1. Introduction
Short-lived radionuclides such as
54Mn,
60Co, and
65Zn enter the environment through nuclear explosions, accidents, and regulated emissions from nuclear facilities and mining plants [
1]. Their accumulation in fish is critical for understanding ecological and human health risks. These radionuclides vary in half-lives, making their study in nature challenging:
60Co (5.27 years),
54Mn (312 days), and
65Zn (245 days) [
2].
Metals enter fish via gills, skin, and the gastrointestinal tract, transporting through the bloodstream and binding to proteins. This results in differential accumulation across organs, with external tissues acting as primary entry points, while internal organs like the liver and kidneys show the highest concentrations [
3,
4].
Manganese plays a role in metabolic functions, influencing respiration, bone formation, and reproduction. It remains relatively stable in fish tissues, irrespective of external concentrations [
3,
5]. Cobalt, vital for hemoglobin synthesis, aids in iron assimilation and influences calcium and phosphorus metabolism [
5]. Zinc is crucial for enzymatic reactions, tissue oxygenation, and redox processes, accumulating predominantly in bones and scales [
3,
5].
One of the earliest studies on
54Mn and
65Zn accumulation in marine organisms was conducted in the Atlantic Ocean, near Beaufort, North Carolina, between 1964 and 1966. The research revealed that levels of these radionuclides varied among different aquatic organisms. For example, the highest concentration of
65Zn was observed in the mollusk
Crassostrea virginica (Gmelin, 1791), while
Argopecten irradians (Lamarck, 1819) showed the highest levels of
54Mn [
6]. Similarly, research on the genus
Oncorhynchus fish on the U.S. Pacific coast during the 1960s detected high
60Co and
65Zn levels in liver and roe, exceeding muscle concentrations [
7].
Similar studies were carried out in freshwater ecosystems. For instance, between 1963 and 1967,
65Zn was found in the body of
Acipenser transmontanus (Richardson, 1836) sampled from the Hanford Reach River and the McNary Pool (USA) [
8]. By 1990, this radionuclide was undetectable in the species. Poston T.M. and Klopfer D.C [
3] concluded that these isotopes accumulate unevenly in different organs and tissues of fish. The highest concentrations of
60Co were found in the kidneys, while the lowest levels of
54Mn were observed in muscle tissue, with bone tissue showing the highest levels of this radionuclide. The distribution of
65Zn in fish organs and tissues was as follows: kidneys = gonads > liver > gills > muscles = heart.
The radiation risk to humans from consuming fish containing short-lived isotopes (e.g.,
65Zn) was assessed in the United States in the mid-1960s. During an experiment, volunteers consumed the muscle tissue of
Coregonus houi (Linnaeus, 1758) from the Columbia River daily for 11 weeks. The concentration of
65Zn in the fish muscle was 5 nCi/200 g (i.e., 185 Bq/200 g) of fresh weight. The researchers concluded that this concentration of
65Zn in the fish muscle posed no significant health risk to humans [
9]. Although global fallout decreased after atmospheric nuclear test bans, research on
54Mn,
60Co, and
65Zn continued, focusing on uptake mechanisms.
Despite the rapid disappearance of manganese, cobalt, and zinc radionuclides from the environment following the ban on atmospheric nuclear tests and the subsequent decline in global fallout, interest in studying the accumulation of 54Mn, 60Co, and 65Zn by fish persisted. Throughout the 1970s and 1980s, researchers conducted laboratory studies to investigate the primary pathways through which these radionuclides enter fish and the variations in their accumulation across different organs and tissues.
Laboratory experiments confirmed food as the primary uptake pathway for
54Mn and
65Zn, while
60Co is absorbed from both food and water [
10,
11,
12,
13]. Pentreath [
14] found that waterborne cobalt contributes minimally to fish uptake. Studies with
Seriola quinqueradiata (Temminck and Schlegel, 1845) showed that
60Co concentrates in blood-rich organs, while
54Mn accumulates in bones, and
65Zn distributes evenly across organs and muscles [
10].
