Genetic Structuring and Connectivity of European Squid Populations in the Mediterranean Sea Based on Mitochondrial COI Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Molecular Procedures
2.3. Statistical Analysis
3. Results
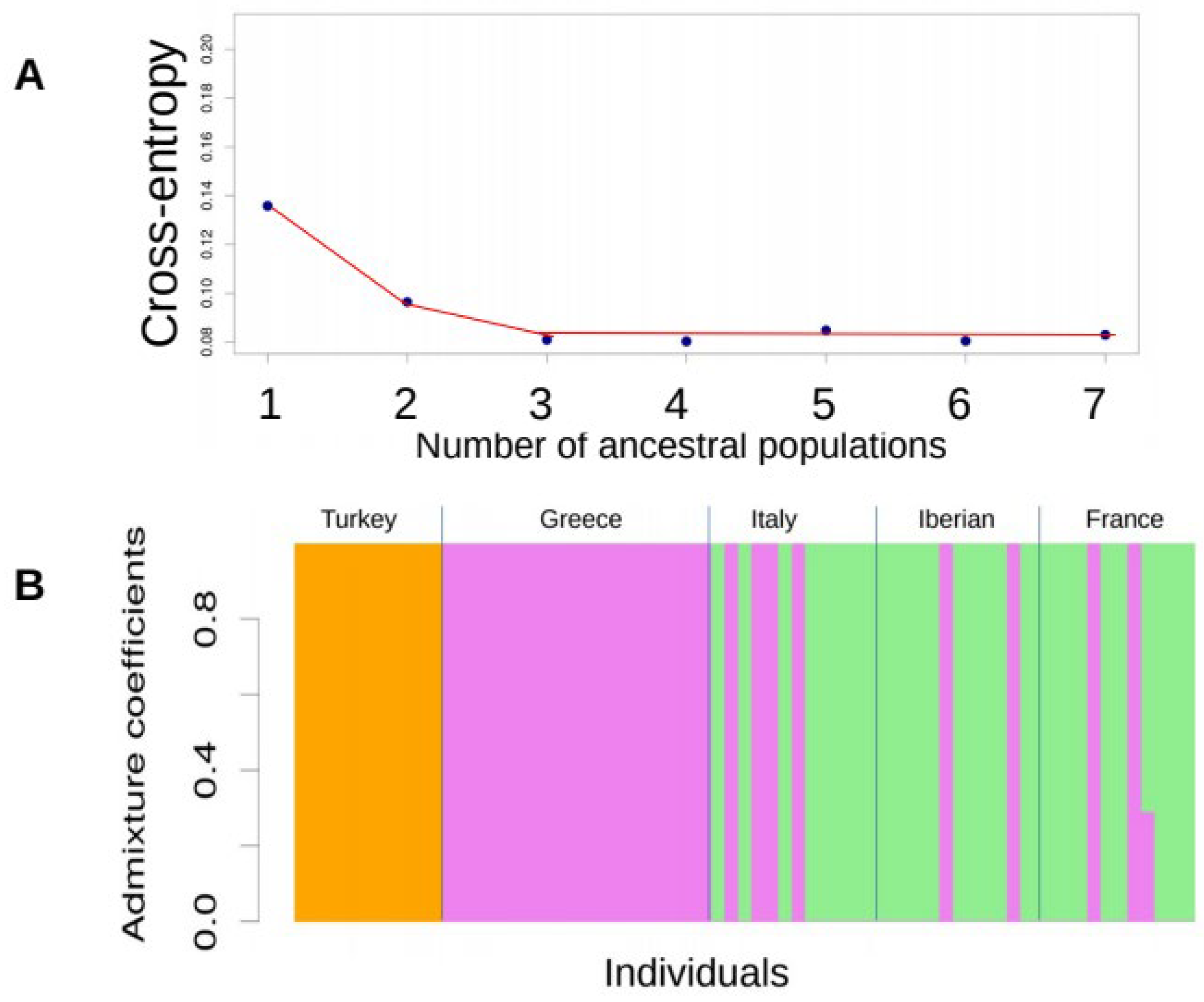
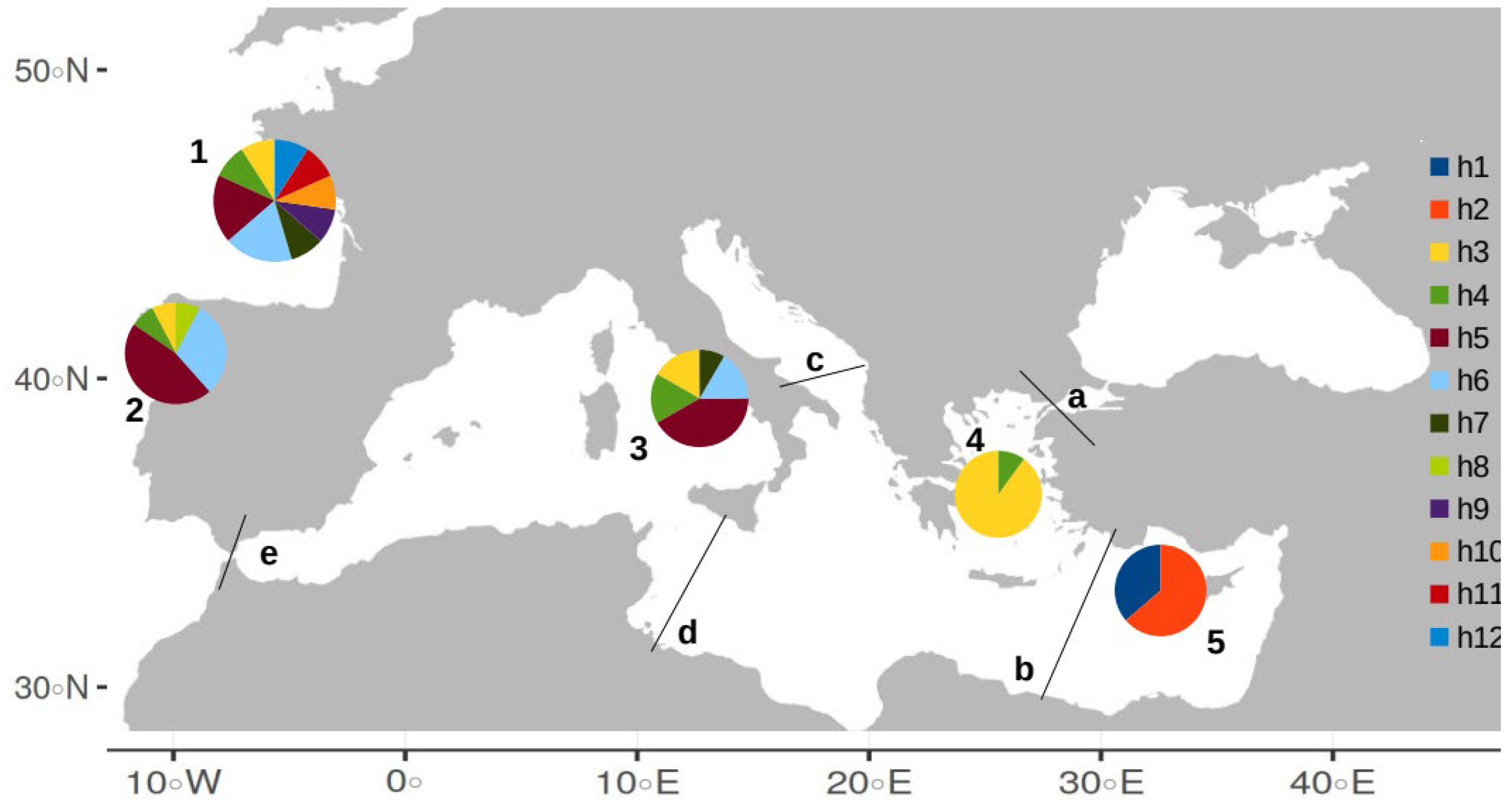
3.1. Haplotype Diversity
3.2. Nucleotide Diversity Analysis
3.3. Genetic Differentiation (ΦST and FST Values)
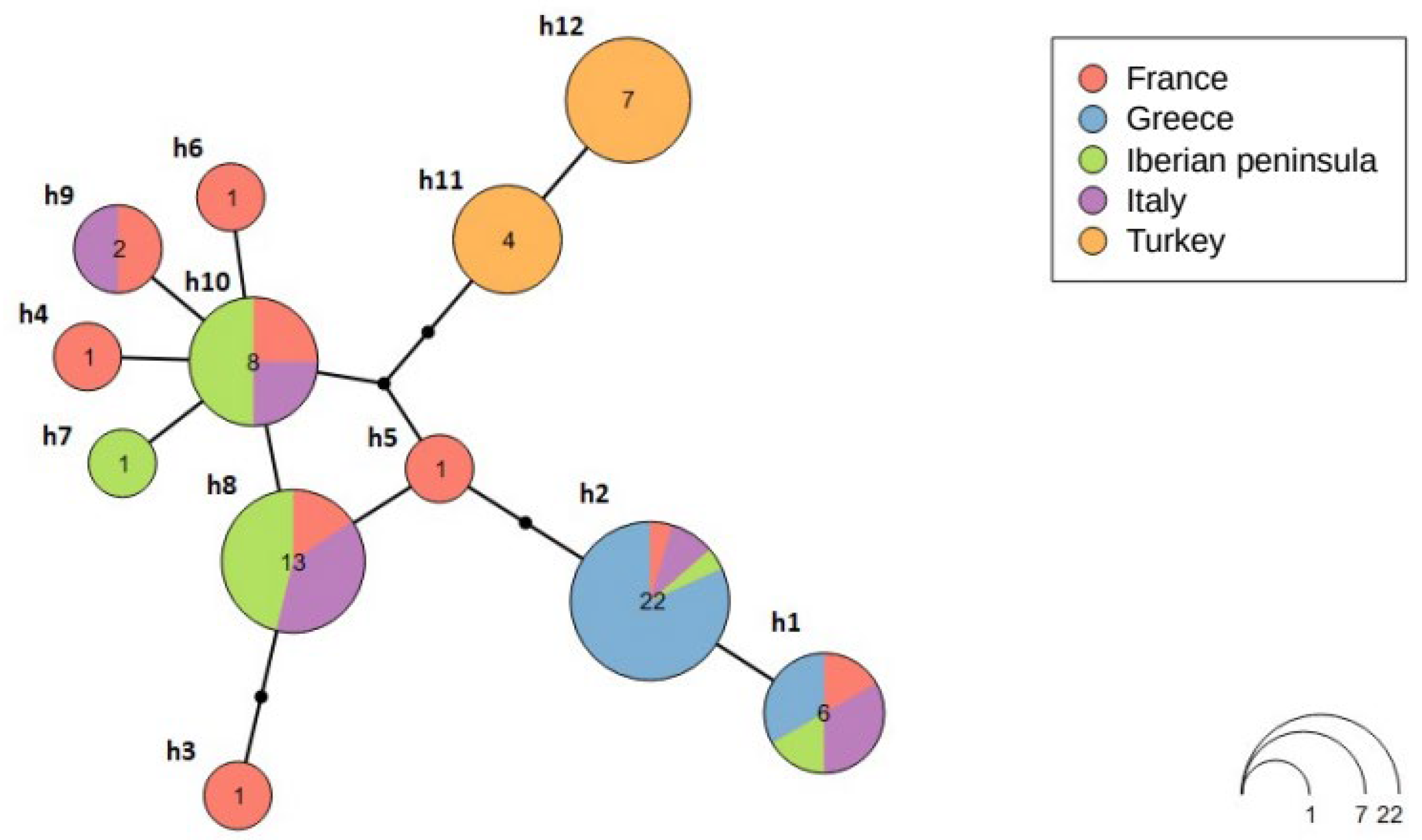
3.4. Median-Joining Network
3.5. Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roper, C.F.E.; Jereb, P. Cephalopods of the World. An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date. In Volume 2. Myopsid and Oegopsid Squids; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Vafidis, D.; Koutsoubas, D.; Chartosia, N.; Koukouras, A. The Teuthoidea (Cephalopoda, Mollusca) Fauna of the Aegean Sea: Comparison with the Neighbouring Seas and Notes on Their Diet Composition. J. Biol. Res. 2008, 10, 191–205. [Google Scholar]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine Heatwaves Drive Recurrent Mass Mortalities in the Mediterranean Sea. Diversity 2022, 14, 123. [Google Scholar] [CrossRef]
- Šifner, S.K.; Vrgoč, N.; Krstulović-Šifner, S. Climate-Driven Shifts in Squid Spawning Phenology: Evidence from the Adriatic Sea. Water 2023, 15, 456. [Google Scholar] [CrossRef]
- Laptikhovsky, V.; Allcock, L.A.; Barnwall, L.; Barrett, C.; Cooke, G.; Drerup, C.; Firmin, C. Spatial and temporal variability of spawning and nursery grounds of Loligo forbesii and Loligo vulgaris squids in ecoregions of Celtic Seas and Greater North Sea. ICES J. Mar. Sci. 2022, 79, 1918–1930. [Google Scholar] [CrossRef]
- Rosa, R.; Pissarra, V.; Borges, F.O.; Xavier, J.; Gleadall, I.G.; Golikov, A.; Bello, G.; Morais, L.; Lishchenko, F.; Roura, Á.; et al. Global Patterns of Species Richness in Coastal Cephalopods. Front. Mar. Sci. 2019, 6, 469. [Google Scholar] [CrossRef]
- Kaplan, M.B.; Mooney, T.A.; McCorkle, D.C.; Cohen, A.L. Adverse Effects of Ocean Acidification on Early Development of Squid (Doryteuthis pealeii). PLoS ONE 2013, 8, e63714. [Google Scholar] [CrossRef] [PubMed]
- Spady, B.L.; Watson, S.A.; Chase, T.J.; Munday, P.L. Projected Ocean Acidification and Seasonal Temperature Alter the Behaviour and Growth of a Tropical Squid. ICES J. Mar. Sci. 2022, 79, 1553–1563. [Google Scholar] [CrossRef]
- Belkin, I.M. Rapid Warming of Large Marine Ecosystems. Prog. Oceanogr. 2009, 81, 207–213. [Google Scholar] [CrossRef]
