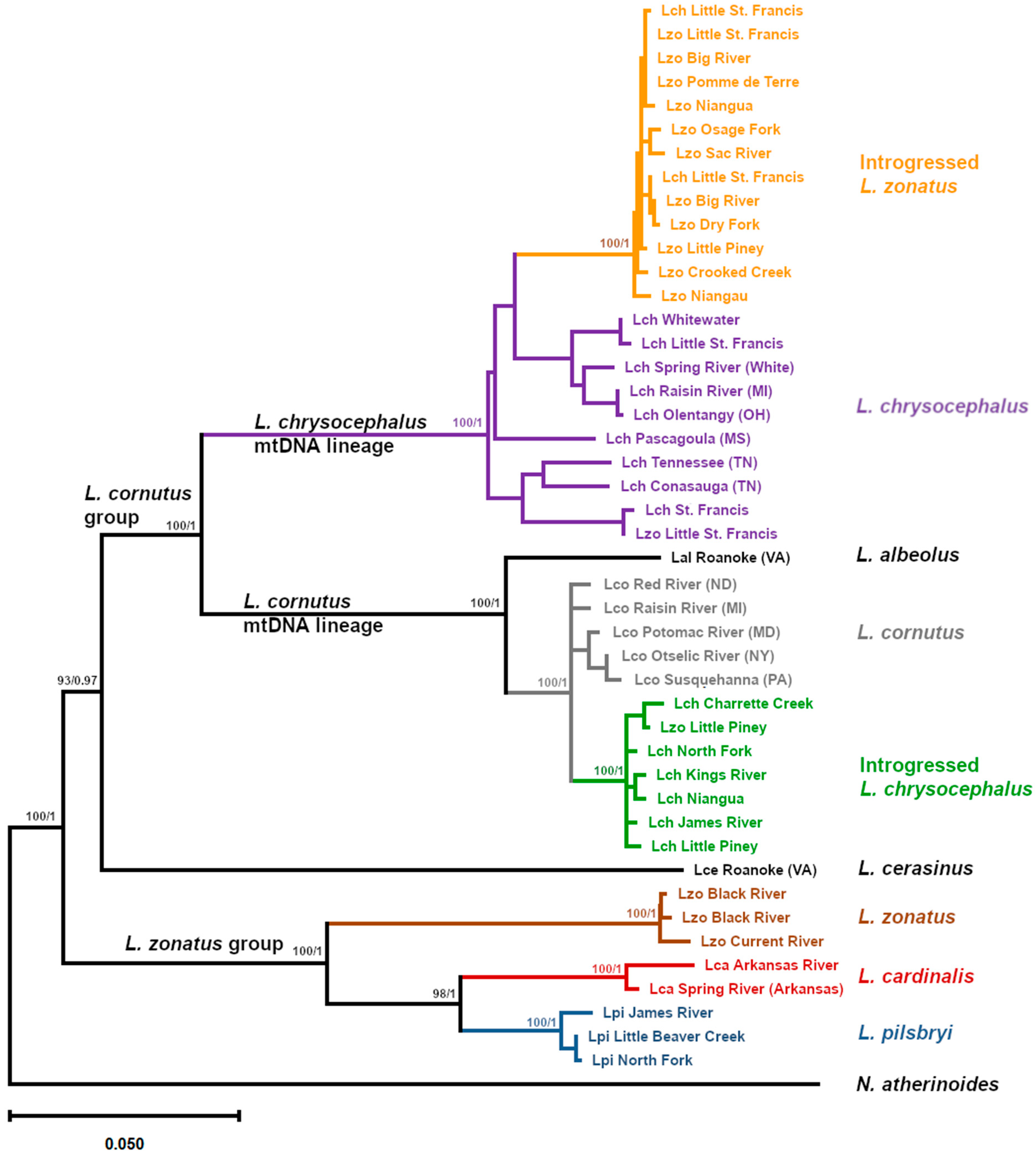
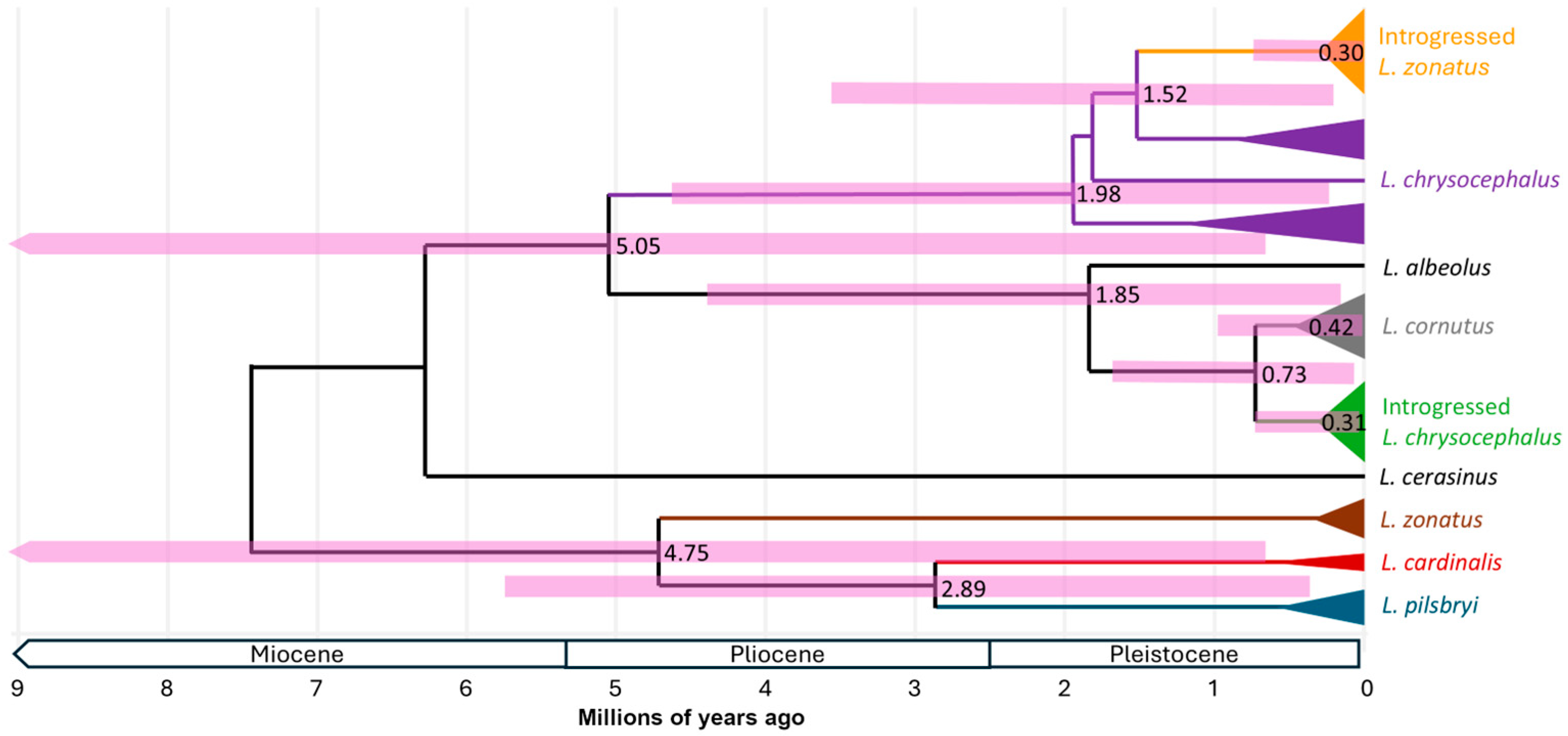
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mtDNA | Mitochondrial DNA |
| ML | Maximum Likelihood |
| PCR | Polymerase Chain Reaction |
| RFLP | Restriction Fragment Length Polymorphism |
| HPD | Highest Posterior Density |
References
- Arnold, M.L. Natural Hybridization and Evolution; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Dowling, T.E.; Secor, C.L. The Role of Hybridization and Introgression in the Diversification of Animals. Annu. Rev. Ecol. Syst. 1997, 28, 593–619. [Google Scholar] [CrossRef]
- Edelman, N.B.; Mallet, J. Prevalence and Adaptive Impact of Introgression. Annu. Rev. Genet. 2025, 55, 265–283. [Google Scholar] [CrossRef]
- Hedrick, P.W. Adaptive Introgression in Animals: Examples and Comparison to New Mutation and Standing Variation as Sources of Adaptive Variation. Mol. Ecol. 2013, 22, 4606–4618. [Google Scholar] [CrossRef]
- Mallet, J. Hybridization as an Invasion of the Genome. Trends Ecol. Evol. 2005, 20, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Toews, D.P.L.; Brelsford, A. The Biogeography of Mitochondrial and Nuclear Discordance in Animals. Mol. Ecol. 2012, 21, 3907–3930. [Google Scholar] [CrossRef]
- Komarova, V.A.; Lavrenchenko, L.A. Approaches to the Detection of Hybridization Events and Genetic Introgression upon Phylogenetic Incongruence. Biol. Bull. Rev. 2022, 12, 240–253. [Google Scholar] [CrossRef]
- Bonnet, T.; Leblois, R.; Rousset, F.; Crochet, P.A. A Reassessment of Explanations for Discordant Introgressions of Mitochondrial and Nuclear Genomes. Evolution 2017, 71, 2140–2158. [Google Scholar] [CrossRef]
- Wielstra, B. Historical Hybrid Zone Movement: More Pervasive than Appreciated. J. Biogeogr. 2019, 46, 1300–1305. [Google Scholar] [CrossRef]
- Sloan, D.B.; Havird, J.C.; Sharbrough, J. The On-Again, off-Again Relationship between Mitochondrial Genomes and Species Boundaries. Mol. Ecol. 2017, 26, 2212–2236. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.E. Reconciling the Mitonuclear Compatibility Species Concept with Rampant Mitochondrial Introgression. Integr. Comp. Biol. 2019, 59, 912–924. [Google Scholar] [CrossRef]
- Burton, R.S. The Role of Mitonuclear Incompatibilities in Allopatric Speciation. Cell. Mol. Life Sci. 2022, 79, 103. [Google Scholar] [CrossRef]
- Dowling, T.E.; Hoeh, W.R. The Extent of Introgression Outside the Contact Zone between Notropis cornutus and Notropis chrysocephalus (Teleostei: Cyprinidae). Evolution 1991, 45, 944–956. [Google Scholar] [CrossRef]
