Spatio-Temporal Habitat Dynamics of Migratory Small Yellow Croaker (Larimichthys polyactis) in Hangzhou Bay, China
Abstract
1. Introduction
2. Materials and Methods
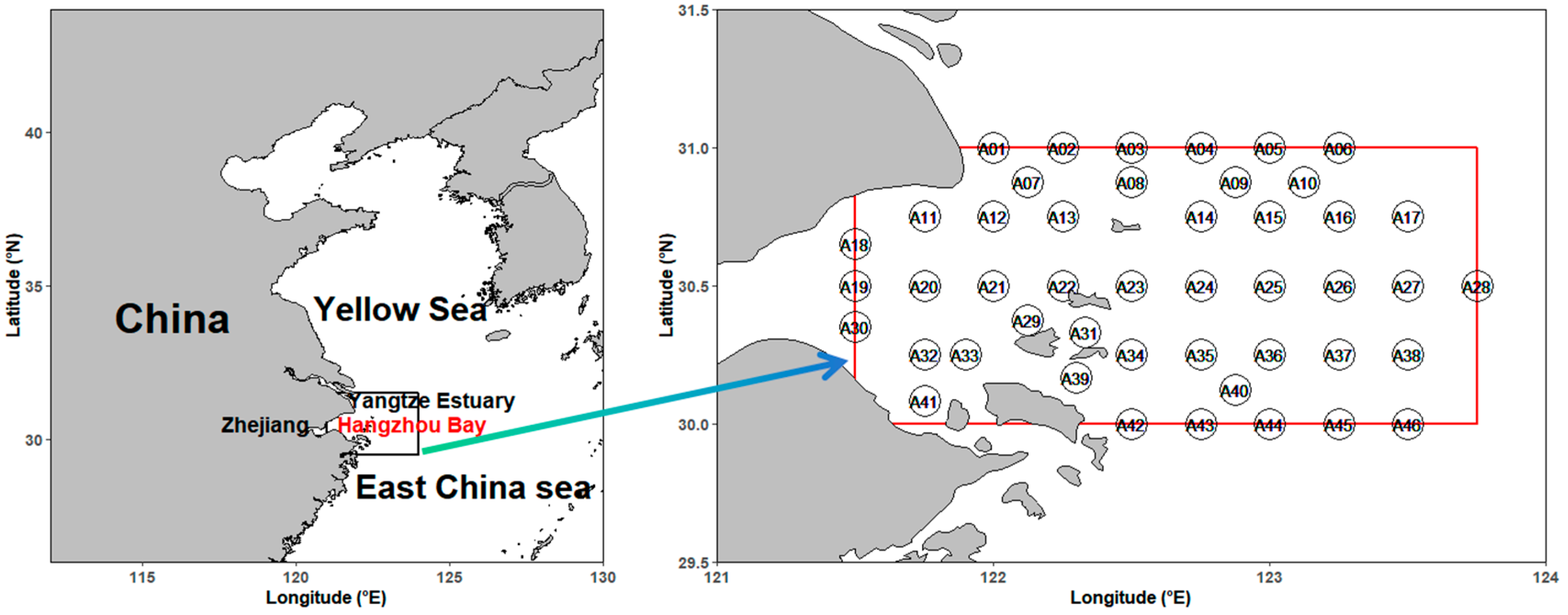
2.1. Study Area and Sampling
2.2. Environmental Variables Selection
2.3. Data Analyses
3. Results
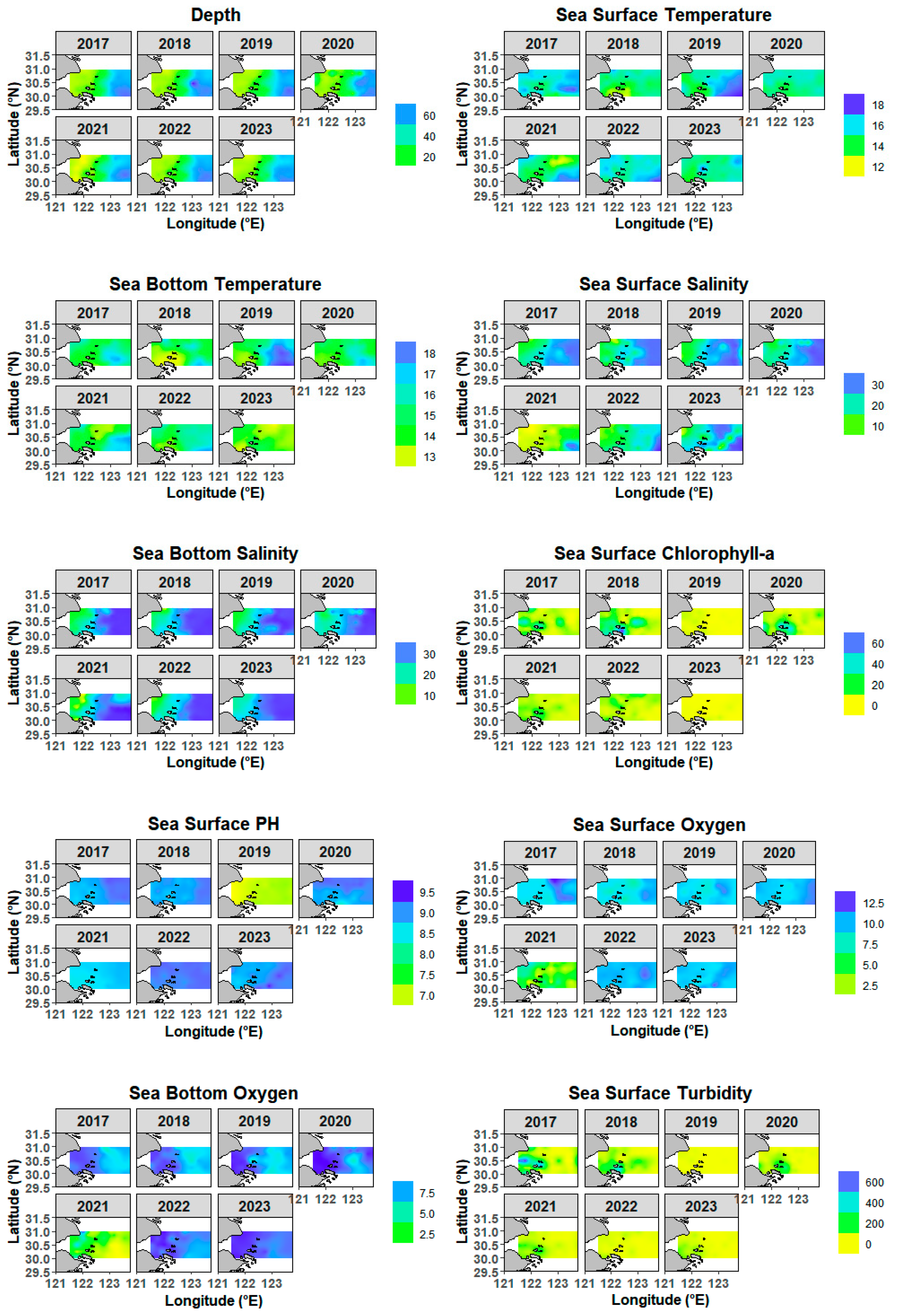
3.1. Characteristics of Environmental Factors
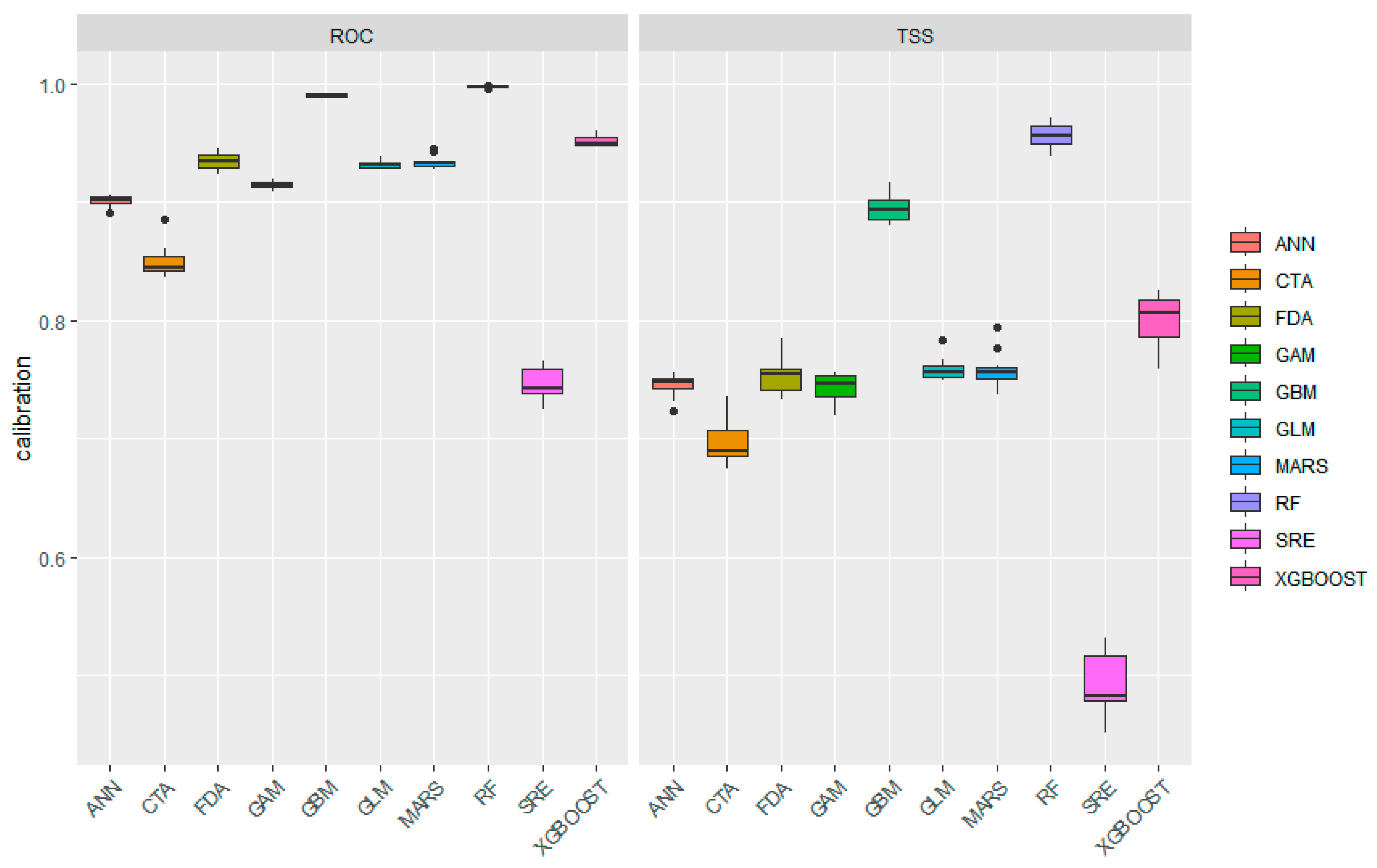
3.2. Model Performance
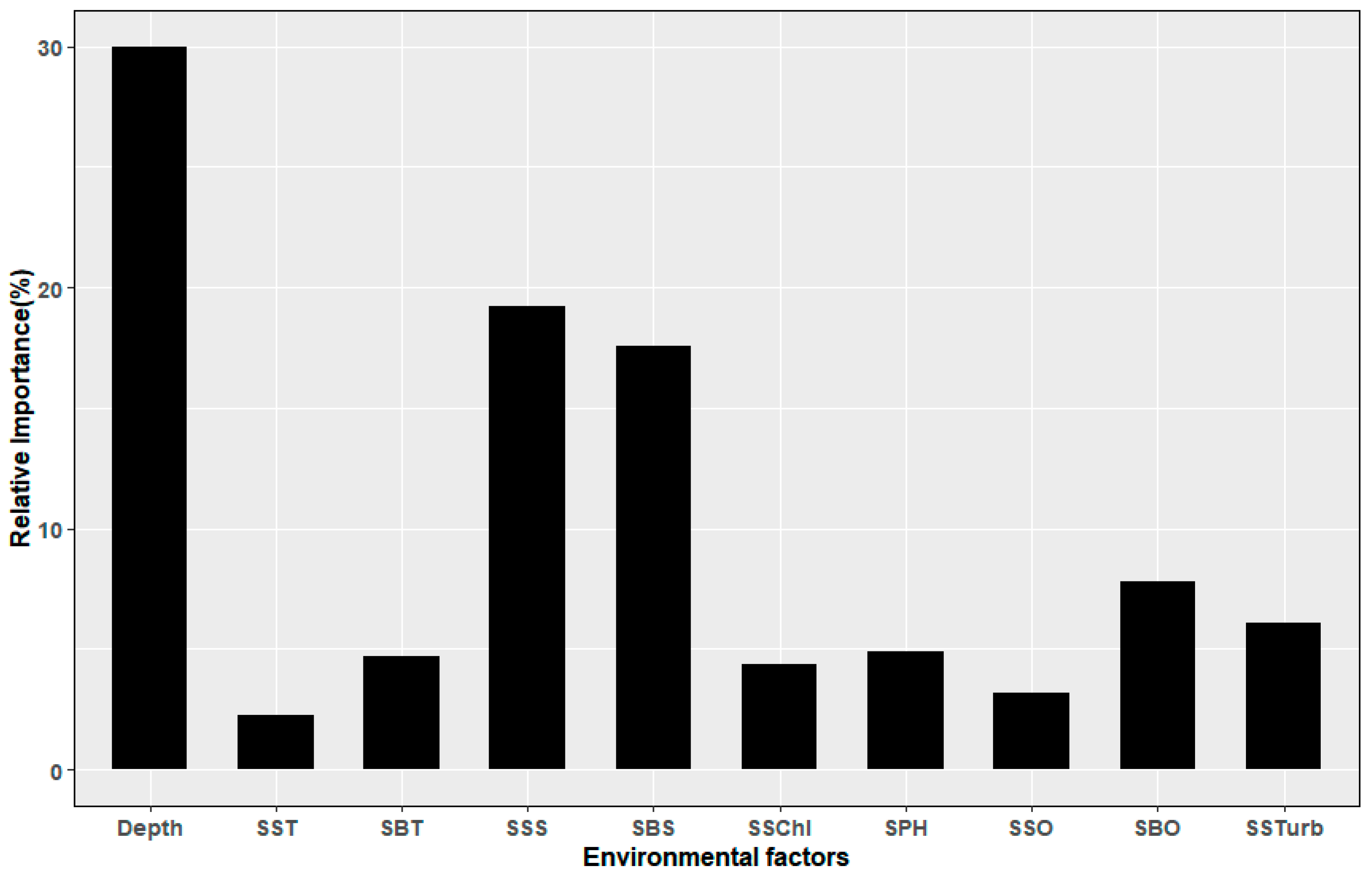
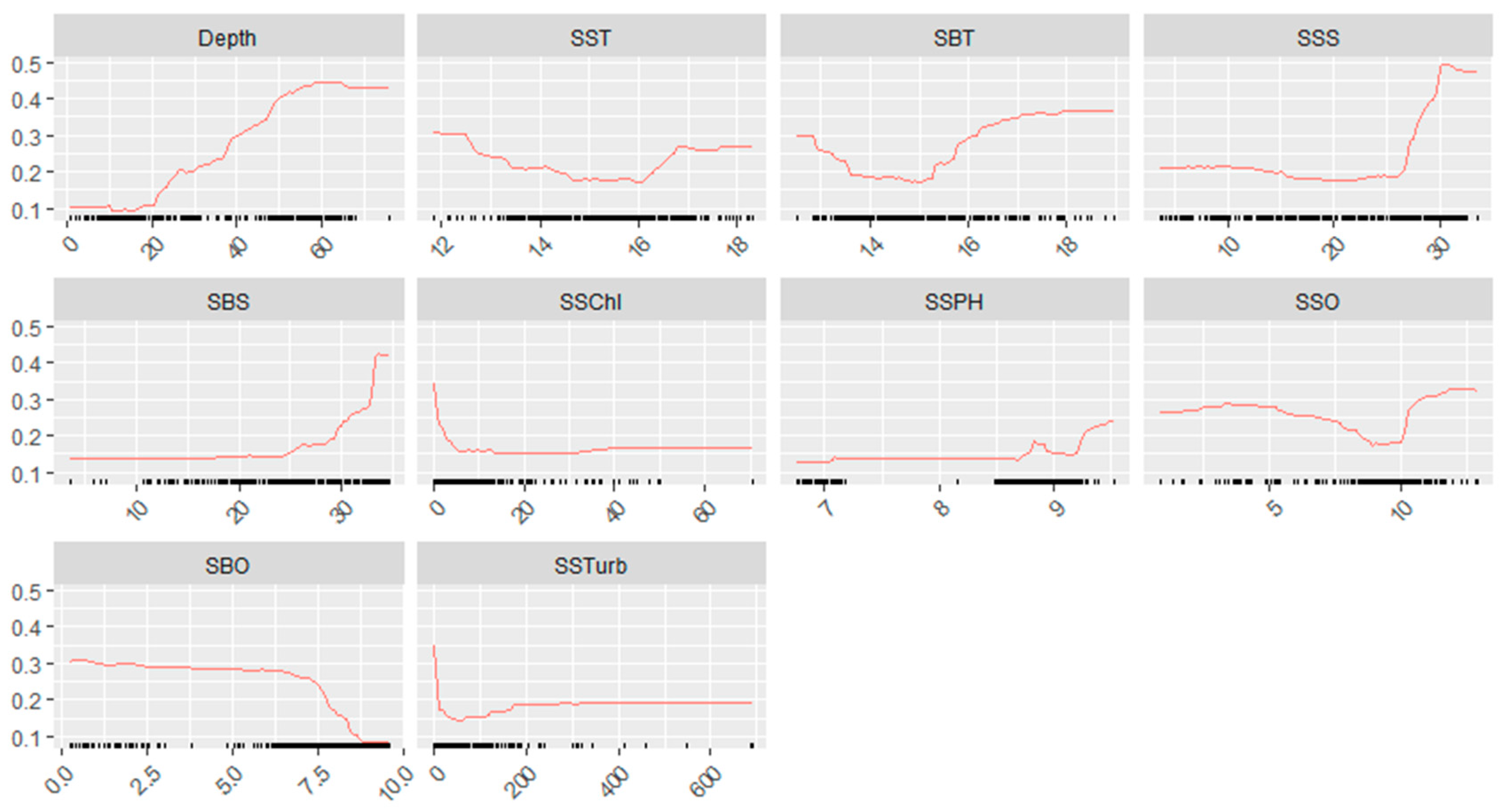
3.3. The Relationship Between L. polyactis Distribution and Environmental Factors
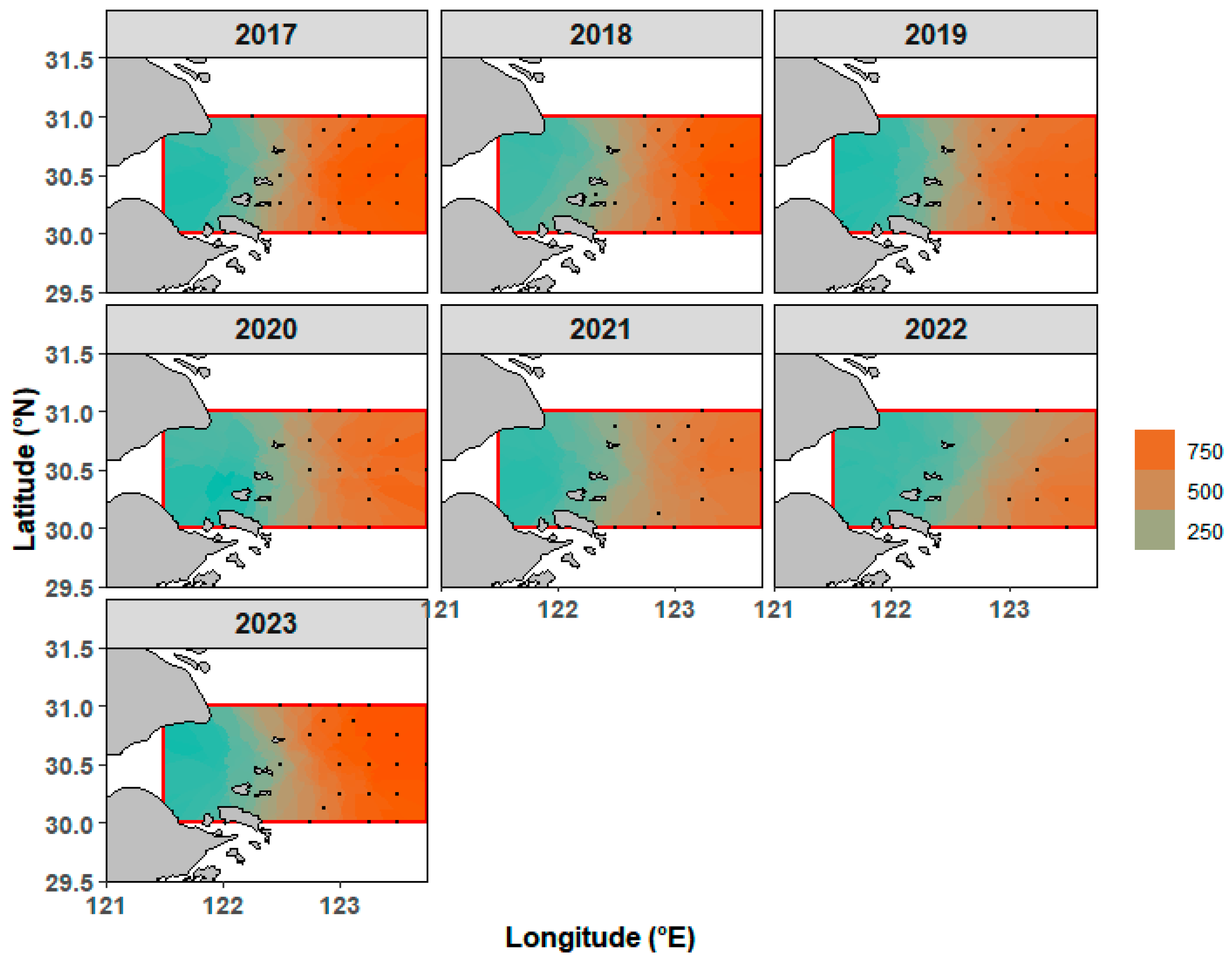
3.4. Spatio-Temporal Distribution of L. polyactis
4. Discussion
4.1. Interpretation of Model Results
4.2. Environmental Variable Predictors
4.3. Spatio-Temporal Dynamics
4.4. Implications for Conservation and Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, L.S.; Liu, Z.L.; Jiang, Y.Z.; Huang, W.; Gao, T.X. Current status of small yellow croaker resources in the southern Yellow Sea and the East China Sea. Chin. J. Oceanol. Limnol. 2011, 29, 547–555. [Google Scholar] [CrossRef]
