Lysozyme Activity in the Hemolymph of Octopus vulgaris (Cuvier, 1797) Following Challenge with Gram-Negative Bacteria: Insights into Temperature-Driven Innate Immune Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Aquaria Systems
2.3. Bacteria, Media, and Culture Conditions
2.4. Challenge/Injections of Octopods
2.5. Hemolymph Sampling
2.6. Lysozyme Activity (Test)
- ΔA450 is the change in absorbance between 0 and 5 min;
- df represents the dilution factor;
- (mL) corresponds to the volume of the M. lysodeikticus (0.4 mg/mL) suspension in the reaction mixture;
- Corresponds to the absorbance decrease defined as one Unit of lysozyme activity (one unit of lysozyme causes a decrease in absorbance ΔA450 of 0.001/min).
2.7. Statistical Analysis
- (i)
- Temperatures for the same bacterium, day, and injection route;
- (ii)
- Time points for the same bacterium, route, and temperature;
- (iii)
- Challenged vs. control (non-challenged) specimens at the same route, day, and temperature.
3. Results
3.1. Lysozyme Activity (Results)
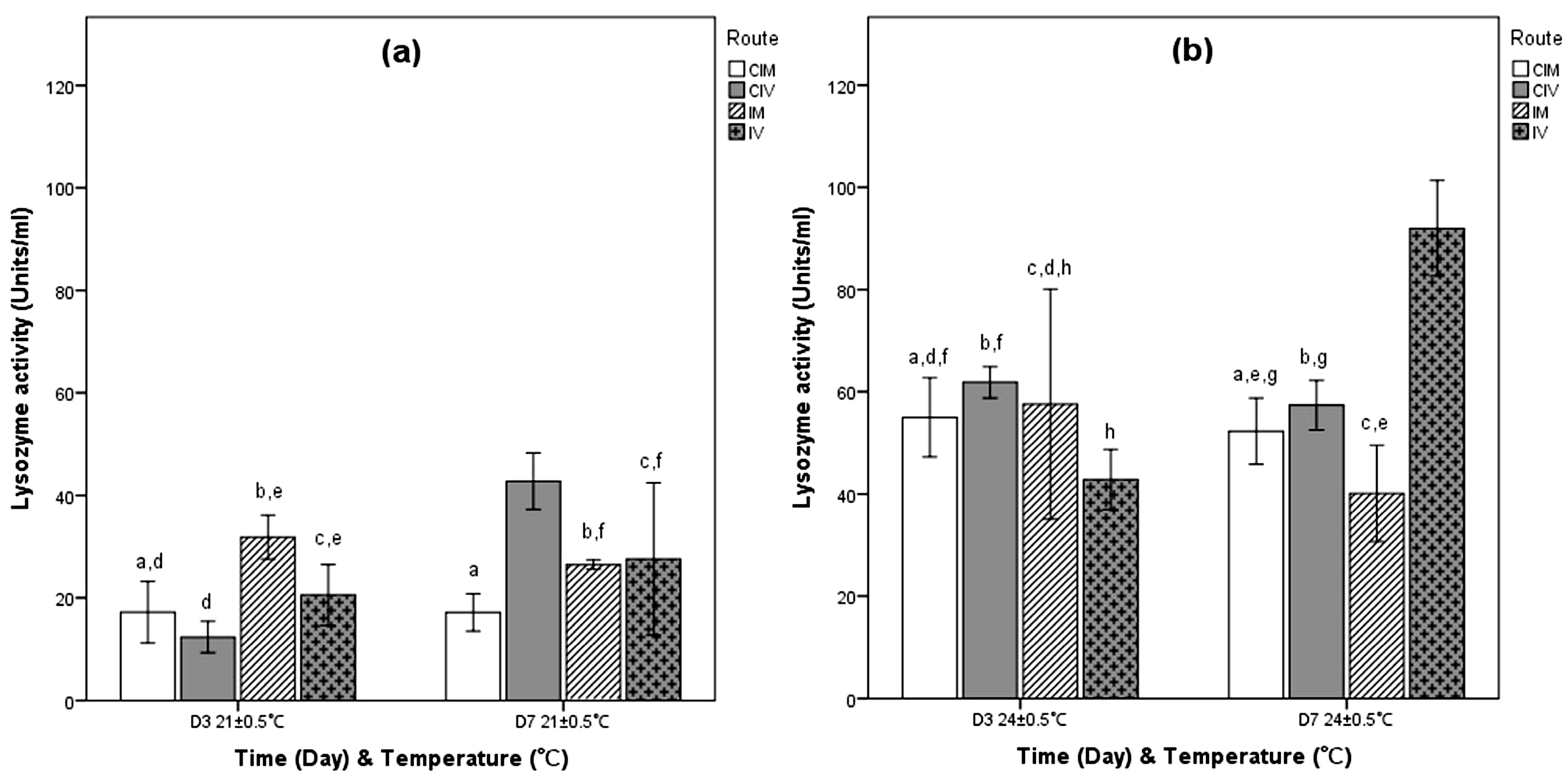
3.1.1. Challenge with Phdp
3.1.2. Challenge with Phdd
3.1.3. Challenge with VA
3.1.4. Challenge with VAO1
4. Discussion
5. Conclusions
- This study highlights the significance of lysozyme activity as a potential biomarker of innate immune function in O. vulgaris under pathogenic challenge and varying environmental temperatures.