- Sacchi, J.; Fabi, G.; Sartor, P. Impact of Bottom Trawling on Loligo vulgaris Stocks: A Case Study from the NW Mediterranean. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Sbragaglia, V.; Azzurro, E.; Mariani, S.; Fanelli, E.; Del Rio Fernandez, J.; Aguzzi, J. Automated Image Analysis for the Detection of Benthic Crustaceans and Bacterial Mat Coverage Using the Deep Learning Tool DeepLabCut. Sci. Rep. 2021, 11, 14129. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Watson, R.; Pauly, D. Signature of Ocean Warming in Global Fisheries Catch. Nature 2013, 497, 365–368. [Google Scholar] [CrossRef]
- Lotze, H.K.; Tittensor, D.P.; Bryndum-Buchholz, A.; Eddy, T.D.; Cheung, W.W.L.; Galbraith, E.D.; Barange, M.; Barrier, N.; Bianchi, D.; Blanchard, J.L.; et al. Global Ensemble Projections Reveal Trophic Amplification of Ocean Biomass Declines with Climate Change. Proc. Natl. Acad. Sci. USA 2019, 116, 12907–12912. [Google Scholar] [CrossRef]
- Storelli, M.M.; Barone, G.; Storelli, A.; Marcotrigiano, G.O. Trace Metals in Loligo vulgaris from the Ionian Sea (Italy). J. Food Prot. 2006, 69, 682–686. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in Mussels and Fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Vasilakopoulos, P.; Maravelias, C.D.; Tserpes, G. The Alarming Decline of Mediterranean Fish Stocks. Fishes 2017, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Gaines, S.D.; Costello, C.; Owashi, B.; Mangin, T.; Bone, J.; Molinos, J.G.; Burden, M.; Dennis, H.; Halpern, B.S.; Kappel, C.V.; et al. Improved Fisheries Management Could Offset Many Negative Effects of Climate Change. Sci Adv. 2018, 4, eaao1378. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Boavida-Portugal, J.; Pimentel, M.; Pereira, J.; Rosa, R. Loligo Vulgaris, European Squid. In Advances in Squid Biology, Ecology and Fisheries. Part I: Myopsid Squids; Nova Science Publishers: New York, NY, USA, 2013; pp. 3–32. [Google Scholar]
- FAO. Fishery and Aquaculture Statistics—Yearbook 2020; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Cheng, S.H.; Gold, M.; Rodriguez, N.; Barber, P.H. Genome-Wide SNPs Reveal Complex Fine Scale Population Structure in the California Market Squid Fishery (Doryteuthis opalescens). Conserv. Genet. 2021, 22, 97–110. [Google Scholar] [CrossRef]
- García-Mayoral, E.; Silva, C.N.S.; Ramilo, A.; Roura, Á.; Moreno, A.; Strugnell, J.M.; González, Á.F. Population Connectivity of the European Squid Loligo Vulgaris along the West Iberian Peninsula Coast: Comparing MtDNA and SNPs. Mar. Biol. 2024, 171, 237. [Google Scholar] [CrossRef]
