Histological and Transcriptomic Profiling Reveals Metabolic and Immune Responses to Ammonia Stress in Scatophagus argus
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Experimental Conditions
2.2. Acute Toxicity Test
2.3. Histological Observation
2.4. Transcriptome Analysis
2.4.1. Sample Collection and RNA Extraction
2.4.2. cDNA Library Preparation and Sequencing
2.4.3. Differential Expression and Enrichment Analysis
2.4.4. qRT-PCR Validation
2.5. Ethical Statement
3. Results
3.1. Acute Toxicity of Ammonia to Scatophagus argus
3.2. Histological Changes in the Gill and Liver of Scatophagus argus Under Ammonia Stress
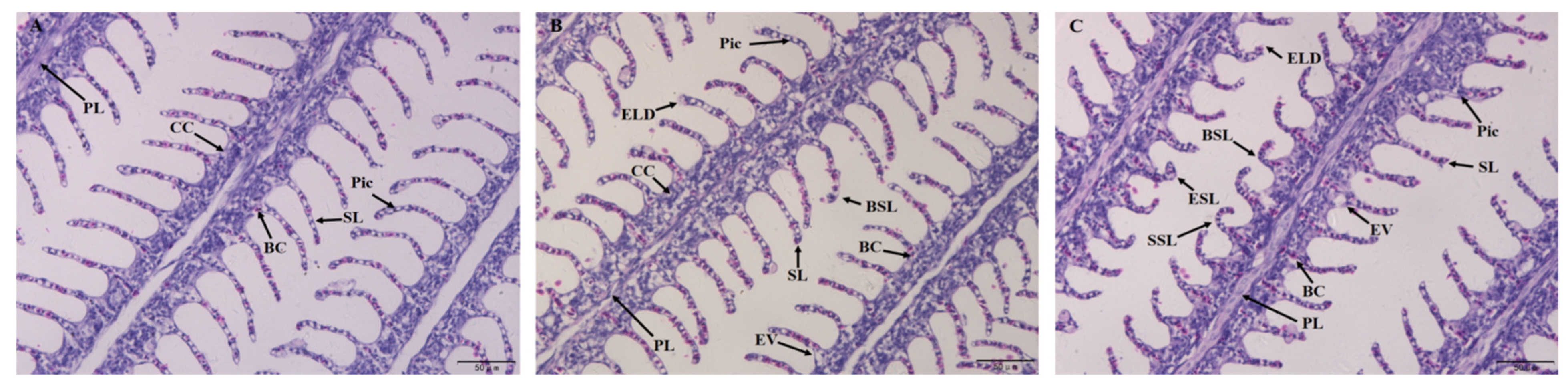
3.2.1. Gill Morphology
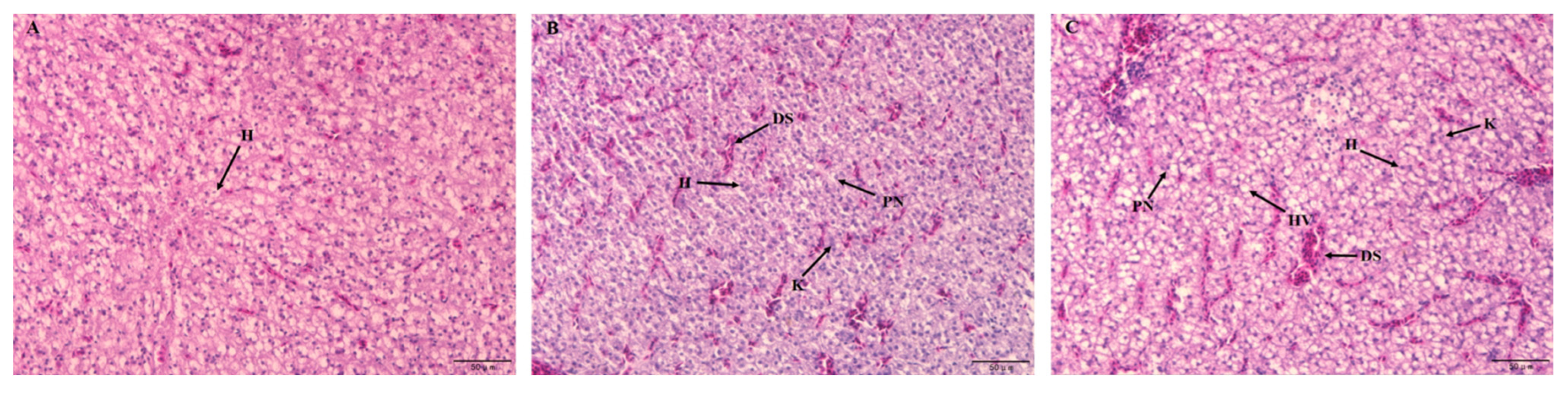
3.2.2. Liver Histopathology
3.3. Transcriptomic Response of Scatophagus argus Gills to Ammonia Stress
3.3.1. Sequencing Quality and Mapping Statistics
3.3.2. Identification of Differentially Expressed Genes
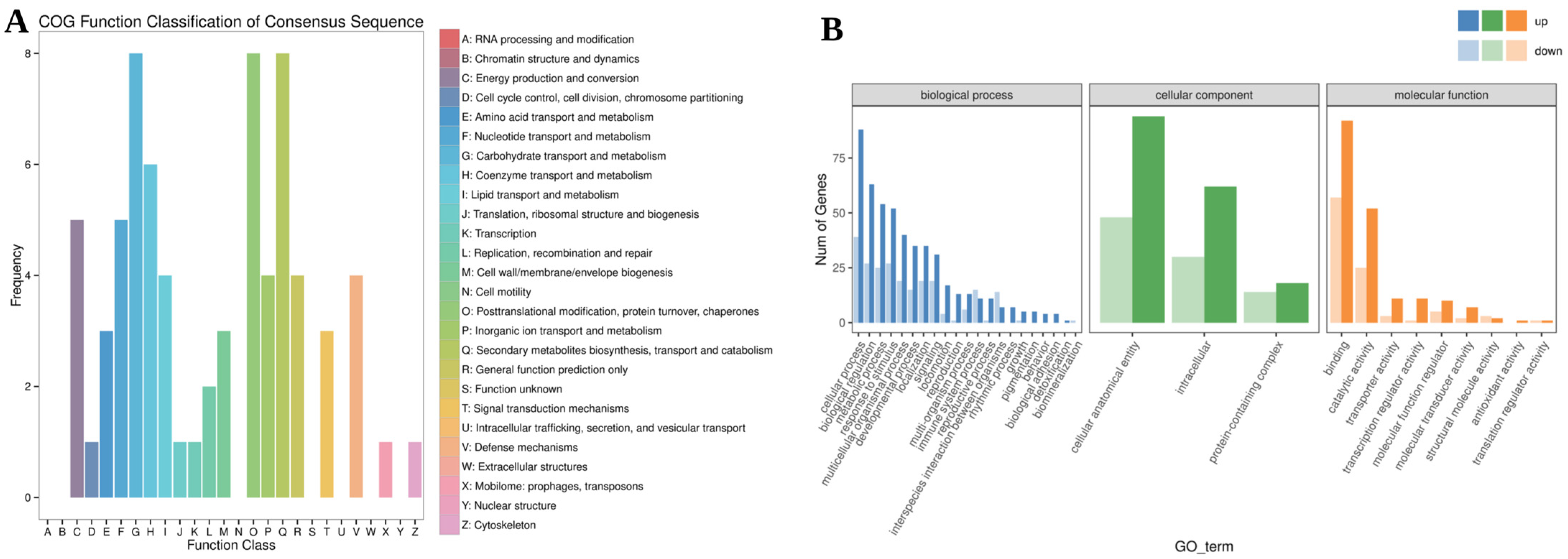
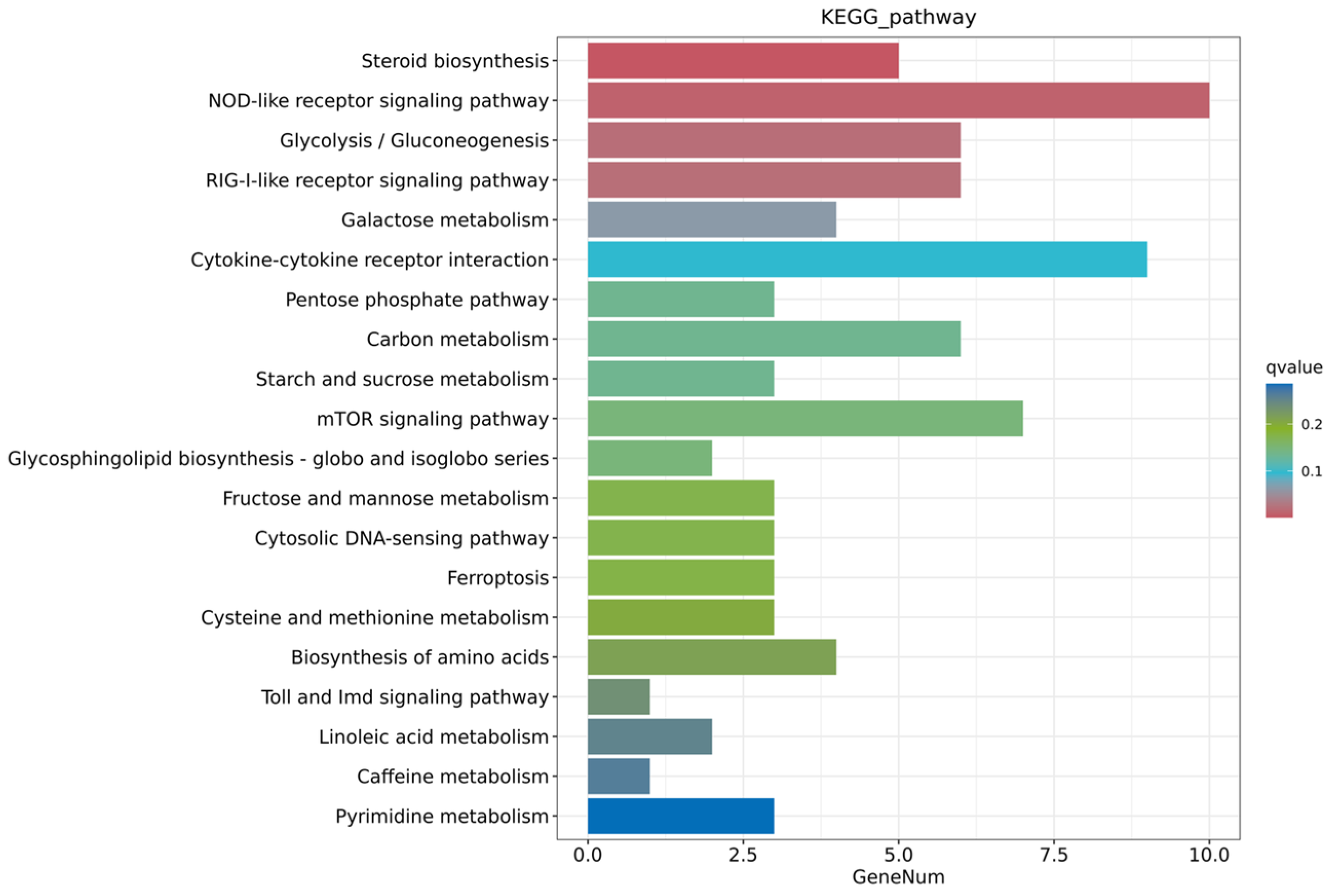
3.3.3. Functional Enrichment Analysis of DEGs
3.3.4. Expression Changes in Representative Energy Metabolism-Related Genes
3.3.5. Expression Changes in Representative Immune-Related Genes
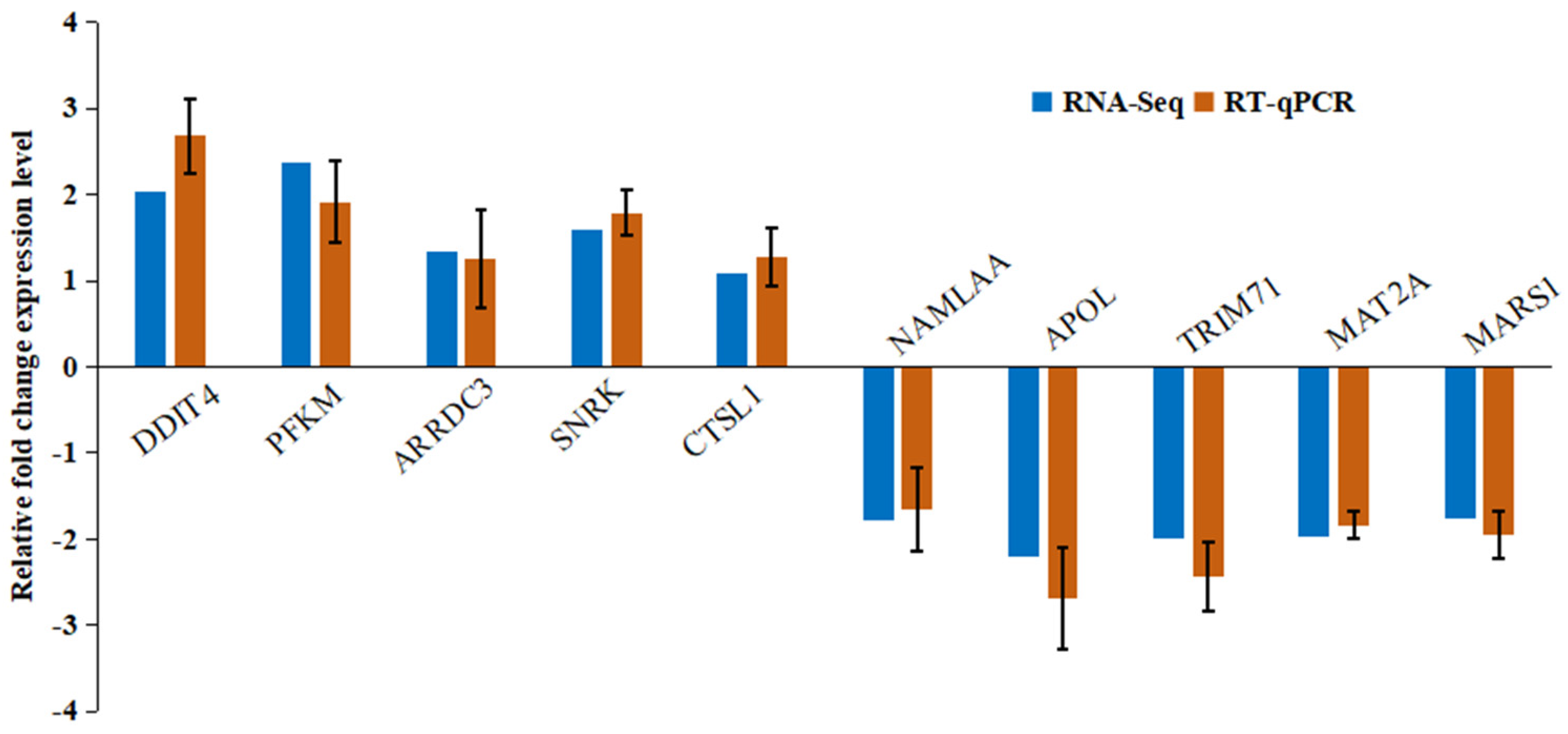
3.3.6. Validation of DEGs by qRT-PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, T.M.; Puglis, H.J.; Kent, D.B.; Durán, J.L.; Bradshaw, L.M.; Farag, A.M. Ammonia and Aquatic Ecosystems—A Review of Global Sources, Biogeochemical Cycling, and Effects on Fish. Sci. Total Environ. 2024, 907, 167911. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Barbieri, E.; Aydın, B.; Yousefi, M. A Review of Dietary Approaches for Ammonia Toxicity Remediation in Fish. Aquacult. Int. 2024, 32, 5639–5675. [Google Scholar] [CrossRef]
- Randall, D.J.; Tsui, T.K.N. Ammonia Toxicity in Fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á. Ecological and Toxicological Effects of Inorganic Nitrogen Pollution in Aquatic Ecosystems: A Global Assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.A.; Wood, C.M. Seven Things Fish Know about Ammonia and We Don’t. Respir. Physiol. Neurobiol. 2012, 184, 231–240. [Google Scholar] [CrossRef]
- Ip, A.Y.K.; Chew, S.F. Ammonia Production, Excretion, Toxicity, and Defense in Fish: A Review. Front. Physiol. 2010, 1, 2118. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.K.; Chew, S.F.; Randall, D.J. Ammonia Toxicity, Tolerance, and Excretion. Fish Physiol. 2001, 20, 109–148. [Google Scholar]