- Lysozyme activity in cell-free hemolymph varied significantly depending on the following:
- −
- Pathogen type;
- −
- Route of challenge;
- −
- Time point post-injection;
- −
- Environmental temperature.
- Specimens challenged intramuscularly, especially with Photobacterium damselae subsp. piscicida and damselae, induce stronger lysozyme responses, especially at 21 ± 0.5 °C on day 3 post-injection.
- −
- The observed peak activity suggests a temperature- and time-dependent activation pattern of O. vulgaris immunity.
- Notably, lysozyme activity in cephalopods appears to persist longer than expected and previously assumed, possibly indicating a more prolonged role in immune defense.
- −
- This sustained enzymatic response might be central to bacterial neutralization in cephalopods.
- Lysozyme serves as a non-specific yet informative immune indicator of immune status, although environmental land biological variability must be taken into account.
- Future research should focus on the following:
- −
- Long-term patterns of lysozyme activity beyond early-phase responses;
- −
- A wider array of pathogens and challenge methods;
- −
- Molecular pathways regulating lysozyme expression and interaction with other immune components;
- −
- Additional environmental factors, such as salinity, pollution, and ocean acidification.
- Overall, applying lysozyme activity as a biomarker for health monitoring in octopus aquaculture could contribute meaningfully to developing sustainable and resilient farming practices.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garlock, T.M.; Asche, F.; Anderson, J.L.; Eggert, H.; Anderson, T.M.; Che, B.; Chávez, C.A.; Chu, J.; Chukwuone, N.; Dey, M.M.; et al. Environmental, economic, and social sustainability in aquaculture: The aquaculture performance indicators. Nat. Commun. 2024, 15, 527, Erratum in Nat. Commun. 2024, 15, 5965. [Google Scholar] [CrossRef]
- Vaz-Pires, P.; Seixas, P.; Barbosa, A. Aquaculture potential of the common octopus (Octopus vulgaris Cuvier, 1797): A review. Aquaculture 2004, 238, 221–238. [Google Scholar] [CrossRef]
- Almeida, D.; Domínguez-Pérez, D.; Matos, A.; Agüero-Chapin, G.; Osório, H.; Vasconcelos, V.; Campos, A.; Antunes, A. Putative antimicrobial peptides of the posterior salivary glands from the cephalopod Octopus vulgaris revealed by exploring a composite protein database. Antibiotics 2020, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- EUWFOP. European Union World Fisheries Overview Platform; EUWFOP: Brussels, Belgium, 2023; Available online: https://www.reportlinker.com/dataset/15d0ef2740e108bb3c5bb5a19cc3e1bdad910695 (accessed on 16 July 2025).