- Hauser, L.; Carvalho, G.R. Paradigm Shifts in Marine Fisheries Genetics: Ugly Hypotheses Slain by Beautiful Facts. Fish Fish. 2008, 9, 333–362. [Google Scholar] [CrossRef]
- Rothschild, B.J.; Beamish, R.J. On the Future of Fisheries Science. In The Future of Fisheries Science in North America; Springer: Dordrecht, The Netherlands, 2009; pp. 1–11. [Google Scholar] [CrossRef]
- Tintore, J.; La Violette, P.E.; Blade, I.; Cruzado, A. A Study of an Intense Density Front in the Eastern Alboran Sea: The Almeria–Oran Front. J. Phys. Oceanogr. 1988, 18, 1384–1397. [Google Scholar] [CrossRef]
- Guarnieo, I.; Franzellitti, S.; Ungaro, N.; Tommasini, S.; Piccinetti, C.; Tinti, F. Control Region Haplotype Variation in the Central Mediterranean Common Sole Indicates Geographical Isolation and Population Structuring in Italian Stocks. J. Fish. Biol. 2002, 60, 1459–1474. [Google Scholar] [CrossRef]
- Natoli, A.; Birkun, A.; Aguilar, A.; Lopez, A.; Hoelzel, A.R. Habitat Structure and the Dispersal of Male and Female Bottlenose Dolphins (Tursiops Truncatus). Proc. R. Soc. B Biol. Sci. 2005, 272, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Lascaratos, A.; Salusti, E.; Papageorgaki, G. Wind-Induced Upwellings and Currents in the Gulfs of Patras, Nafpaktos and Korinthos, Western Greece. Oceanol. Acta 1989, 12, 159–164. [Google Scholar]
- Exadactylos, A.; Geffen, A.J.; Thorpe, J.P. Population Structure of the Dover Sole, Solea solea L., in a Background of High Gene Flow. J. Sea Res. 1998, 40, 117–129. [Google Scholar] [CrossRef]
- Bahri-Sfar, L.; Lemaire, C.; Ben Hassine, O.K.; Bonhomme, F. Fragmentation of Sea Bass Populations in the Western and Eastern Mediterranean as Revealed by Microsatellite Polymorphism. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 929–935. [Google Scholar] [CrossRef]
- Quesada, H.; Zapata, C.; Alvarez, G. A Multilocus Allozyme Discontinuity in the Mussel Mytilus Galloprovincialis: The Interaction of Ecological and Life-History Factors. Mar. Ecol. Prog. Ser. 1995, 116, 99–115. [Google Scholar] [CrossRef]
- Fontaine, M.C.; Roland, K.; Calves, I.; Austerlitz, F.; Palstra, F.P.; Tolley, K.A.; Ryan, S.; Ferreira, M.; Jauniaux, T.; Llavona, A.; et al. Postglacial Climate Changes and Rise of Three Ecotypes of Harbour Porpoises, Phocoena phocoena, in Western Alearctic Waters. Mol. Ecol. 2014, 23, 3306–3321. [Google Scholar] [CrossRef]