Research on
Phoxinus laevis (Kessler, 1879) found that kidneys retained the highest
60Co levels, while
54Mn accumulated mostly in external organs [
15]. Another study on
Pleuronectes platessa (Linnaeus, 1758) confirmed that
58Co concentrates in intestines and gills, with minimal muscle accumulation [
14]. In experiments conducted by Koyanagi T. et al. [
16], radionuclides
54Mn,
60Co, and
65Zn were introduced directly into the stomach of
Kareius bicoloratus (Basilewsky, 1855) in the form of gelatin capsules filled with moist bottom sediments containing these radionuclides. The distribution of radionuclides across organs and tissues was determined one week after introduction. The highest concentrations of
54Mn,
60Co, and
65Zn were detected in the intestines. High levels in internal organs (liver and kidneys) indicated a relatively rapid transfer of radionuclides from the stomach to these organs. Muscles generally contain the lowest levels of radionuclides.
Pentreath [
11] found that
65Zn in
P. platessa distributes evenly across bones, skin, and muscles, with high gonadal levels. Studies on
Raja clouata (Linnaeus, 1758) showed
65Zn concentration in the liver and spleen, while
54Mn accumulated in cartilage and the highest
58Co levels were in gills and heart [
12].
Different aquatic species accumulate radionuclides at varying rates. Experiments showed that algae rapidly absorb and release
60Co, while mollusks and fish retain it longer [
13]. These findings underline the need for continued research into radionuclide behavior in aquatic ecosystems and their implications for environmental safety.
The study of short-lived radionuclides is particularly important due to their ecological and human health implications. These radionuclides can enter the environment following nuclear accidents, such as the Chornobyl disaster, leading to contamination of aquatic ecosystems. Understanding the behavior of these radionuclides in fish populations is crucial for assessing their potential risks to ecosystem stability and human consumers.
One of the key challenges in studying short-lived radionuclides is their rapid decay, which makes long-term tracking and analysis difficult. This has resulted in inconsistencies in the literature regarding organ-specific accumulation and uptake pathways. Additionally, differences in experimental conditions, species physiology, and environmental factors further complicate the interpretation of results, leading to a lack of consensus on the distribution of these radionuclides in fish tissues.