- Sauer, W.H.H.; Gleadall, I.G.; Downey-Breedt, N.; Doubleday, Z.; Gillespie, G.; Haimovici, M.; Ibáñez, C.M.; Katugin, O.N.; Leporati, S.; Lipinski, M.R.; et al. World octopus fisheries. Rev. Fish. Sci. Aquac. 2019, 29, 279–429. [Google Scholar] [CrossRef]
- Gestal, C.; Castellanos-Martínez, S. Understanding the cephalopod immune system based on functional and molecular evidence. Fish Shellfish Immunol. 2015, 46, 120–130. [Google Scholar] [CrossRef]
- Sacchi, S.; Malagoli, D.; Franchi, N. The invertebrate immunocyte: A complex and versatile model for immunological, developmental, and environmental research. Cells 2024, 13, 2106. [Google Scholar] [CrossRef]
- Rodríguez-Domínguez, H.; Soto-Búa, M.; Iglesias-Blanco, R.; Crespo-González, C.; Arias-Fernández, C.; García-Estévez, J. Preliminary study on the phagocytic ability of Octopus vulgaris Cuvier, 1797 (Mollusca: Cephalopoda) haemocytes in vitro. Aquaculture 2006, 254, 563–570. [Google Scholar] [CrossRef]
- White, D.M. Studies on Pathology and Immunology of the Common Octopus (Octopus vulgaris Cuvier, 1797). Ph.D. Thesis, University of the Aegean, Mytilini, Greece, 2023. Available online: https://www.didaktorika.gr/eadd/handle/10442/54760 (accessed on 4 August 2025).
- Van Herreweghe, J.M.; Michiels, C.W. Invertebrate lysozymes: Diversity and distribution, molecular mechanism and in vivo function. J. Biosci. 2012, 37, 327–348. [Google Scholar] [CrossRef]
- Matozzo, V.; Chinellato, A.; Munari, M.; Finos, L.; Bressan, M.; Marin, M.G. First evidence of immunomodulation in bivalves under seawater acidification and increased temperature. PLoS ONE 2012, 7, e33820. [Google Scholar] [CrossRef]
- Locatello, L.; Fiorito, G.; Finos, L.; Rasotto, M.B. Behavioural and immunological responses to an immune challenge in Octopus vulgaris. Physiol. Behav. 2013, 122, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wei, X.; Yang, J.; Zhang, R.; Zhang, Q.; Yang, J. The bacteriolytic mechanism of an invertebrate-type lysozyme from mollusk Octopus ocellatus. Fish Shellfish Immunol. 2019, 93, 232–239. [Google Scholar] [CrossRef]
- Hu, F.; Wang, Y.; Hu, J.; Bao, Z.; Wang, M. A novel c-type lysozyme from Litopenaeus vannamei exhibits potent antimicrobial activity. Fish Shellfish Immunol. 2022, 131, 108485. [Google Scholar] [CrossRef]
- Marra, A.; Hanson, M.A.; Kondo, S.; Erkosar, B.; Lemaitre, B. Drosophila antimicrobial peptides and lysozymes regulate gut microbiota composition and abundance. mBio 2021, 12, e0082421. [Google Scholar] [CrossRef]
- Montoya, L.N.F.; Martins, T.P.; Gimbo, R.Y.; Zanuzzo, F.S.; Urbinati, E.C. β-Glucan-induced cortisol levels improve the early immune response in matrinxã (Brycon amazonicus). Fish Shellfish Immunol. 2017, 60, 197–204. [Google Scholar] [CrossRef]
- Amphan, S.; Unajak, S.; Printrakoon, C.; Areechon, N. Feeding-regimen of β-glucan to enhance innate immunity and disease resistance of Nile tilapia, Oreochromis niloticus Linn., against Aeromonas hydrophila and Flavobacterium columnare. Fish Shellfish Immunol. 2019, 87, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.; Polycarpo, G.; Moromizato, B.S.; Sidekerskis, A.; Silva, T.; Reis, I.; Fierro-Castro, C. Lysozyme activity as an indicator of innate immunity of tilapia (Oreochromis niloticus) when challenged with LPS and Streptococcus agalactiae. Rev. Bras. Zootec. 2021, 50, e20210053. [Google Scholar] [CrossRef]
- FAO. Expanding Sustainable Aquaculture Production; FAO: Rome, Italy, 2025; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/9df19f53-b931-4d04-acd3-58a71c6b1a5b/content/sofia/2022/expanding-sustainable-aquaculture-production.html (accessed on 15 July 2025).