- Zhang, C.; Ye, Z.J.; Wan, R.; Ma, Q.Y.; Li, Z.G. Investigating the population structure of small yellow croaker (Larimichthys polyactis) using internal and external features of otoliths. Fish. Res. 2014, 153, 41–47. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.H.; Liu, Z.L.; Liu, Y.; Zhang, Y.; Yang, L.L.; Wang, F.; Wu, H.; Cheng, J.H. Seasonal distribution of the early life stages of the small yellow croaker (Larimichthys polyactis) and its dynamic controls adjacent to the Changjiang River Estuary. Fish. Oceanogr. 2023, 32, 390–404. [Google Scholar] [CrossRef]
- Ma, Q.; Yan, J.; Ren, Y. Linear mixed-effects models to describe length-weight relationships for yellow croaker (Larimichthys polyactis) along the north coast of China. PLoS ONE 2017, 12, e0171811. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, T.X.; Yan, Y.R.; Song, N. Genetic variation of the small yellow croaker (Larimichthys polyactis) inferred from mitochondrial DNA provides novel insight into the fluctuation of resources. Acta Oceanol. Sin. 2022, 41, 88–95. [Google Scholar] [CrossRef]
- Tang, Y.L.; Ma, S.Y.; Liu, C.D.; Wang, X.; Cheng, S. Influence of spatial-temporal and environmental factors on Larimichthys polyactis, Octopus variabilis, and species aggregated set-net CPUEs in Haizhou Bay, China. J. Ocean Univ. China 2018, 17, 973–982. [Google Scholar] [CrossRef]
- Rubec, P.J.; Santi, C.E.; Ault, J.S.; Monaco, M.E. Development of modelling and mapping methods to predict spatial distributions and abundance of estuarine and coastal fish species life-stages in Florida. Aquac. Fish Fish. 2023, 3, 1–22. [Google Scholar] [CrossRef]
- Barletta, M.; Barletta-Bergan, A.; Saint-Paul, U.; Hubold, G. The role of salinity in structuring the fish assemblages in a tropical estuary. J. Fish Bio. 2005, 66, 45–72. [Google Scholar] [CrossRef]
- Elliott, M.; Whitfield, A.K. Challenging paradigms in estuarine ecology and management. Estuar. Coast. Shelf Sci. 2011, 94, 306–314. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, H.; Jiang, K.J.; Xiang, D.L.; Shi, Y.C.; Huang, S.S.; Li, Y.; Han, H.B. Simulating the changes of the habitats suitability of chub mackerel (Scomber japonicus) in the high seas of the North Pacific Ocean using ensemble models under medium to long-term future climate scenarios. Mar. Pollut. Bull. 2024, 207, 12. [Google Scholar] [CrossRef]