- Gkafas, G.A.; Exadactylos, A.; Rogan, E.; Raga, J.A.; Reid, R.; Hoelzel, A.R. Biogeography and Temporal Progression during the Evolution of Striped Dolphin Population Structure in European Waters. J. Biogeogr. 2017, 44, 2681–2691. [Google Scholar] [CrossRef]
- Guarneros-Narváez, P.V.; Rodríguez-Canul, R.; De Silva-Dávila, R.; Zamora-Briseño, J.A.; Améndola-Pimenta, M.; Souza, A.J.; Ordoñez, U.; Velázquez-Abunader, I. Loliginid Paralarvae from the Southeastern Gulf of Mexico: Abundance, Distribution, and Genetic Structure. Front. Mar. Sci. 2022, 9, 941908. [Google Scholar] [CrossRef]
- Lambeck, K.; Purcell, A. Sea-Level Change in the Mediterranean Sea since the LGM: Model Predictions for Tectonically Stable Areas. Quat. Sci. Rev. 2005, 24, 1969–1988. [Google Scholar] [CrossRef]
- Hewitt, G.M. Genetic Consequences of Climatic Oscillations in the Quaternary. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.-X.; Wang, H.-Y.; Zhang, T.; Liu, J.-X. Population Genetic Structure and Demographic History of Atrina pectinata Based on Mitochondrial DNA and Microsatellite Markers. PLoS ONE 2014, 9, e95436. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, S.; Azzellino, A.; Airoldi, S.; Hoelzel, A.R. Social Kin Associations and Genetic Structuring of Striped Dolphin Populations (Stenella coeruleoalba) in the Mediterranean Sea. Mol. Ecol. 2007, 16, 2922–2933. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn, M.; Hoelzel, A.R.; Carvalho, G.R.; Hofreiter, M. Faunal Histories from Holocene Ancient DNA. Trends Ecol. Evol. 2011, 26, 405–413. [Google Scholar] [CrossRef]
- Garoia, F.; Guarniero, I.; Ramšak, A.; Ungaro, N.; Landi, M.; Piccinetti, C.; Mannini, P.; Tinti, F. Microsatellite DNA Variation Reveals High Gene Flow and Panmictic Populations in the Adriatic Shared Stocks of the European Squid and Cuttlefish (Cephalopoda). Heredity 2004, 93, 166–174. [Google Scholar] [CrossRef]
- García-Mayoral, E.; Roura, Á.; Ramilo, A.; González, Á.F. Spatial Distribution and Genetic Structure of Loliginid Paralarvae along the Galician Coast (NW Spain). Fish. Res. 2020, 222, 105406. [Google Scholar] [CrossRef]
- Olmos-Pérez, L.; Pierce, G.J.; Roura, Á.; González, Á.F. Barcoding and Morphometry to Identify and Assess Genetic Population Differentiation and Size Variability in Loliginid Squid Paralarvae from NE Atlantic (Spain). Mar. Biol. 2018, 165, 136. [Google Scholar] [CrossRef]