- Hanlon, R.T.; Forsythe, J.W.; Cooper, K.M.; Dinuzzo, A.R.; Folse, D.S.; Kelly, M.T. Fatal penetrating skin ulcers in laboratory-reared octopuses. J. Invertebr. Pathol. 1984, 44, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Rigos, G.; Katharios, P. Pathological obstacles of newly-introduced fish species in Mediterranean mariculture: A review. Rev. Fish Biol. Fish. 2010, 20, 47–70. [Google Scholar] [CrossRef]
- Gestal, G.; Pascual, S.; Guerra, A.; Fiorito, G.; Vieites, J.M. Handbook of Pathogens and Diseases in Cephalopods; Springer Open: Berlin/Heidelberg, Germany, 2019; pp. 1–234. [Google Scholar] [CrossRef]
- Farto, R.; Fichi, G.; Gestal, C.; Pascual, S.; Nieto, T.P. Bacteria-Affecting Cephalopods. In Handbook of Pathogens and Diseases in Cephalopods; Gestal, C., Pascual, S., Guerra, Á., Fiorito, G., Vieites, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 127–142. [Google Scholar] [CrossRef]
- Pascual, C.; Angeles-González, L.E.; Rodríguez-Flores, C.; Rosas, C. Sea surface temperature modulates physiological and immunological condition of Octopus maya. Front. Physiol. 2019, 10, 739. [Google Scholar] [CrossRef]
- Ángeles-González, L.E.; Pascual, C.; Mascaró, M.; Sánchez, A.; Gallardo, P.; Rodríguez-Flores, C.; Rosas, C. Linking inferred laboratory-derived temperature stress to immunocompetence in the Mayan octopus (Octopus maya). Front. Mar. Sci. 2025, 12, 1524296. [Google Scholar] [CrossRef]
- Castillo, M.G.; Goodson, M.S.; McFall-Ngai, M.J. The immune response of cephalopods from head to foot. Fish Shellfish Immunol. 2015, 46, 145–160. [Google Scholar] [CrossRef]
- Day, R.J.; Doubleday, Z.A.; Semmens, J.M. Exercise stress affects immune and metabolic physiology in the pale octopus (Octopus pallidus). Fish Shellfish Immunol. 2024, 145, 108896. [Google Scholar] [CrossRef]
- Paillard, C.; Allam, B.; Oubella, R. Effect of temperature on defense parameters in Manila clam Ruditapes philippinarum challenged with Vibrio tapetis. Dis. Aquat. Org. 2004, 59, 249–262. [Google Scholar] [CrossRef]
- Papadopoulos, M.; Bort, J.; Theodorou, J.A.; Katsiadaki, I. Molecular immune responses of Ruditapes decussatus to Vibrio splendidus: Effects of temperature on lysozyme gene expression. Fishes 2025, 10, 72. [Google Scholar] [CrossRef]
- White, D.M.; Valsamidis, M.A.; Bakopoulos, V. In vitro hemocyte phagocytosis activation after experimental infection of common octopus, Octopus vulgaris (Cuvier, 1797) with Photobacterium damselae subsp. piscicida or Vibrio alginolyticus at different temperatures and infection routes. J. Invertebr. Pathol. 2022, 191, 107754. [Google Scholar] [CrossRef]
- White, D.M.; Valsamidis, M.A.; Kokkoris, G.D.; Bakopoulos, V. The effect of temperature and challenge route on in vitro hemocyte phagocytosis activation after experimental challenge of common octopus, Octopus vulgaris (Cuvier, 1797) with either Photobacterium damselae subsp. damselae or Vibrio anguillarum O1. Microb. Pathog. 2023, 174, 105955. [Google Scholar] [CrossRef]
- White, D.M.; Valsamidis, M.A.; Bakopoulos, V. Hemolymph activities after experimental infection of common octopus, Octopus vulgaris (Cuvier, 1797) with Photobacterium damselae subsp. piscicida. In Proceedings of the “HydroMediT” 4th International Congresses on Applied Ichthyology, Oceanography, and Aquatic Environment, Mytilene, Lesvos, Greece, 30 May–2 June 2021. [Google Scholar]
- Miliou, H.; Fintikaki, M.; Tzitzinakis, M.; Kountouris, T.; Verriopoulos, G. Fatty acid composition of the common octopus, Octopus vulgaris, in relation to rearing temperature and body weight. Aquaculture 2006, 256, 311–322. [Google Scholar] [CrossRef]
- Prato, E.; Portacci, G.; Biandolino, F. Effect of diet on growth performance, feed efficiency and nutritional composition of Octopus vulgaris. Aquaculture 2010, 309, 203–211. [Google Scholar] [CrossRef]
- Petza, D.; Katsanevakis, S.; Lykouri, N.; Spiliotis, V.; Verriopoulos, G. Investigation of the potential effect of diet, body mass and maturity on growth and feed performance of common Octopus vulgaris: An information theory approach. Aquac. Nutr. 2011, 17, e348–e361. [Google Scholar] [CrossRef]
- Weather Stars. 2022. Available online: https://seatemperature.info/mytilini-water-temperature.html#google_vignette (accessed on 3 June 2022).