- Sun, H.; Wang, W.; Li, J.; Yang, Z. Growth, Oxidative Stress Responses, and Gene Transcription of Juvenile Bighead Carp (Hypophthalmichthys nobilis) under Chronic-Term Exposure of Ammonia. Environ. Toxicol. Chem. 2014, 33, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Xu, Z.; Zhang, H.; Mei, J.; Xie, J. The Effects of Acute Exposure to Ammonia on Oxidative Stress, Hematological Parameters, Flesh Quality, and Gill Morphological Changes of the Large Yellow Croaker (Larimichthys crocea). Animals 2023, 13, 2534. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, M.; Xia, Y.; Sun, W.; Xiong, G.; Shi, L.; Qiao, Y.; Wu, W.; Ding, A.; Chen, L.; et al. Deterioration of Muscle Quality Caused by Ammonia Exposure in Rainbow Trout (Oncorhynchus mykiss). Food Biosci. 2023, 53, 102609. [Google Scholar] [CrossRef]
- Hongxing, G.; Xiafei, L.; Jialing, L.; Zhenquan, C.; Luoyu, G.; Lei, L.; Yuxuan, S.; Zhiguo, D.; Min, W. Effects of Acute Ammonia Exposure on Antioxidant and Detoxification Metabolism in Clam Cyclina sinensis. Ecotoxicol. Environ. Saf. 2021, 211, 111895. [Google Scholar] [CrossRef] [PubMed]
- Loong, A.M.; Tan, J.Y.L.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Defense against Environmental Ammonia Toxicity in the African Lungfish, Protopterus aethiopicus: Bimodal Breathing, Skin Ammonia Permeability and Urea Synthesis. Aquat. Toxicol. 2007, 85, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, M.P. Ammonia Excretion and Urea Handling by Fish Gills: Present Understanding and Future Research Challenges. J. Exp. Zool. 2002, 293, 284–301. [Google Scholar] [CrossRef]
- Gupta, S. An Overview on Morphology, Biology, and Culture of Spotted Scat Scatophagus argus (Linnaeus 1766). Rev. Fish. Sci. Aquac. 2016, 24, 203–212. [Google Scholar] [CrossRef]
- Su, M.; Feng, Z.; Zhong, Y.; Ye, Z.; Zhang, J. Insights into Salinity Effect on Growth of the Spotted Scat (Scatophagus argus): Exploring the Optimum Salinity for Its Culture. Aquac. Rep. 2025, 41, 102667. [Google Scholar] [CrossRef]
- Su, M.; Liu, N.; Zhang, Z.; Zhang, J. Osmoregulatory Strategies of Estuarine Fish Scatophagus argus in Response to Environmental Salinity Changes. BMC Genom. 2022, 23, 545. [Google Scholar] [CrossRef]