- Song, X.; Hu, F.; Xu, M.; Zhang, Y.; Jin, Y.; Gao, X.; Liu, Z.; Ling, J.; Li, S.; Cheng, J. Spatiotemporal distribution and dispersal pattern of early life stages of the small yellow croaker (Larimichthys polyactis) in the southern Yellow Sea. Diversity 2024, 16, 521. [Google Scholar] [CrossRef]
- Yin, J.; Wang, J.; Zhang, C.L.; Xu, B.D.; Xue, Y.; Ren, Y.P. Spatial and temporal distribution characteristics of Larimichthys polyactis eggs in Haizhou Bay and adjacent regions based on two- stage GAM. J. Fish. Sci. China 2019, 26, 1164–1174. [Google Scholar]
- Wan, R.; Song, P.; Li, Z.; Long, X.; Wang, D.; Zhai, L. Use of ensemble model for modeling the larval fish habitats of different ecological guilds in the Yangtze Estuary. Fishes 2023, 8, 209. [Google Scholar] [CrossRef]
- Brunel, T.; van Damme, C.J.G.; Samson, M.; Dickey-Collas, M. Quantifying the influence of geography and environment on the northeast Atlantic mackerel spawning distribution. Fish. Oceanogr. 2017, 27, 159–173. [Google Scholar] [CrossRef]