- Sauer, W.H.H.; Lipinski, M.R.; Augustyn, C.J. Tag Recapture Studies of the Chokka Squid Loligo Vulgaris Reynaudii D′Orbigny, 1845 on Inshore Spawning Grounds on the South-East Coast of South Africa. Fish. Res. 2000, 45, 283–289. [Google Scholar] [CrossRef]
- González, Á.F.; Otero, J.; Pierce, G.J.; Guerra, Á. Age, Growth, and Mortality of Loligo Vulgaris Wild Paralarvae: Implications for Understanding of the Life Cycle and Longevity. ICES J. Mar. Sci. 2010, 67, 1119–1127. [Google Scholar] [CrossRef]
- Moreno, A.; Pierce, G.J.; Azevedo, M.; Pereira, J.; Santos, A.M.P. The Effect of Temperature on Growth of Early Life Stages of the Common Squid Loligo Vulgaris. J. Mar. Biol. Assoc. UK 2012, 92, 1619–1628. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Sun, C.; Dumont, H.; Han, B.-P. A New Set of Highly Efficient Primers for COI Amplification in Rotifers. Mitochondrial DNA Part. B 2021, 6, 636–640. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Ratnasingham, S.; de Waard, J.R. Barcoding Animal Life: Cytochrome c Oxidase Subunit 1 Divergences among Closely Related Species. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Villesen, P. FaBox: An Online Toolbox for FASTA Sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin Suite Ver 3.5: A New Series of Programs to Perform Population Genetics Analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Vences, M.; Patmanidis, S.; Schmidt, J.-C.; Matschiner, M.; Miralles, A.; Renner, S.S. Hapsolutely: A User-Friendly Tool Integrating Haplotype Phasing, Network Construction, and Haploweb Calculation. Bioinform. Adv. 2024, 4, vbae083. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Rohl, A. Median-Joining Networks for Inferring Intraspecific Phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Melis, R.; Vacca, L.; Cuccu, D.; Mereu, M.; Cau, A.; Follesa, M.C.; Cannas, R. Genetic Population Structure and Phylogeny of the Common Octopus Octopus Vulgaris Cuvier, 1797 in the Western Mediterranean Sea through Nuclear and Mitochondrial Markers. Hydrobiologia 2018, 807, 277–296. [Google Scholar] [CrossRef]
- Bessa-Silva, A. Fasta2Structure: A user-friendly tool for converting multiple aligned FASTA files to STRUCTURE format. BMC Bioinform. 2024, 25, 73. [Google Scholar] [CrossRef]
- Frichot, E.; François, O. LEA: An R package for landscape and ecological association studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Frichot, E.; Mathieu, F.; Trouillon, T.; Bouchard, G.; François, O. Fast and efficient estimation of individual ancestry coefficients. Genetics 2014, 196, 973–983. [Google Scholar] [CrossRef]