- Lin, X.; Hu, S.; Liu, S.; Huang, H. Unexpected Prey of Juvenile Spotted Scat (Scatophagus Argus) near a Wharf: The Prevalence of Fouling Organisms in Stomach Contents. Ecol. Evol. 2018, 8, 8547–8554. [Google Scholar] [CrossRef]
- Shi, S.; Sun, X.; Zhang, C.; Lv, C.; Liu, Y.; Du, J.; Qi, Q. Transcriptome Analysis of the Effect of Acute Ammonia Stress on Pseudobagrus ussuriensis Liver Tissue. Fishes 2025, 10, 17. [Google Scholar] [CrossRef]
- Liu, M.-J.; Guo, H.-Y.; Zhu, K.-C.; Liu, B.-S.; Liu, B.; Guo, L.; Zhang, N.; Yang, J.-W.; Jiang, S.-G.; Zhang, D.-C. Effects of Acute Ammonia Exposure and Recovery on the Antioxidant Response and Expression of Genes in the Nrf2-Keap1 Signaling Pathway in the Juvenile Golden Pompano (Trachinotus ovatus). Aquat. Toxicol. 2021, 240, 105969. [Google Scholar] [CrossRef]
- Motamedi-Tehrani, J.; Peyghan, R.; Shahriari, A.; Razijalali, M.; Ebrahimi, E. The Influence of Ammonia-N and Salinity Levels on Oxidative Stress Markers, Hepatic Enzymes, and Acid Phosphatase Activity in Nile Tilapia (Oreochromis niloticus). Sci. Rep. 2025, 15, 559. [Google Scholar] [CrossRef] [PubMed]
- Egnew, N.; Renukdas, N.; Ramena, Y.; Yadav, A.K.; Kelly, A.M.; Lochmann, R.T.; Sinha, A.K. Physiological insights into largemouth bass (Micropterus salmoides) survival during long-term exposure to high environmental ammonia. Aquat. Toxicol. 2019, 207, 72–82. [Google Scholar] [CrossRef]
- Jin, J.; Amenyogbe, E.; Yang, Y.; Wang, Z.; Lu, Y.; Xie, R.; Droepenu, E.K.; Huang, J. Effects of Ammonia Nitrogen Stress on the Physiological, Biochemical, and Metabolic Levels of the Gill Tissue of Juvenile Four-Finger Threadfin (Eleutheronema tetradactylum). Aquat. Toxicol. 2024, 274, 107049. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, J.; Qin, X.; Qiu, W.; Mei, J.; Xie, J. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Tissue Structure in Fish Exposed to Ammonia Nitrogen: A Review. Animals 2021, 11, 3304. [Google Scholar] [CrossRef]
- Lin, W.; Luo, H.; Wu, J.; Hung, T.-C.; Cao, B.; Liu, X.; Yang, J.; Yang, P. A Review of the Emerging Risks of Acute Ammonia Nitrogen Toxicity to Aquatic Decapod Crustaceans. Water 2022, 15, 27. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Huang, J.; Yang, L.; Jiang, S.; Yang, Q.; He, J.; Jiang, S. Transcriptome Reveals Involvement of Immune Defense, Oxidative Imbalance, and Apoptosis in Ammonia-Stress Response of the Black Tiger Shrimp (Penaeus monodon). Fish Shellfish Immunol. 2018, 83, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L. Acute toxic effects of ammonia and nitrite on Epinephelus coioides fry. Mar. Sci. 2012, 36, 81–86, (In Chinese with English abstract). [Google Scholar]