- Gerber, A.S.; Loggins, R.; Kumar, S.; Dowling, T.E. Does Nonneutral Evolution Shape Observed Patterns of DNA Variation in Animal Mitochondrial Genomes? Annu. Rev. Genet. 2001, 35, 539–566. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, N.; Champion, C.P.; Weir, L.K.; Dalziel, A.C. Reproductive Isolating Mechanisms Contributing to Asymmetric Hybridization in Killifishes (Fundulus spp.). J. Evol. Biol. 2023, 36, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, K.S. Biased Hybridization and Its Impact on Adaptive Introgression. Trends Ecol. Evol. 2021, 36, 488–497. [Google Scholar] [CrossRef]
- Wielstra, B.; Arntzen, J.W. Postglacial Species Displacement in Triturus Newts Deduced from Asymmetrically Introgressed Mitochondrial DNA and Ecological Niche Models. BMC Evol. Biol. 2012, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Currat, M.; Ruedi, M.; Petit, R.J.; Excoffier, L. The Hidden Side of Invasions: Massive Introgression by Local Genes. Evolution 2008, 62, 1908–1920. [Google Scholar] [CrossRef]
- Hubbs, C.L. Society of Systematic Biologists Hybridization between Fish Species in Nature. Syst. Zool. 1955, 4, 1–20. [Google Scholar] [CrossRef]
- Zbinden, Z.D.; Douglas, M.R.; Chafin, T.K.; Douglas, M.E. A Community Genomics Approach to Natural Hybridization. Proc. R. Soc. B Biol. Sci. 2023, 290, 20230768. [Google Scholar] [CrossRef]
- Scribner, K.T.; Page, K.S.; Bartron, M.L. Hybridization in Freshwater Fishes: A Review of Case Studies and Cytonuclear Methods of Biological Inference. Rev. Fish. Biol. Fish. 2001, 10, 293–323. [Google Scholar] [CrossRef]
- Meuser, A.V.; Pitura, A.R.; McFarlane, S.E.; Mandeville, E.G.D. Extensive multi-species hybridization between Leuciscidae minnow species. bioRxiv 2025. [Google Scholar] [CrossRef]
- Svensson, O.; Kvarnemo, C. How Sexual and Natural Selection Interact and Shape the Evolution of Nests and Nesting Behaviour in Fishes. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220139. [Google Scholar] [CrossRef]
- Corush, J.B.; Fitzpatrick, B.M.; Wolfe, E.L.; Keck, B.P. Breeding Behaviour Predicts Patterns of Natural Hybridization in North American Minnows (Cyprinidae). J. Evol. Biol. 2021, 34, 486–500. [Google Scholar] [CrossRef]
- Wilson, C.C.; Bernatchez, L. The Ghost of Hybrids Past: Fixation of Arctic Charr (Salvelinus alpinus) Mitochondrial DNA in an Introgressed Population of Lake Trout (S. namaycush). Mol. Ecol. 1998, 7, 127–132. [Google Scholar] [CrossRef]