The objective of this review is to synthesize the available literature on the accumulation and distribution of 54Mn, 60Co, and 65Zn in fish, highlighting key findings and unresolved questions. It aims to discuss pathways of uptake, organ-specific behavior, and potential implications for both ecological stability and human health. By identifying existing knowledge gaps, this review seeks to inform future research directions and enhance risk assessment methodologies. Understanding these processes is essential for developing improved environmental monitoring strategies and ensuring the safety of aquatic food resources.
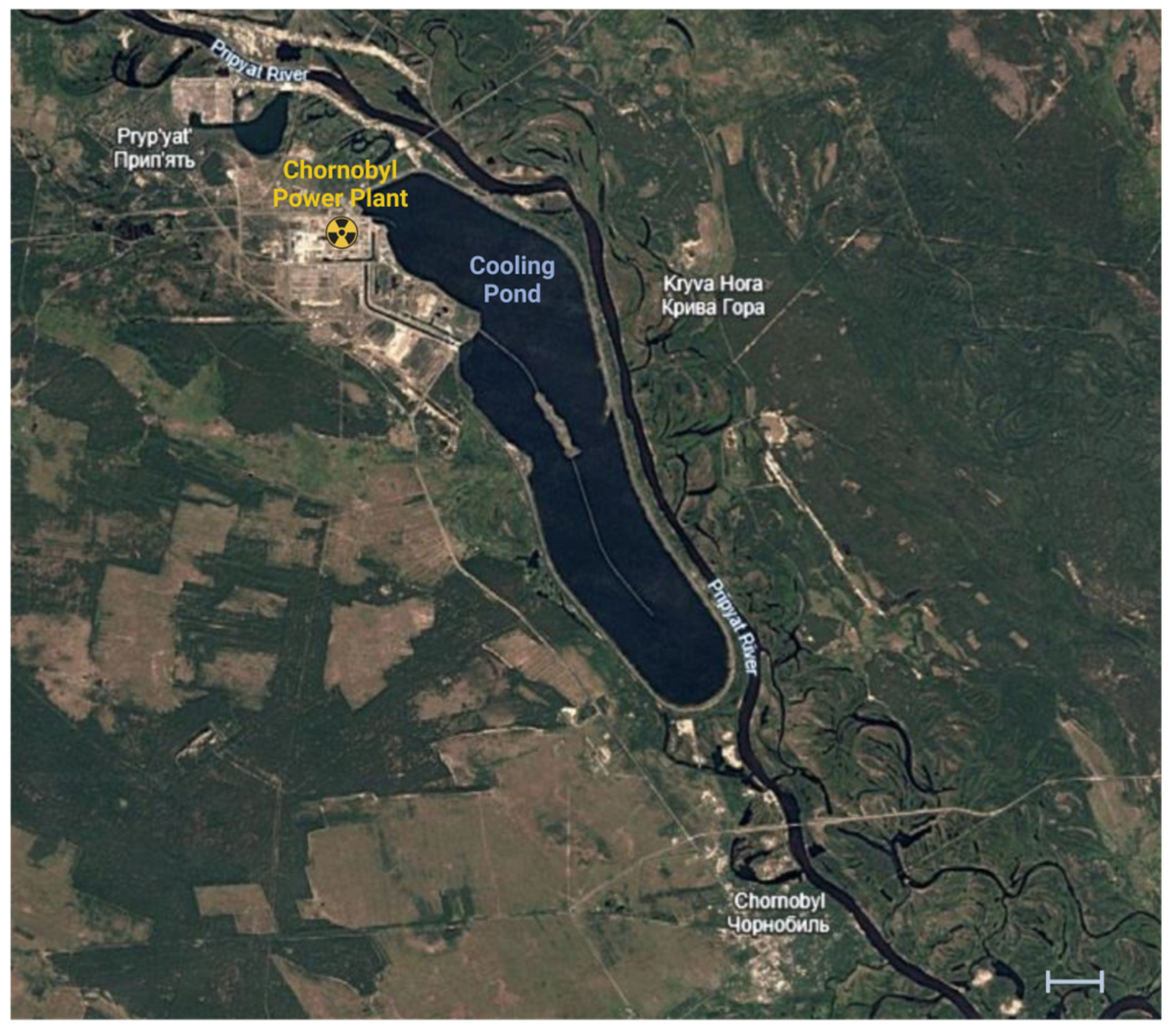
4. Radionuclide Contamination in Chornobyl NPP Cooling Pond: Effects on Water and Aquatic Biota
The primary source of radionuclide contamination in the CP under normal plant operating conditions was liquid effluents, including imbalanced water and waste from specialized laundries and sanitary checkpoints. The greatest volume of imbalanced water discharge typically occurred during the plant’s initial operating period [
20]. Before the 1986 accident, the presence of
54Mn and
60Co in CP water was regularly detected, although at low concentrations—no more than 0.003 Bq/L in 1980. These radionuclides were quickly removed from the water, predominantly accumulating in the sediment and aquatic vegetation. For example, sediment samples taken in the fall of 1981 showed radionuclide levels of 243 Bq/kg for
54Mn and 500 Bq/kg for
60Co, while
65Zn was not detected in either water or sediment before the accident [
21].
Manganese (
54Mn): Before the accident,
54Mn levels in free-living fish species were relatively low, with the highest concentration in muscle tissue at 8.5 Bq/kg. Levels in the head and internal organs did not exceed 23.5 Bq/kg. The peak influx likely occurred in 1980–1981, and by 1985, due to its short half-life,
54Mn levels had decreased. It was primarily found in non-predatory planktophages and benthophages, with significantly lower levels observed in fish raised in tanks on clean, artificial feeds. After the accident,
54Mn concentrations in all the CP’s components increased significantly but were detected in only 3–4% of approximately 5000 radionuclide measurements. The highest specific activity of
54Mn was observed in the water’s vegetation in March 1987, at 2000 Bq/kg dry weight. In fish,
54Mn levels were orders of magnitude lower than those of other radionuclides, generally ranging between 10 and 500 Bq/kg fresh weight, with the highest concentration (561 Bq/kg) found in the roe of
Perca fluviatilis (Linnaeus, 1758) in March 1988. Post-accident,
54Mn was still primarily detected in benthophages and planktophages, but unlike pre-accident data, it was now mainly found in the internal organs, comprising over 50% of the detections. Its distribution across fish tissues was relatively uniform, with no single organ or tissue identified as a specific accumulator of this radionuclide [
22].