- Liu, M.-J.; Guo, H.-Y.; Liu, B.; Zhu, K.-C.; Guo, L.; Liu, B.-S.; Zhang, N.; Yang, J.-W.; Jiang, S.-G.; Zhang, D.-C. Gill Oxidative Damage Caused by Acute Ammonia Stress Was Reduced through the HIF-1α/NF-κb Signaling Pathway in Golden Pompano (Trachinotus ovatus). Ecotoxicol. Environ. Saf. 2021, 222, 112504. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Liang, X.; Yang, S.; Ge, H.; Dong, Z. Molecular and Physiological Responses in the Ammonia Transport Pathways in Clam Cyclina sinensis Exposed to Chronic Ammonia Nitrogen. Aquac. Rep. 2024, 35, 101952. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, Z.; Liu, X.; Ma, Z. The Impact of Acute Ammonia Nitrogen Stress on the Gill Tissue Structure and Antioxidant Ability of Gills and Red and White Muscle in Juvenile Yellowfin Tuna (Thunnus albacares). Antioxidants 2024, 13, 1357. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, X.; Geng, Z.; Chen, C.; Zhang, Y.; Liu, S.; Song, J. Effects of Chronic Ammonia Nitrogen Toxicity on the Gill Tissues and Non-Specific Immune Indexes of Juvenile Carassius auratus. Freshw. Fish. 2024, 54, 59–68, (In Chinese with English abstract). [Google Scholar]
- Wang, Z.; Chen, S.; Cao, D.; Lu, B.; Chang, Q.; Liu, C.; Yan, J. Effects of Acute Ammonia Nitrogen Stress on Histopathology of Gill and Liver and Enzyme Activities of Juvenile Verasper variegatus. Prog. Fish. Sci. 2017, 38, 59–69, (In Chinese with English abstract). [Google Scholar]
- Zhang, W.; Xia, S.; Zhu, J.; Miao, L.; Ren, M.; Lin, Y.; Ge, X.; Sun, S. Growth Performance, Physiological Response and Histology Changes of Juvenile Blunt Snout Bream, Megalobrama amblycephala exposed to Chronic Ammonia. Aquaculture 2019, 506, 424–436. [Google Scholar] [CrossRef]
- Lin, S.; Nascimento, E.M.; Gajera, C.R.; Chen, L.; Neuhöfer, P.; Garbuzov, A.; Wang, S.; Artandi, S.E. Distributed Hepatocytes Expressing Telomerase Repopulate the Liver in Homeostasis and Injury. Nature 2018, 556, 244–248. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, L.; Fish, M.; Logan, C.Y.; Nusse, R. Self-Renewing Diploid Axin2(+) Cells Fuel Homeostatic Renewal of the Liver. Nature 2015, 524, 180–185. [Google Scholar] [CrossRef]