- Doiron, S.; Bernatchez, L.; Blier, P.U. A Comparative Mitogenomic Analysis of the Potential Adaptive Value of Arctic Charr MtDNA Introgression in Brook Charr Populations (Salvelinus fontinalis Mitchill). Mol. Biol. Evol. 2002, 19, 1902–1909. [Google Scholar] [CrossRef]
- Englmaier, G.K.; Rodríguez, N.V.; Bravničar, J.; Zangl, L.; Persat, H.; Marić, S.; Ratschan, C.; Delling, B.; Gonçalves, D.V.; Secci-Petretto, G.; et al. SNP-Based Analysis of European Thymallus spp. (Salmonidae) Reveals Extensive Mito-Nuclear Discordance Relevant for Biogeographic Inferences, Taxonomy and Conservation. Divers. Distrib. 2024, 30, e13845. [Google Scholar] [CrossRef]
- Nevado, B.; KoblmÜller, S.; Sturmbauer, C.; Snoeks, J.; Usano-Alemany, J.; Verheyen, E. Complete Mitochondrial DNA Replacement in a Lake Tanganyika Cichlid Fish. Mol. Ecol. 2009, 18, 4240–4255. [Google Scholar] [CrossRef] [PubMed]
- Bossu, C.M.; Near, T.J. Gene Trees Reveal Repeated Instances of Mitochondrial DNA Introgression in Orangethroat Darters (Percidae: Etheostoma). Syst. Biol. 2009, 58, 114–129. [Google Scholar] [CrossRef]
- Duvernell, D.D.; Schaefer, J.F. Variation in Contact Zone Dynamics between Two Species of Topminnows, Fundulus notatus and F. olivaceus, across Isolated Drainage Systems. Evol. Ecol. 2014, 28, 37–53. [Google Scholar] [CrossRef]
- Berbel-Filho, W.M.; Pacheco, G.; Tatarenkov, A.; Lira, M.G.; Garcia de Leaniz, C.; Rodríguez López, C.M.; Lima, S.M.Q.; Consuegra, S. Phylogenomics Reveals Extensive Introgression and a Case of Mito-Nuclear Discordance in the Killifish Genus Kryptolebias. Mol. Phylogenet. Evol. 2022, 177, 107617. [Google Scholar] [CrossRef]
- Carson, E.W.; Dowling, T.E. Influence of Hydrogeographic History and Hybridization on the Distribution of Genetic Variation in the Pupfishes Cyprinodon atrorus and C. bifasciatus. Mol. Ecol. 2006, 15, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Duvernell, D.D.; Aspinwall, N. Introgression of Luxilus cornutus MtDNA into Allopatric Populations of Luxilus chrysocephalus (Teleostei: Cyprinidae) in Missouri and Arkansas. Mol. Ecol. 1995, 4, 173–181. [Google Scholar] [CrossRef]
- Dowling, T.E.; Naylor, G.J.P. Evolutionary Relationships of Minnows in the Genus Luxilus (Teleostei: Cyprinidae) as Determined from Cytochrome b Sequences. Copeia 1997, 1997, 758–765. [Google Scholar] [CrossRef]
- Dowling, T.E.; Moore, W.S. Level of Reproductive Isolation between Two Cyprinid Fishes, Notropis cornutus and N. chrysocephalus. Copeia 1984, 1984, 617–628. [Google Scholar] [CrossRef]