- Alexander, D.H.; Lange, K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinform. 2011, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.W.; Pierce, G.J.; Boyle, P.R. Subtle Population Structuring within a Highly Vagile Marine Invertebrate, the Veined Squid Loligo forbesi, Demonstrated with Microsatellite DNA Markers. Mol. Ecol. 1999, 8, 407–417. [Google Scholar] [CrossRef]
- Theocharis, A.; Georgopoulos, D.; Lascaratos, A.; Nittis, K. Water Masses and Circulation in the Central Region of the Eastern Mediterranean: Eastern Ionian, South Aegean and Northwest Levantine, 1986–1987. Stud. Oceanogr. 1993, 40, 1121–1142. [Google Scholar] [CrossRef]
- Nikula, R.; Väinölä, R. Phylogeography of Cerastoderma Glaucum (Bivalvia: Cardiidae) across Europe: A Major Break in the Eastern Mediterranean. Mar. Biol. 2003, 143, 339–350. [Google Scholar] [CrossRef]
- Natoli, A.; Cañadas, A.; Peddemors, V.M.; Aguilar, A.; Vaquero, C.; Fernández-Piqueras, P.; Hoelzel, A.R. Phylogeography and Alpha Taxonomy of the Common Dolphin (Delphinus sp.). J. Evol. Biol. 2006, 19, 943–954. [Google Scholar] [CrossRef]
- Viñas, J.; Alvarado Bremer, J.; Pla, C. Phylogeography of the Atlantic Bonito (Sarda Sarda) in the Northern Mediterranean: The Combined Effects of Historical Vicariance, Population Expansion, Secondary Invasion, and Isolation by Distance. Mol. Phylogenet. Evol. 2004, 33, 32–42. [Google Scholar] [CrossRef]
- Voultsiadou, E.; Dailianis, T.; Antoniadou, C.; Vafidis, D.; Dounas, C.; Chintiroglou, C.C. Aegean Bath Sponges: Historical Data and Current Status. Rev. Fish. Sci. Aquac. 2011, 19, 34–51. [Google Scholar] [CrossRef]
- Patarnello, T.; Volckaert, F.A.M.J.; Castolho, R. Pillars of Hercules: Is the Atlantic–Mediterranean Transition a Phylogeographical Break? Mol. Ecol. 2007, 16, 4426–4444. [Google Scholar] [CrossRef]
- Pascual, M.; Rives, B.; Schunter, C.; Macpherson, E. Impact of Life History Traits on Gene Flow: A Multispecies Systematic Review across Oceanographic Barriers in the Mediterranean Sea. PLoS ONE 2017, 12, e0176419. [Google Scholar] [CrossRef] [PubMed]
- Avtzis, D.N.; Markoudi, V.; Mizerakis, V.; Devalez, J.; Nakas, G.; Poulakakis, N.; Petanidou, T. The Aegean Archipelago as Cradle: Divergence of the Glaphyrid Genus Pygopleurus and Phylogeography of P. foina. Syst. Biodivers. 2021, 19, 346–358. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Poulos, S.E. Water Masses of the Mediterranean Sea and Black Sea: An Overview. Water 2023, 15, 3194. [Google Scholar] [CrossRef]