- Font-Burgada, J.; Shalapour, S.; Ramaswamy, S.; Hsueh, B.; Rossell, D.; Umemura, A.; Taniguchi, K.; Nakagawa, H.; Valasek, M.A.; Ye, L.; et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell 2015, 162, 766–779. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, E.; Li, C.; Pan, C.; Zhao, X.; Wang, Y.; Ling, Q. Effects of Heat Stress on Histopathology, Antioxidant Enzymes, and Transcriptomic Profiles in Gills of Pikeperch Sander lucioperca. Aquaculture 2021, 534, 736277. [Google Scholar] [CrossRef]
- Mangang, Y.A.; Pandey, P.K. Hemato-Biochemical Responses and Histopathological Alterations in the Gill and Kidney Tissues of Osteobrama belangeri (Valenciennes, 1844) Exposed to Different Sub-Lethal Unionized Ammonia. Aquaculture 2021, 542, 736887. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Liu, Q.; Sun, J.; He, K.; Adam, A.A.; Luo, J.; Li, Z.; Wang, Y.; Yang, S.; et al. Combined Exposure to Hypoxia and Ammonia Aggravated Biological Effects on Glucose Metabolism, Oxidative Stress, Inflammation and Apoptosis in Largemouth Bass (Micropterus salmoides). Aquat. Toxicol. 2020, 224, 105514. [Google Scholar] [CrossRef]
- Zou, J.; Hu, P.; Wang, M.; Chen, Z.; Wang, H.; Guo, X.; Gao, J.; Wang, Q. Liver Injury and Metabolic Dysregulation in Largemouth Bass (Micropterus salmoides) after Ammonia Exposure. Metabolites 2023, 13, 274. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose Metabolism in Fish: A Review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, C.; Wang, J.; Yang, L.; Yan, X.; Zhi, S.; Nie, G. Phosphofructokinase Family Genes in grass carp: Molecular Identification and Tissue-Specific Expression in Response to Glucose, Insulin and Glucagon. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2024, 269, 110898. [Google Scholar] [CrossRef] [PubMed]
- Wamelink, M.M.C.; Struys, E.A.; Jakobs, C. The Biochemistry, Metabolism and Inherited Defects of the Pentose Phosphate Pathway: A Review. J. Inherit. Metab. Dis. 2008, 31, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.; Krüger, A.; Tauqeer Alam, M.; et al. The Return of Metabolism: Biochemistry and Physiology of the Pentose Phosphate Pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- González-Ruiz, R.; Leyva-Carrillo, L.; Peregrino-Uriarte, A.B.; Yepiz-Plascencia, G. The Combination of Hypoxia and High Temperature Affects Heat Shock, Anaerobic Metabolism, and Pentose Phosphate Pathway Key Components Responses in the White Shrimp (Litopenaeus vannamei). Cell Stress Chaperones 2023, 28, 493–509. [Google Scholar] [CrossRef]