- Li, M.; Wang, B.; Li, Y. Influence of suspended particulate matters on P dynamics and eutrophication in the highly turbid estuary: A case study in Hangzhou Bay, China. Mar. Pollut. Bull. 2024, 207, 116793. [Google Scholar] [CrossRef]
- Longhurst, A.R.; Harrison, W.G. The biological pump: Profiles of plankton production and consumption in the upper ocean. Prog. Oceanogr. 1989, 22, 47–123. [Google Scholar] [CrossRef]
- Jin, Z.H.; Xu, K.D.; Zhang, H.L.; Zhou, Y.D.; Long, X.Y.; Zhan, W.; Ma, W.J.; Xu, F. Resource assessment of the near-shore Sciaenidae fishes in Zhejiang Province, China. J. Dalian Fish. Univ. 2025, 1–16. [Google Scholar]
- Lu, Z.H.; Miao, Z.Q.; Lin, N. The structure and diversity fish communities in Spring in the middle sea area of Zhejiang Province and adjacent region. J. Zhejiang Ocean Univ. 2009, 28, 51–56. [Google Scholar]
- Ren, J.S.; Jin, X.S.; Yang, T.; Kooijman, S.A.L.M.; Shan, X.J. A dynamic energy budget model for small yellow croaker Larimichthys polyactis: Parameterisation and application in its main geographic distribution waters. Ecol. Model. 2020, 427, 109051. [Google Scholar] [CrossRef]
- Rao, W.B.; Mao, C.P.; Wang, Y.G.; Su, J.B.; Balsam, W.; Ji, J.F. Geochemical constraints on the provenance of surface sediments of radial sand ridges off the Jiangsu coastal zone, East China. Mar. Geol. 2015, 359, 35–49. [Google Scholar] [CrossRef]
- Liu, Z.L.; Jin, Y.; Yang, L.L.; Yan, L.P.; Zhang, Y.; Xu, M.; Tang, J.H.; Zhou, Y.D.; Hu, F.; Cheng, J.H. Incorporating egg-transporting pathways into conservation plans of spawning areas: An example of small yellow croaker (Larimichthys polyactis) in the East China Sea zone. Front. Mar. Sci. 2022, 9, 941411. [Google Scholar] [CrossRef]
- Zhou, F.; Su, J.L.; Huang, D.J. Study on the intrusion of coastal low salinity water in the west of southern Huanghai Sea during spring and summer. Haiyang Xuebao 2004, 26, 34–44. [Google Scholar]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. biomod2: Ensemble Platform for Species Distribution Modeling; R Package: Vienna, Austria, 2016; Available online: https://CRAN.R-project.org/package=biomod2 (accessed on 1 January 2020).