- Sayın, E.; Eronat, C.; Uçkaç, Ş.; Beşiktepe, Ş.T. Hydrography of the Eastern Part of the Aegean Sea during the Eastern Mediterranean Transient (EMT). J. Mar. Syst. 2011, 88, 502–515. [Google Scholar] [CrossRef]
- Zodiatis, G.; Brenner, S.; Gertman, I.; Ozer, T.; Simoncelli, S.; Ioannou, M.; Savva, S. Twenty Years of In-Situ Monitoring in the South-Eastern Mediterranean Levantine Basin: Basic Elements of the Thermohaline Structure and of the Mesoscale Circulation during 1995–2015. Front. Mar. Sci. 2023, 9, 1074504. [Google Scholar] [CrossRef]
- Rossi, V.; Ser-Giacomi, E.; López, C.; Hernández-García, E. Hydrodynamic Provinces and Oceanic Connectivity from a Transport Network Help Designing Marine Reserves. Geophys. Res. Lett. 2014, 41, 2883–2891. [Google Scholar] [CrossRef]
- Riginos, C.; Douglas, K.E.; Jin, Y.; Shanahan, D.F.; Treml, E.A. Effects of Geography and Life History Traits on Genetic Differentiation in Benthic Marine Fishes. Ecography. 2011, 34, 566–575. [Google Scholar] [CrossRef]
- Sabatés, A.; Masó, M. Effect of a Shelf-Slope Front on the Spatial Distribution of Mesopelagic Fish Larvae in the Western Mediterranean. Deep Sea Res. Part A Oceanogr. Res. Pap. 1990, 37, 1085–1098. [Google Scholar] [CrossRef]
- Sabatés, A.; Salat, J.; Olivar, M.P. Advection of Continental Water as an Export Mechanism for Anchovy, Engraulis encrasicolus, Larvae. Sci. Mar. 2001, 65, 77–88. [Google Scholar] [CrossRef]
- Sabatés, A.; Olivar, M.P.; Salat, J.; Palomera, I.; Alemany, F. Physical and Biological Processes Controlling the Distribution of Fish Larvae in the NW Mediterranean. Prog. Oceanogr. 2007, 74, 355–376. [Google Scholar] [CrossRef]
- Siokou-Frangou, I.; Christaki, U.; Mazzocchi, M.G.; Montresor, M.; Ribera d’Alcalá, M.; Vaqué, D.; Zingone, A. Plankton in the Open Mediterranean Sea: A Review. Biogeosciences 2010, 7, 1543–1586. [Google Scholar] [CrossRef]
- Thunell, R.C.; Williams, D.F.; Howell, M. Atlantic-Mediterranean Water Exchange during the Late Neocene. Paleoceanography 1987, 2, 661–678. [Google Scholar] [CrossRef]
- Göpel, A.; Oesterwind, D.; Barrett, C.; Cannas, R.; Caparro, L.S.; Carbonara, P.; Donnaloia, M.; Follesa, M.C.; Larivain, A.; Laptikhovsky, V.; et al. Phylogeography of the Veined Squid, Loligo forbesii, in European Waters. Sci. Rep. 2022, 12, 7817. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Nolte, M.J.; Crandall, K.A.; Shaw, P.W. Testing Hypotheses of Population Structuring in the Northeast Atlantic Ocean and Mediterranean Sea Using the Common Cuttlefish Sepia officinalis. Mol. Ecol. 2007, 16, 2667–2679. [Google Scholar] [CrossRef]