- Li, T.Y.; Sun, Y.; Liang, Y.; Liu, Q.; Shi, Y.; Zhang, C.-S.; Zhang, C.; Song, L.; Zhang, P.; Zhang, X.; et al. ULK1/2 Constitute a Bifurcate Node Controlling Glucose Metabolic Fluxes in Addition to Autophagy. Mol. Cell 2016, 62, 359–370. [Google Scholar] [CrossRef]
- Ni, Q.; Li, W.; Liang, X.; Liu, J.; Ge, H.; Dong, Z. Gill Transcriptome Analysis Reveals the Molecular Response to the Acute Low-Salinity Stress in Cyclina sinensis. Aquac. Rep. 2021, 19, 100564. [Google Scholar] [CrossRef]
- Shafqat, N.; Muniz, J.R.C.; Pilka, E.S.; Papagrigoriou, E.; von Delft, F.; Oppermann, U.; Yue, W.W. Insight into S-adenosylmethionine biosynthesis from the crystal structures of the human methionine adenosyltransferase catalytic and regulatory subunits. Biochem. J. 2013, 452, 27–36. [Google Scholar] [CrossRef]
- Cuaz-Pérolin, C.; Furman, C.; Larigauderie, G.; Legedz, L.; Lasselin, C.; Copin, C.; Jaye, M.; Searfoss, G.; Yu, K.T.; Duverger, N.; et al. REDD2 Gene Is Upregulated by Modified LDL or Hypoxia and Mediates Human Macrophage Cell Death. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1830–1835. [Google Scholar] [CrossRef]
- Simonson, B.; Subramanya, V.; Chan, M.C.; Zhang, A.; Franchino, H.; Ottaviano, F.; Mishra, M.K.; Knight, A.C.; Hunt, D.; Ghiran, I.; et al. DDiT4L Promotes Autophagy and Inhibits Pathological Cardiac Hypertrophy in Response to Stress. Sci. Signal. 2017, 10, eaaf5967. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, Z.-H.; Wu, J.; Han, J. Ribosome-Rescuer PELO Catalyzes the Oligomeric Assembly of NOD-like Receptor Family Proteins via Activating Their ATPase Enzymatic Activity. Immunity 2023, 56, 926–943.e7. [Google Scholar] [CrossRef]
- Schnell, S.; Démollière, C.; Van Den Berk, P.; Jacobs, H. Gimap4 Accelerates T-Cell Death. Blood 2006, 108, 591–599. [Google Scholar] [CrossRef] [PubMed]