- Dowling, T.E.; Smith, G.R.; Brown, W.M. Reproductive Isolation and Introgression between Notropis cornutus and Notropis chrysocephalus (Family Cyprinidae): Comparison of Morphology, Allozymes, and Mitochondrial DNA. Evolution 1989, 43, 620–634. [Google Scholar] [CrossRef]
- Meagher, S.; Dowling, T.E. Hybridization between the Cyprinid Fishes Luxilus albeolus, L. cornutus, and L. cerasinus with Comments on the Proposed Hybrid Origin of L. albeolus. Copeia 1991, 1991, 979–991. [Google Scholar] [CrossRef]
- Pflieger, W.L. The Fishes of Missouri, 2nd ed.; Missouri Department of Conservation: Jefferson City, MO, USA, 1997. [Google Scholar]
- Gleason, C.A.; Berra, T.M. Demonstration of Reproductive Isolation and Observation of Mismatings in Luxilus. Copeia 1993, 1993, 614–628. [Google Scholar] [CrossRef]
- Dowling, T.E.; Broughton, R.E.; Demarais, B.D. Significant Role for Historical Effects in the Evolution of Reproductive Isolation: Evidence from Patterns of Introgression between the Cyprinid Fishes, Luxilus cornutus and Luxilus chrysocephalus. Evolution 1997, 51, 1574–1583. [Google Scholar] [CrossRef]
- Halas, D. Assessing the Prevalence of Common Patterns and Unique Events in the Formation of Biotas: A Study of Fish Taxa of the North American Central Highlands. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2011. [Google Scholar]
- Jerde, C.L.; Mahon, A.R.; Campbell, T.; McElroy, M.E.; Pin, K.; Childress, J.N.; Armstrong, M.N.; Zehnpfennig, J.R.; Kelson, S.J.; Koning, A.A.; et al. Are Genetic Reference Libraries Sufficient for Environmental DNA Metabarcoding of Mekong River Basin Fish? Water 2021, 13, 1767. [Google Scholar] [CrossRef]
- Dziedzic, E.; Sidlauskas, B.; Cronn, R.; Anthony, J.; Cornwell, T.; Friesen, T.A.; Konstantinidis, P.; Penaluna, B.E.; Stein, S.; Levi, T. Creating, Curating and Evaluating a Mitogenomic Reference Database to Improve Regional Species Identification Using Environmental DNA. Mol. Ecol. Resour. 2023, 23, 1880–1904. [Google Scholar] [CrossRef]
- Mayden, R.L. Systematics of the Notropis zonatus Species Group, with Description of a New Species from the Interior Highlands of North America. Copeia 1988, 1988, 153–173. [Google Scholar] [CrossRef]
- Dowling, T.E.; Hoeh, W.R.; Smith, G.R.; Brown, W.M. Evolutionary Relationships of Shiners in the Genus Luxilus (Cyprinidae) as Determined by Analysis of Mitochondrial DNA. Copeia 1992, 1992, 306–322. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Stout, C.; Schonhuth, S.; Mayden, R.; Garrison, N.L.; Armbruster, J.W. Phylogenomics and Classification of Notropis and Related Shiners (Cypriniformes: Leuciscidae) and the Utility of Exon Capture on Lower Taxonomic Groups. PeerJ 2022, 10, e14072. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An Advanced Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Smith, G.R.; Dowling, T.E.; Gobalet, K.W.; Lugaski, T.; Shiozawa, D.K.; Evans, R.P. Biogeography and Rates of Evolution of Great Basin Fishes. In Great Basin Aquatic Systems History; Her, R., Madsen, D.B., Currey, D.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2002; pp. 175–234. [Google Scholar]
- Smith, G.R.; Dowling, T.E. Correlating Hydrographic Events and Divergence Times of Speckled Dace (Rhinichthys: Teleostei: Cyprinidae) in the Colorado River Drainage. In Proceedings of the Special Paper of the Geological Society of America; Geological Society of America: Boulder, CO, USA, 2008; Volume 439, pp. 301–317. [Google Scholar]
- Spencer, J.E.; Smith, G.R.; Dowling, T.E. Middle to Late Cenozoic Geology, Hydrography, and Fish Evolution in the American Southwest. In Proceedings of the Special Paper of the Geological Society of America; Geological Society of America: Boulder, CO, USA, 2008; Volume 439, pp. 279–299. [Google Scholar]
- Unmack, P.J.; Dowling, T.E.; Laitinen, N.J.; Secor, C.L.; Mayden, R.L.; Shiozawa, D.K.; Smith, G.R. Influence of Introgression and Geological Processes on Phylogenetic Relationships of Western North American Mountain Suckers (Pantosteus, Catostomidae). PLoS ONE 2014, 9, e90061. [Google Scholar] [CrossRef]
- Smith, G.R.; Chow, J.; Unmack, P.J.; Markle, D.F.; Dowling, T.E.; Arbor, A. Evolution of the Rhinichthys osculus Complex (Teleostei: Cyprinidae) in Western North America. In Fishes of the Mio-Pliocene Western Snake River Plain and Vicinity; Miscellaneous Publications Museum of Zoology, University of Michigan: Ann Arbor, MI, USA, 2017; Volume 2, pp. 45–84. [Google Scholar]
- Estabrook, G.F.; Smith, G.R.; Dowling, T.E. Body Mass and Temperature Influence Rates of Mitochondrial DNA Evolution in North American Cyprinid Fish. Evolution 2007, 61, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian Evolutionary Analysis by Sampling Trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef]
- Lee, V.M.; Berkman, L.K.; Geheber, A.D.; Landwer, B.; Ludwig, E.J.; Duvernell, D.D. Putting EDNA to the Test: A Field Comparison of EDNA Metabarcoding to Established Protocols for Assessing Biodiversity in Missouri’s Ozark Highland Streams. Environ. DNA 2024, 6, e510. [Google Scholar] [CrossRef]
- Rovey, C.W.I.; Balco, G. Summary of Early and Middle Pleistocene Glaciations in Northern Missouri, USA. In Developments in Quarternary Sciences; Ehlers, J., Gibbard, P.L., Hughes, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 15, pp. 553–561. [Google Scholar]