- Pierce, G.J.; Allcock, L.; Bruno, I.; Bustamante, P.; González, Á.; Guerra, Á.; Jereb, P.; Lefkaditou, E.; Malham, S.; Moreno, A.; et al. (Eds.) Cephalopod biology and fisheries in Europe. In ICES Cooperative Research Report No. 303; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2010; 181p. [Google Scholar] [CrossRef]
- Lefkaditou, E.; Peristeraki, P.; Bekas, P.; Tserpes, G.; Politou, C.; Petrakis, G. Cephalopods distribution in the southern Aegean Sea. Mediterr. Mar. Sci. 2003, 4, 79–86. [Google Scholar] [CrossRef]
| Population | Sample Size | Polymorphic Sites (S) | Nucleotide Diversity |
|---|---|---|---|
| France | 11 | 9 | 0.0126 +/− 0.008 |
| Greece | 20 | 1 | 0.0009 +/− 0.001 |
| Italy | 12 | 6 | 0.0110 +/− 0.007 |
| Iberian | 13 | 0.0078 +/− 0.006 | |
| Turkey | 11 | 1 | 0.0024 +/− 0.002 |
| Italy | France | Iberian Peninsula | Turkey | Greece | |
|---|---|---|---|---|---|
| 1.305 * | 1.914 * | 2.233 * | 5.387 * | - | Greece |
| 3.548 * | 3.218 * | 3.472 * | - | 0.947 * | Turkey |
| −0.026 | −0.116 | - | 0.752 * | 0.748 * | Iberian Peninsula |
| −0.103 | - | −0.054 | 0.668 * | 0.652 * | France |
| - | −0.042 | −0.012 | 0.707 * | 0.579 * | Italy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertesi, V.; Sarantopoulou, J.; Exadactylos, A.; Vafidis, D.; Gkafas, G.A. Genetic Structuring and Connectivity of European Squid Populations in the Mediterranean Sea Based on Mitochondrial COI Data. Fishes 2025, 10, 394. https://doi.org/10.3390/fishes10080394
Pertesi V, Sarantopoulou J, Exadactylos A, Vafidis D, Gkafas GA. Genetic Structuring and Connectivity of European Squid Populations in the Mediterranean Sea Based on Mitochondrial COI Data. Fishes. 2025; 10(8):394. https://doi.org/10.3390/fishes10080394
Chicago/Turabian StylePertesi, Vasiliki, Joanne Sarantopoulou, Athanasios Exadactylos, Dimitrios Vafidis, and Georgios A. Gkafas. 2025. "Genetic Structuring and Connectivity of European Squid Populations in the Mediterranean Sea Based on Mitochondrial COI Data" Fishes 10, no. 8: 394. https://doi.org/10.3390/fishes10080394
APA StylePertesi, V., Sarantopoulou, J., Exadactylos, A., Vafidis, D., & Gkafas, G. A. (2025). Genetic Structuring and Connectivity of European Squid Populations in the Mediterranean Sea Based on Mitochondrial COI Data. Fishes, 10(8), 394. https://doi.org/10.3390/fishes10080394