- La Marca, J.E.; Aubrey, B.J.; Yang, B.; Chang, C.; Wang, Z.; Kueh, A.; Tai, L.; Wilcox, S.; Milla, L.; Heinzel, S.; et al. Genome-Wide CRISPR Screening Identifies a Role for ARRDC3 in TRP53-Mediated Responses. Cell Death Differ. 2024, 31, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, L.; Sun, Y.; Liu, T.; Hou, F.; Liu, X. Comparison of Immune Response in Pacific White Shrimp, Litopenaeus Vannamei, after Knock down of Toll and IMD Gene in Vivo. Dev. Comp. Immunol. 2016, 60, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Qi, J.; Echtenkamp, S.F.; Chatterjee, R.; Wang, M.; Boons, G.-J.; Dziarski, R.; Gupta, D. Zebrafish Peptidoglycan Recognition Proteins Are Bactericidal Amidases Essential for Defense against Bacterial Infections. Immunity 2007, 27, 518–529. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′-3′) | |
|---|---|---|
| Forward | Reverse | |
| DDIT4 | TGTACCAAACTTCTCATCCCGG | GCTGAAAGGTGGGTACAAGGTA |
| PFKM | GTCGTATCTTTGCTAACACACCG | TTCATGATGGGCCTGATCTTCAA |
| ARRDC3 | GGTCCTATTTCCCTAAGTGCCAA | TGACCTCCTTCATCTTCCCTTTG |
| SNRK | CGACACACAACAAGCCCAAG | GCTCTTGTGCCTCGAAGTCT |
| CTSL1 | CCCAAATACAACTCTGCCAACG | CTTGCCATCTACATCCTCTCCC |
| NAMLAA | TTCCTTGGTGACGCTGACTC | GTGGAGCTGTGTCTGAACGA |
| APOL | CCGGATGGTGTTCTCATTCCA | CACTGGAGATCTTTGCCCCTT |
| TRIM71 | ATCCAAACACAGCACACACATG | CCAGTAACAACGTCCAGTCAGA |
| MAT2A | GACCGAACAGTTAGACCAGCA | TCTTCTTCCATGTCAGCCCAC |
| MARS1 | TCCCATCATCAGCTCCTCCA | TGATGCAGCGAGGTTTGAGT |
| β-actin | TCATGAAGATCCTGACAGAGCG | TGATGCTGTTGTAGGTGGTCTC |
| LC50-96 h (mg/L) | SC (mg/L) | 95% Confidence Interval | |
|---|---|---|---|
| TAN | 59.43 | 5.94 | 58.27–60.61 |
| NH3 | 3.93 | 0.39 |
| Samples | Clean Reads | GC Content | % ≥ Q30 | Genome Mapping Ratio |
|---|---|---|---|---|
| An0-1 | 21,332,348 | 45.77% | 93.48% | 84.76% |
| An0-2 | 19,319,538 | 47.37% | 93.73% | 87.94% |
| An0-3 | 19,690,111 | 47.66% | 93.83% | 86.89% |
| An60-1 | 21,218,424 | 47.07% | 94.40% | 85.96% |
| An60-2 | 21,061,737 | 47.54% | 93.73% | 86.46% |
| An60-3 | 22,226,345 | 46.05% | 93.75% | 86.83% |
| Gene ID | Gene Name | KEGG Pathway | Log2FC | FDR |
|---|---|---|---|---|
| EVM0015383 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Glycolysis/gluconeogenesis (ko00010) | 1.64 | 0.04015 |
| EVM0016958 | 6-phosphofructokinase, muscle type (PFKM) | Glycolysis/gluconeogenesis (ko00010) | 2.00 | 0.01206 |
| EVM0022814 | Phosphoglucomutase-1 (PGM1) | Pentose phosphate pathway (ko00030) | 1.04 | 0.04084 |
| EVM0013965 | DNA damage-inducible transcript 4 (DDIT4) | mTOR signaling pathway (ko04150) | 2.03 | 0.01011 |