- Robison, H.W. Zoogeographic Implications of the Mississippi River Basin. In The Zoogeography of North American Freshwater Fishes; Hocutt, E.H., Wiley, E.O., Eds.; Wiley and Sons: New York, NY, USA, 1986; pp. 267–285. [Google Scholar]
- Ray, J.M.; Wood, R.M.; Simons, A.M. Phylogeography and Post-Glacial Colonization Patterns of the Rainbow Darter, Etheostoma caeruleum (Teleostei: Percidae). J. Biogeogr. 2006, 33, 1550–1558. [Google Scholar] [CrossRef]
- Duvernell, D.D.; Westhafer, E.; Schaefer, J.F. Late Pleistocene Range Expansion of North American Topminnows Accompanied by Admixture and Introgression. J. Biogeogr. 2019, 46, 2126–2140. [Google Scholar] [CrossRef]
- Beeson, H.W.; McCoy, S.W.; Keen-Zebert, A. Geometric Disequilibrium of River Basins Produces Long-Lived Transient Landscapes. Earth Planet. Sci. Lett. 2017, 475, 34–43. [Google Scholar] [CrossRef]
- Hibbins, M.S.; Hahn, M.W. Phylogenomic Approaches to Detecting and Characterizing Introgression. Genetics 2022, 220, iyab173. [Google Scholar] [CrossRef]
- Teletchea, F. Molecular Identification Methods of Fish Species: Reassessment and Possible Applications. Rev. Fish. Biol. Fish. 2009, 19, 265–293. [Google Scholar] [CrossRef]

| Site No. | River | Drainage | Species | Lat/Long |
|---|---|---|---|---|
| 1 | Charrette Creek | Missouri River | L. chrysocephalus | 38.686 N 91.104 W |
| 2 | Big River | Meramec River | L. zonatus | 38.169 N 90.730 W |
| 3 | Crooked Creek | Meramec River | L. zonatus | 37.800 N 91.349 W |
| 4 | Dry Fork | Meramec River | L. zonatus | 37.991 N 91.555 W |
| 5 | Little Piney Creek | Gasconade River | L. zonatus, L. chrysocephalus | 37.910 N 91.903 W |
| 6 | Osage Fork | Gasconade River | L. zonatus | 37.633 N 92.452 W |
| 7 | Niangua River | Osage River | L. zonatus, L. chrysocephalus | 37.642 N 93.044 W |
| 8 | Pomme de Terre | Osage River | L. zonatus | 37.556 N 93.307 W |
| 9 | Sac River | Osage River | L. zonatus | 37.878 N 93.720 W |
| 10 | Little St. Francis River | St. Francis River | L. zonatus, L. chrysocephalus | 37.547 N 90.388 W |
| 11 | St. Francis River | St. Francis River | L. chrysocephalus | 37.595 N 90.498 W |
| 12 | Whitewater River | St. Francis River | L. chrysocephalus | 37.579 N 90.001 W |
| 13 | Black River | Black River | L. zonatus | 37.328 N 90.768 W |
| 14 | Current River | Black River | L. zonatus | 37.287 N 91.410 W |
| 15 | North Fork | White River | L. pilsbryi, L. chrysocephalus | 36.851 N 92.187 W |