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Wang, Y.S.; Xie, B.Y.; Wan, F.H.; Xiao, Q.M.; Dai, L.Y. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodivers. Sci. 2007, 4, 365–372. (In Chinese) [Google Scholar]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling; R Package: Vienna, Austria, 2014; Available online: http://CRAN.Rproject.org/package=raster (accessed on 1 January 2020).
- Deckmyn, A. Maps: Draw Geographical Maps; R Package: Vienna, Austria, 2022; Available online: http://CRAN.R-project.org/package=maps (accessed on 1 January 2020).
- Ginestet, C. ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Li, Z.G.; Ye, Z.J.; Wan, R.; Zhang, C. Model selection between traditional and popular methods for standardizing catch rates of target species: A case study of Japanese Spanish mackerel in the gillnet fishery. Fish. Res. 2015, 161, 312–319. [Google Scholar] [CrossRef]
- Lee-Yaw, J.A.; McCune, J.L.; Pironon, S. Species distribution models rarely predict the biology of real populations. Ecography 2022, 2022, e05877. [Google Scholar] [CrossRef]
- Savage, S.L.; Lawrence, R.L.; Squires, J.R. Predicting relative species composition within mixed conifer forest pixels using zero-inflated models and Landsat imagery. Remote Sens. Environ. 2015, 171, 326–336. [Google Scholar] [CrossRef]
- de la Hoz, C.F.; Ramos, E.; Puente, A.; Juanes, J.A. Temporal transferability of marine distribution models: The role of algorithm selection. Ecol. Indic. 2019, 106, 105499.1–105499.14. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.L.; Xu, B.D. Evaluating the approaches of habitat suitability modelling for whitespotted conger (Conger myriaste). Fish. Res. 2017, 195, 230–237. [Google Scholar] [CrossRef]
- Li, Z.G.; Wan, R.; Ye, Z.J.; Chen, Y.; Ren, Y.P.; Liu, H.; Jiang, Y.Q. Use of random forests and support vector machines to improve annual egg production estimation. Fish. Sci. 2017, 83, 1–11. [Google Scholar] [CrossRef]
- Murase, H.; Nagashima, H.; Yonezaki, H.; Matsukura, R.; Kitakado, T. Application of a generalized additive model (GAM) to reveal relationships between environmental factors and distributions of pelagic fish and krill: A case study in Sendai Bay, Japan. ICES J. Mar. Sci. 2009, 66, 1073–1080. [Google Scholar] [CrossRef]
- Akter, S.; Abhilash, W.K.; Nama, S.; Borah, S.; Angmo, S.; Deshmukhe, G.; Nayak, B.B.; Ramteke, K. Phytoplankton-environment dynamics in a tropical estuary of the northeastern Arabian Sea: A Generalized Additive Model (GAM) approach. Environ. Monit. Assess. 2025, 197, 201. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Liu, X.Y.; Han, Z.Q. Predicting the effects of climate change on the suitable habitat of Japanese Spanish Mackerel (Scomberomorus niphonius) based on the species distribution model. Front. Mar. Sci. 2022, 9, 927790. [Google Scholar] [CrossRef]
- Pang, H.; Lin, A.P.; Holford, M.; Enerson, B.E.; Lu, B.; Lawton, M.P.; Floyd, E.; Zhao, H. Pathway analysis using random forests classification and regression. Bioinformatics 2006, 22, 2028–2036. [Google Scholar] [CrossRef]
- Potter, I.C.; Tweedley, J.R.; Elliott, M.; Whitfield, A.K. The ways in which fish use estuaries: A refinement and expansion of the guild approach. Fish Fish. 2015, 16, 230–239. [Google Scholar] [CrossRef]
- Bento, E.G.; Grilo, T.F.; Nyitrai, D.; Dolbeth, M.; Pardal, M.A.; Martinho, F. Climate influence on juvenile European sea bass (Dicentrarchus labrax, L.) populations in an estuarine nursery: A decadal overview. Mar. Environ. Res. 2016, 122, 93–104. [Google Scholar] [CrossRef]
- Ding, F.Y.; Lin, L.S.; Li, J.S.; Cheng, J.H. Distribution of the spawning population of small yellow croaker (Larimichthys polyactis) in the northern East China Sea and its relationship with water masses. J. Nat. Resour. 2007, 22, 1013–1019. (In Chinese) [Google Scholar]