- UN. UN Environment Programme. Mediterranean Action Plan-Barcelona Convection; UN: New York, NY, USA, 2022; Available online: https://www.unep.org/unepmap/resources/factsheets/climate-change. (accessed on 3 June 2022).
- Katsanevakis, S.; Protopapas, N.; Miliou, H.; Verriopoulos, G. Effect of temperature on specific dynamic action in the common octopus, Octopus vulgaris (Cephalopoda). Mar Biol. 2005, 146, 733–738. [Google Scholar] [CrossRef]
- Noyola, J.; Caamal-Monsreal, C.; Díaz, F.; Re, D.; Sánchez, A.; Rosas, C. Thermopreference, tolerance and metabolic rate of early stages juvenile Octopus maya acclimated to different temperatures. J. Therm. Biol. 2013, 38, 14–19. [Google Scholar] [CrossRef]
- Hanif, A.; Bakopoulos, V.; Dimitriadis, G.J. Maternal transfer of humoral specific and non-specific immune parameters to sea bream (Sparus aurata) larvae. Fish Shellfish Immunol. 2004, 17, 411–435. [Google Scholar] [CrossRef]
- Bakopoulos, V.; White, D.; Valsamidis, M.A.; Vasilaki, F. Experimental infection of Octopus vulgaris (Cuvier, 1797) with Photobacterium damsela subsp. piscicida. Immunohistochemical tracking of antigen and tissue responses. J. Invertebr. Pathol. 2017, 144, 24–31. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assays. In Techniques in Fish Immunology; Stolen, J.S., Fletcher, T.C., Anderson, D.P., Roberson, B.S., van Muiswinkel, W.B., Eds.; SOS Publications: Cambridge, UK, 1990; pp. 101–103. Available online: https://scholar.google.com/scholar_lookup?title=Lysozyme%20assays&author=A.E.%20Ellis&publication_year=1990&pages=101-103 (accessed on 28 August 2025).
- Sigma-Aldrich. Enzymatic Assay of Lysozyme; Sigma-Aldrich: Shanghai, China, 2025; Available online: https://www.sigmaaldrich.com/GR/en/technical-documents/protocol/protein-biology/enzyme-activity-assays/enzymatic-assay-of-lysozyme (accessed on 14 July 2025).
- Cerón, A.; Costa, S.; Imbernon, R.; Queiroz, R.; Castro, J.; Ferraz, H.; Oliveira, R.; Costa, S. Study of stability, kinetic parameters and release of lysozyme immobilized on chitosan microspheres by crosslinking and covalent attachment for cotton fabric functionalization. Process Biochem. 2023, 128, 116–125. [Google Scholar] [CrossRef]
- Norusis, M.J. SPSS, version 12.0 Guide to Data Analysis; Prentice Hall PTR: Centennial, CO, USA, 2004.