Cobalt (
60Co): Pre-accident
60Co levels in fish were generally low, peaking at 22.2 Bq/kg in the second half of 1981, with a minimum specific activity of 0.49 Bq/kg in fish tissue. By 1985,
60Co levels had decreased several times, not exceeding 4.6 Bq/kg, with an average of 1.5 Bq/kg. After the accident,
60Co levels in the biota of the cooling pond increased significantly.
60Co was widely detected in high concentrations in filamentous algae (
Cladophora spp.) and water plants (
Potamogeton spp.) with specific activities of 2300 Bq/kg and 3400 Bq/kg dry weight, respectively. Different fish species showed varied
60Co levels, with the highest found in planktophages
H. molitrix (
Table 1) and
A. nobilis. Benthophages such as
C. carpio (
Table 2),
Rutilus rutilus (Linnaeus, 1758), and
Abramis brama (Linnaeus, 1758) also had significant levels of
60Co. In fish with a mixed type of nutrition (
Silurus glanis (Linnaeus, 1758),
I. punctatus, and
P. fluviatilis), the
60Co content is similar in value to the benthophages—
R. rutilus and
A. brama. The lowest concentrations were found in ichthyophages
Sander lucioperca (Linnaeus, 1758) (Table 4) and
Aspius aspius (Linnaeus, 1758). Ingestion is proposed as the primary pathway for cobalt uptake in fish. The contribution of the nutrition pathway to the accumulation of
60Co by fish of low trophic levels is probably more significant than the accumulation of this radionuclide by ichthyophages. However, studies have suggested that
60Co can enter the organisms of the studied fish species both through the trophic chain and directly from the water.
60Co distribution within fish bodies was uneven, with the highest levels found in the kidneys, followed by the liver, swim bladder, eggs, spine, fat, and red muscles. Scales and heads in direct contact with water also frequently showed high cobalt levels, while the lowest concentrations were detected in the heart, stomach, ribs, and fat [
23].
Zinc (
65Zn): Unlike manganese and cobalt, zinc was more frequently detected in fish samples before the accident. The range of
65Zn concentrations across different organs and tissues varied by more than tenfold, with the lowest levels found in scales, internal organs, and muscles, and the highest (up to 500.8 Bq/kg fresh weight) in the spine and head.
65Zn was detected in fish of all studied ecological groups, including obligate phytophages (
C. idella), benthophages (
Blicca bjoerkna (Linnaeus, 1758),
A. brama,
R. rutilus,
Carassius carassius (Linnaeus, 1758), and
C. carpio), planktophages (
A. nobilis and
H. molitrix), facultative planktophages (
Abramis ballerus (Linnaeus, 1758)), and obligate ichthyophages (
S. lucioperca and
Esox lucius (Linnaeus, 1758)). Post-accident,
65Zn was predominantly found in fish, with specific activities reaching 6000 Bq/kg fresh weight. It was detected in only one water sample and one sample of periphyton, represented by filamentous algae (
Cladophora spp.). Unlike this radionuclide’s relatively uniform pre-accident distribution among ecological fish groups, it was more frequently detected in peaceful species post-accident. Approximately 85% of fish samples detected
65Zn were benthophages (
B. bjoerkna (
Table 3),
A. brama,
C. carpio, and
R. rutilus) and planktophages (
H. molitrix and
A. nobilis). Around 15% of all samples that had
65Zn were from fish with mixed types of nutrition (
S. glanis,
I. punctatus, and
P. fluviatilis), with only about 8% of all samples in obligate ichthyophages (
L. lucioperca and
A. aspius). Post-accident, the distribution of
65Zn in fish tissues differed significantly from pre-accident patterns, with its presence in muscles and bones becoming rare and in lower concentrations than in other organs and tissues. The highest frequency of
65Zn detection occurred in organs and tissues directly exposed to water, such as fins, scales, gills, skin, and head, indicating that these tissues are reliable indicators of
65Zn presence in the water [
24].