| EVM0012705 | Succinate dehydrogenase iron–sulfur subunit (SDHB) | Citrate cycle (TCA cycle) (ko00020) | 1.29 | 0.00077 |
| EVM0021624 | Betaine-homocysteine S-methyltransferase 1 (BHMT1) | Glycine, serine, and threonine metabolism (ko00260) | 1.30 | 0.01799 |
| EVM0007678 | S-adenosylmethionine synthase isoform type-2 (MAT2A) | Cysteine and methionine metabolism (ko00270) | −1.98 | 0.00012 |
| EVM0022587 | Malate dehydrogenase 1 (MDH1) | Citrate cycle (TCA cycle) (ko00020) | −1.28 | 0.01748 |
| Gene ID | Gene Name | KEGG Pathway | Log2FC | FDR |
|---|---|---|---|---|
| EVM0005729 | Arrestin domain-containing protein 3 (ARRDC3) | NOD-like receptor signaling pathway (ko04621) | 1.34 | 0.00825 |
| EVM0018062 | GTPase IMAP family member 4 (GIMAP4) | NOD-like receptor signaling pathway (ko04621) | 2.12 | 0.01906 |
| EVM0023124 | Cathepsin L1 (CTSL1) | Lysosome (ko04142) | 1.10 | 4.07 × 10−5 |
| EVM0007718 | Tumor necrosis factor receptor superfamily member 21 (TNFRSF21) | Cytokine–cytokine receptor interaction (ko04060) | 1.39 | 0.01206 |
| EVM0002453 | N-acetylmuramoyl-L-alanine amidase (NAMLAA) | Toll and Imd signaling pathway (ko04624) | −1.77 | 0.00713 |
| EVM0023302 | Stimulator of interferon genes protein (STING) | NOD-like receptor signaling pathway (ko04621) | −1.81 | 0.03222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Zhang, Z.; Zhu, H.; Xu, Q.; Li, S.; Chen, J. Histological and Transcriptomic Profiling Reveals Metabolic and Immune Responses to Ammonia Stress in Scatophagus argus. Fishes 2025, 10, 412. https://doi.org/10.3390/fishes10080412
Xu H, Zhang Z, Zhu H, Xu Q, Li S, Chen J. Histological and Transcriptomic Profiling Reveals Metabolic and Immune Responses to Ammonia Stress in Scatophagus argus. Fishes. 2025; 10(8):412. https://doi.org/10.3390/fishes10080412
Chicago/Turabian StyleXu, Haixin, Zitao Zhang, Honggeng Zhu, Qisheng Xu, Shihu Li, and Jianhua Chen. 2025. "Histological and Transcriptomic Profiling Reveals Metabolic and Immune Responses to Ammonia Stress in Scatophagus argus" Fishes 10, no. 8: 412. https://doi.org/10.3390/fishes10080412
APA StyleXu, H., Zhang, Z., Zhu, H., Xu, Q., Li, S., & Chen, J. (2025). Histological and Transcriptomic Profiling Reveals Metabolic and Immune Responses to Ammonia Stress in Scatophagus argus. Fishes, 10(8), 412. https://doi.org/10.3390/fishes10080412