- Galaiduk, R.; Radford, B.T.; Saunders, B.J.; Newman, S.J.; Harvey, E.S. Characterizing ontogenetic habitat shifts in marine fishes: Advancing nascent methods for marine spatial management. Ecol. Appl. 2017, 27, 1776–1788. [Google Scholar] [CrossRef]
- Parsons, D.F.; Suthers, I.M.; Cruz, D.O.; Smith, J.A. Effects of habitat on fish abundance and species composition on temperate rocky reefs. Mar. Ecol. Prog. Ser. 2016, 561, 155–171. [Google Scholar] [CrossRef]
- Dambrine, C.; Woillez, M.; Huret, M.; de Pontual, H. Characterising Essential Fish Habitat using spatio-temporal analysis of fishery data: A case study of the European seabass spawning areas. Fish. Oceanogr. 2021, 30, 413–428. [Google Scholar] [CrossRef]
- Zhong, X.M.; Zhang, H.; Tang, J.H.; Zhong, F.; Zhong, J.S.; Xiong, Y.; Gao, Y.S.; Ge, K.K.; Yu, W.W. Temporal and spatial distribution of Larimichthys polyactis Bleeker resources in offshore areas of Jiangsu Province. J. Fish. China 2011, 235, 238–246. [Google Scholar]
- Lin, N.; Chen, Y.G.; Jin, Y.; Yuan, X.W.; Ling, J.Z.; Jiang, Y.Z. Distribution of the early life stages of small yellow croaker in the Yangtze River estuary and adjacent waters. Fish. Sci. 2018, 84, 357–363. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hu, C.L.; Xu, K.D.; Zhou, Y.D.; Jiang, R.J.; Li, Z.H.; Li, X.F. Reproductive status of Larimichthys polyactis in Zhoushan fishing ground and adjacent waters from 2020 to 2021. Ocean Dev. Manag. 2022, 39, 53–57. [Google Scholar]
- Benedetti-Cecchi, L.; Bertocci, I.; Micheli, F.; Maggi, E.; Fosella, T.; Vaselli, S. Implications of spatial heterogeneity for management of marine protected areas (MPAs): Examples from assemblages of rocky coasts in the northwest Mediterranean. Mar. Environ. Res. 2003, 55, 429–458. [Google Scholar] [CrossRef]
- Garcia-Charton, J.A.; Perez-Ruzafa, A.; Marcos, C.; Claudet, J.; Badalamenti, F.; Benedetti-Cecchi, L.; Falcón, J.M.; Milazzo, M.; Schembri, P.J.; Stobart, B.; et al. Effectiveness of European Atlanto-Mediterranean MPAs: Do they accomplish the expected effects on populations, communities and ecosystems? J. Nat. Conserv. 2008, 16, 193–221. [Google Scholar] [CrossRef]
- Roberts, C.M.; Bohnsack, J.A.; Gell, F.; Hawkins, J.P.; Goodridge, R. Effects of marine reserves on adjacent fisheries. Science 2001, 294, 1920–1923. [Google Scholar] [CrossRef]
- Greenberg, P. Four Fish: The Future of the Last Wild Food; Penguin: New York, NY, USA, 2010; 285p. [Google Scholar]
| Variable | Depth | SST | SBT | SSS | SBS | SSChl | SBChl | SSPH | SBPH | SSO | SBO | SSTurb | SBTurb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before filtering | 4.59 | 2.34 | 2.90 | 2.97 | 3.85 | 3.78 | 5.94 | 47.25 | 46.93 | 3.83 | 4.25 | 3.51 | 6.34 |
| After filtering | 4.14 | 2.27 | 2.81 | 2.80 | 3.73 | 2.61 | / | 1.24 | / | 3.58 | 4.12 | 2.59 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Wang, D.; Song, P.; Han, M.; Jiang, R.; Zhou, Y. Spatio-Temporal Habitat Dynamics of Migratory Small Yellow Croaker (Larimichthys polyactis) in Hangzhou Bay, China. Fishes 2025, 10, 298. https://doi.org/10.3390/fishes10060298
Long X, Wang D, Song P, Han M, Jiang R, Zhou Y. Spatio-Temporal Habitat Dynamics of Migratory Small Yellow Croaker (Larimichthys polyactis) in Hangzhou Bay, China. Fishes. 2025; 10(6):298. https://doi.org/10.3390/fishes10060298
Chicago/Turabian StyleLong, Xiangyu, Dong Wang, Pengbo Song, Mengwen Han, Rijin Jiang, and Yongdong Zhou. 2025. "Spatio-Temporal Habitat Dynamics of Migratory Small Yellow Croaker (Larimichthys polyactis) in Hangzhou Bay, China" Fishes 10, no. 6: 298. https://doi.org/10.3390/fishes10060298
APA StyleLong, X., Wang, D., Song, P., Han, M., Jiang, R., & Zhou, Y. (2025). Spatio-Temporal Habitat Dynamics of Migratory Small Yellow Croaker (Larimichthys polyactis) in Hangzhou Bay, China. Fishes, 10(6), 298. https://doi.org/10.3390/fishes10060298