| 16 | Little Beaver Creek | White River | L. pilsbryi | 36.801 N 92.909 W |
| 17 | James River | White River | L. pilsbryi, L. chrysocephalus | 37.156 N 93.199 W |
| 18 | Spring River | White River | L. chrysocephalus | 36.317 N 91.493 W |
| 19 | Kings River | White River | L. chrysocephalus | 36.137 N 93.582 W |
| 20 | Spring River | Arkansas River | L. cardinalis | 37.150 N 94.062 W |
| 21 | Arkansas River | Arkansas River | L. cardinalis | NA |
| Species | River | Introgressed L. zonatus | L. chry | Introgressed L. chry | L. zon. | L. pil. | L. car. |
|---|---|---|---|---|---|---|---|
| L. chrysocephalis | |||||||
| Charrette Creek | 1 | ||||||
| Little Piney Creek | 1 | ||||||
| Niangua River | 1 | ||||||
| Little St. Francis River | 9 | 1 | |||||
| St. Francis River | 1 | ||||||
| Whitewater River | 1 | ||||||
| North Fork | 1 | ||||||
| James River | 1 | ||||||
| Kings River | 1 | ||||||
| L. zonatus | |||||||
| Big River | 14 | ||||||
| Crooked Creek | 1 | ||||||
| Dry Fork | 3 | ||||||
| Little Piney Creek | 4 | 1 | |||||
| Osage Fork | 3 | ||||||
| Niangua River | 5 | ||||||
| Pomme de Terre | 6 | ||||||
| Sac River | 4 | ||||||
| Little St. Francis River | 3 | 1 | |||||
| Black River | 17 | ||||||
| Current River | 4 | ||||||
| L. pilsbryi | |||||||
| North Fork | 2 | ||||||
| Little Beaver Creek | 1 | ||||||
| James River | 2 | ||||||
| L. cardinalis | |||||||
| Spring River | 2 | ||||||
| Arkansas River | 1 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duvernell, D.D.; Arnold, C.; Koju, S.; Wicks, A.J.; Dowling, T.E. A Complicated History of Mitogenome Introgression Among Luxilus Species (Teleostei, Family Leuciscidae) in the Ozark Highlands. Fishes 2025, 10, 443. https://doi.org/10.3390/fishes10090443
Duvernell DD, Arnold C, Koju S, Wicks AJ, Dowling TE. A Complicated History of Mitogenome Introgression Among Luxilus Species (Teleostei, Family Leuciscidae) in the Ozark Highlands. Fishes. 2025; 10(9):443. https://doi.org/10.3390/fishes10090443
Chicago/Turabian StyleDuvernell, David D., Carson Arnold, Shila Koju, Abby J. Wicks, and Thomas E. Dowling. 2025. "A Complicated History of Mitogenome Introgression Among Luxilus Species (Teleostei, Family Leuciscidae) in the Ozark Highlands" Fishes 10, no. 9: 443. https://doi.org/10.3390/fishes10090443
APA StyleDuvernell, D. D., Arnold, C., Koju, S., Wicks, A. J., & Dowling, T. E. (2025). A Complicated History of Mitogenome Introgression Among Luxilus Species (Teleostei, Family Leuciscidae) in the Ozark Highlands. Fishes, 10(9), 443. https://doi.org/10.3390/fishes10090443