- Sealey, W.M.; Gatlin, D.M. Dietary vitamin C and vitamin E interact to influence growth and tissue composition of juvenile hybrid striped bass (Morone chrysops X M. saxatilis) head–kidney cells. J. Nutr. 2002, 132, 748–755. [Google Scholar] [CrossRef]
- Ji, S.C.; Takaoka, O.; Jeong, G.S.; Lee, S.W.; Ishimaru, K.; Seoka, M.; Takii, K. Dietary medicinal herbs improve growth and some non-specific immunity of red sea bream Pagrus major. Fish. Sci. 2007, 73, 63–69. [Google Scholar] [CrossRef]
- Ren, T.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Micheal, F.R.; Uyan, O.; Tung, H.T. Influence of dietary vitamin C and bovine lactoferrin on blood chemistry and non-specific immune responses of Japanese eel, Anguilla japonica. Aquaculture 2007, 267, 31–37. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Le Pabic, C.; Safi, G.; Serpentini, A.; Lebel, J.M.; Robin, J.P.; Koueta, N. Prophenoloxidase system, lysozyme and protease inhibitor distribution in the common cuttlefish Sepia officinalis. Comp. Biochem. Physiol. B 2014, 172–173, 96–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, T.C. The role of lysosomes in molluscan inflammation. Am. Zool. 1983, 23, 129–144. [Google Scholar] [CrossRef]
- Malham, S.K.; Runham, N.W. A brief review of the immunobiology of Eledone cirrhosa. S. Afr. J. Mar. Sci. 1998, 20, 385–391. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Belcari, P.; Pagano, E.; Cacialli, F.; Locatello, L. Immune responses of Octopus vulgaris (Mollusca: Cephalopoda) exposed to titanium dioxide nanoparticles. J. Exp. Mar. Biol. Ecol. 2013, 447, 123–127. [Google Scholar] [CrossRef]
- Mukherjee, K.; Moroz, L.L. Evolution of g-type lysozymes in metazoa: Insights into immunity and digestive adaptations. Front. Cell Dev. Biol. 2024, 12, 1487920. [Google Scholar] [CrossRef] [PubMed]



| Bacterial Pathogen | Sampling Period |
|---|---|
| Photobacterium damselae subsp. Piscicida 21 ± 0.5 °C or 24 ± 0.5 °C | October 2017 (n = 27; 21 ± 0.5 °C) |
| November 2017 (n = 27; 24 ± 0.5 °C) | |
| Photobacterium damselae subsp. Damselae 21 ± 0.5 °C or 24 ± 0.5 °C | April 2019 (n = 27; 21 ± 0.5 °C) |
| May 2019 (n = 27; 24 ± 0.5 °C) | |
| Vibrio alginolyticus 21 ± 0.5 °C or 24 ± 0.5 °C | February 2018 (n = 27; 21 ± 0.5 °C) |
| March 2018 (n = 27; 24 ± 0.5 °C) | |
| Vibrio anquillarum O1 21 ± 0.5 °C or 24 ± 0.5 °C | October 2019 (n = 27; 21 ± 0.5 °C) |
| November 2019 (n = 27; 24 ± 0.5 °C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, D.-M.; Anastasiadou, E.; Valsamidis, M.-A.; Bakopoulos, V. Lysozyme Activity in the Hemolymph of Octopus vulgaris (Cuvier, 1797) Following Challenge with Gram-Negative Bacteria: Insights into Temperature-Driven Innate Immune Response. Fishes 2025, 10, 428. https://doi.org/10.3390/fishes10090428
White D-M, Anastasiadou E, Valsamidis M-A, Bakopoulos V. Lysozyme Activity in the Hemolymph of Octopus vulgaris (Cuvier, 1797) Following Challenge with Gram-Negative Bacteria: Insights into Temperature-Driven Innate Immune Response. Fishes. 2025; 10(9):428. https://doi.org/10.3390/fishes10090428
Chicago/Turabian StyleWhite, Daniella-Mari, Eleni Anastasiadou, Michail-Aggelos Valsamidis, and Vasileios Bakopoulos. 2025. "Lysozyme Activity in the Hemolymph of Octopus vulgaris (Cuvier, 1797) Following Challenge with Gram-Negative Bacteria: Insights into Temperature-Driven Innate Immune Response" Fishes 10, no. 9: 428. https://doi.org/10.3390/fishes10090428
APA StyleWhite, D.-M., Anastasiadou, E., Valsamidis, M.-A., & Bakopoulos, V. (2025). Lysozyme Activity in the Hemolymph of Octopus vulgaris (Cuvier, 1797) Following Challenge with Gram-Negative Bacteria: Insights into Temperature-Driven Innate Immune Response. Fishes, 10(9), 428. https://doi.org/10.3390/fishes10090428