Table 1,
Table 2,
Table 3 and
Table 4 summarize the organ-specific content of the discussed short-lived radionuclides in four selected fish species, providing illustrative examples of their distribution and accumulation patterns.
Table 1.
Content of radionuclides (54Mn, 60Co, and 65Zn) in H. molitrix (Bq/kg fresh weight) with years before the Chornobyl accident labelled with an asterisk (*).
Table 1.
Content of radionuclides (54Mn, 60Co, and 65Zn) in H. molitrix (Bq/kg fresh weight) with years before the Chornobyl accident labelled with an asterisk (*).
| Organ | Year | 54Mn | 60Co | 65Zn |
|---|
| Head | 1981 * | 25.3 ± 10.1 | 51.5 ± 15.7 | 500.8 ± 101.4 |
| 1985 * | - | 1.23 ± 0.26 | - |
| 1987 | - | 89.6 ± 10.1 | 204.2 ± 62.2 |
| 1988 | - | 121.5 ± 10.6 | 110.9 ± 13.4 |
| 1990 | - | 18.4 ± 3.4 | - |
| Internal Organs | 1985 * | 0.8 ± 0.2 | 2.7 ± 0.4 | |
| 1987 | | 568.0 ± 54.9 | 519.2 ± 80.6 |
| 1988 | 98.0 ± 17.2 | 977.2 ± 72.3 | 94.8 ± 31.2 |
| Caviar | 1985 * | | 1.67 ± 0.4 | |
| 1987 | | 167.3 ± 23.6 | |
| 1988 | | 152.9 ± 16.6 | 513.4 ± 74.1 |
| 1990 | | 87.8 ± 13.4 | |
| Muscles | 1985 * | - | 0.93 ± 0.26 | |
| 1987 | | 60.2 ± 16.1 | |
| 1988 | 49.1 ± 10.7 | 54.1 ± 11.7 | |
| 1989 | | 42.9 ± 6.4 | |
| 1990 | | 15.9 ± 4.1 | |
Table 2.
Content of radionuclides (54Mn, 60Co, and 65Zn) in C. carpio (Bq/kg fresh weight) after the Chornobyl accident.
Table 2.
Content of radionuclides (54Mn, 60Co, and 65Zn) in C. carpio (Bq/kg fresh weight) after the Chornobyl accident.
| Organ | Year | 54Mn | 60Co | 65Zn |
|---|
| Head | 1986 | - | 140.3 ± 30.3 | 867.8 ± 100.8 |
| 1987 | - | 44.6 ± 8.9 | 279.4 ± 38.2 |
| 1988 | - | 14.9 ± 3.2 | 270.5 ± 29.7 |
| 1989 | - | 74.2 ± 10.1 | 82.8 ± 23.3 |
| 1990 | - | 10.7 ± 2.8 | 33.9 ± 5.8 |
| Fins | 1986 | - | - | 754.0 ± 74.8 |
| 1987 | - | - | 1339.1 ± 124.5 |
| 1988 | - | - | 259.9 ± 28.1 |
| Scales | 1986 | - | 61.6 ± 28.6 | 751.44 ± 122.6 |
| 1987 | - | 191.3 ± 24.7 | 775.2 ± 78.4 |
| 1988 | - | 26.2 ± 5.6 | 70.4 ± 27.7 |
| 1989 | - | 44.1 ± 9.7 | - |
| 1990 | - | 79.7 ± 13.3 | - |
| Internal Organs | 1986 | - | 87.1 ± 20.0 | 626.3 ± 87.2 |
| 1987 | - | 173.4 ± 20.4 | 1385.1 ± 114.0 |
| 1989 | - | 74.2 ± 10.1 | 82.8 ± 23.3 |
| 1990 | - | 12.2 ± 3.6 | - |
| Muscles | 1986 | 168.9 ± 55.3 | 52.2 ± 15.3 | 283.6 ± 96.0 |
| 1987 | - | 415.3 ± 46.3 | 754.2 ± 110.1 |
| 1988 | - | 17.6 ± 3.5 | 63.3 ± 14.7 |
| 1989 | - | 15.8 ± 6.7 | - |
| 1990 | - | 3.5 ± 1.3 | - |
Table 3.
Content of radionuclides (54Mn, 60Co, and 65Zn) in B. bjoerkna (Bq/kg fresh weight) after the Chornobyl accident.
Table 3.
Content of radionuclides (54Mn, 60Co, and 65Zn) in B. bjoerkna (Bq/kg fresh weight) after the Chornobyl accident.
| Organ | Year | 54Mn | 60Co | 65Zn |
|---|
| Head | 1987 | - | 171.9 ± 46.5 | 765.2 ± 135.5 |
| 1988 | - | 36.7 ± 5.0 | 52.9 ± 11.4 |
| 1989 | - | 14.9 ± 4.2 | 21.9 ± 10.2 |
| 1990 | - | 9.8 ± 3.1 | - |
| Fins | 1987 | - | 286.6 ± 73.7 | 478.4 ± 157.9 |
| 1988 | - | 47.6 ± 9.1 | - |
| Internal Organs | 1987 | - | 115.2 ± 30.0 | - |
| 1988 | - | 84.9 ± 10.8 | 178.9 ± 25.7 |
| 1989 | - | 66.3 ± 9.2 | - |
| 1990 | - | 38.4 ± 8.3 | - |
| Muscles | 1987 | - | 64.7 ± 26.2 | - |
| 1988 | - | 28.7 ± 8.44 | - |
| 1989 | - | 12.9 ± 4.1 | - |
| 1990 | - | 4.78 ± 1.2 | - |
Table 4.
Content of radionuclides (54Mn, 60Co, and 65Zn) in S. lucioperca (Bq/kg fresh weight) after the Chornobyl accident.
Table 4.
Content of radionuclides (54Mn, 60Co, and 65Zn) in S. lucioperca (Bq/kg fresh weight) after the Chornobyl accident.
| Organ | Year | 54Mn | 60Co | 65Zn |
|---|
| Fins | 1987 | - | 12.9 ± 5.3 | 73.9 ± 25.2 |
| 1988 | - | 195.2 ± 22.7 | 128.5 ± 22.5 |
| 1989 | - | 17.9 ± 5.4 | - |
| 1990 | - | - | 26.8 ± 15.3 |
| Scales | 1987 | - | - | 529.6 ± 147.7 |
| 1988 | - | - | 304.1 ± 65.8 |
| 1990 | - | 46.3 ± 15.2 | - |
| Internal Organs | 1987 | - | 82.1 ± 2.1 | 378.8 ± 75.9 |
| 1988 | - | 48.2 ± 10.8 | - |
| 1989 | - | 67.2 ± 18.4 | - |
| 1990 | - | 20.4 ± 4.7 | - |
| Muscles | 1987 | 58.2 ± 19.8 | - | 390.6 ± 82.9 |
| 1989 | - | 17.5 ± 8.9 | - |
| 1994 | - | 15.8 ± 4.5 | - |
5. Accumulation of 54Mn, 60Co, and 65Zn in Fish After the Chornobyl Accident
Investigating the accumulation and redistribution of radioactive isotopes such as manganese, cobalt, and zinc in fish persisted following the Chornobyl nuclear accident. The results of the studies [
25,
26] asserted that the belonging of fish to a certain trophic level significantly affects the concentration of isotopes
54Mn,
60Co, and
65Zn in them. These findings are consistent with experimental results obtained in the Chornobyl exclusion zone (
Table 1,
Table 2,
Table 3 and
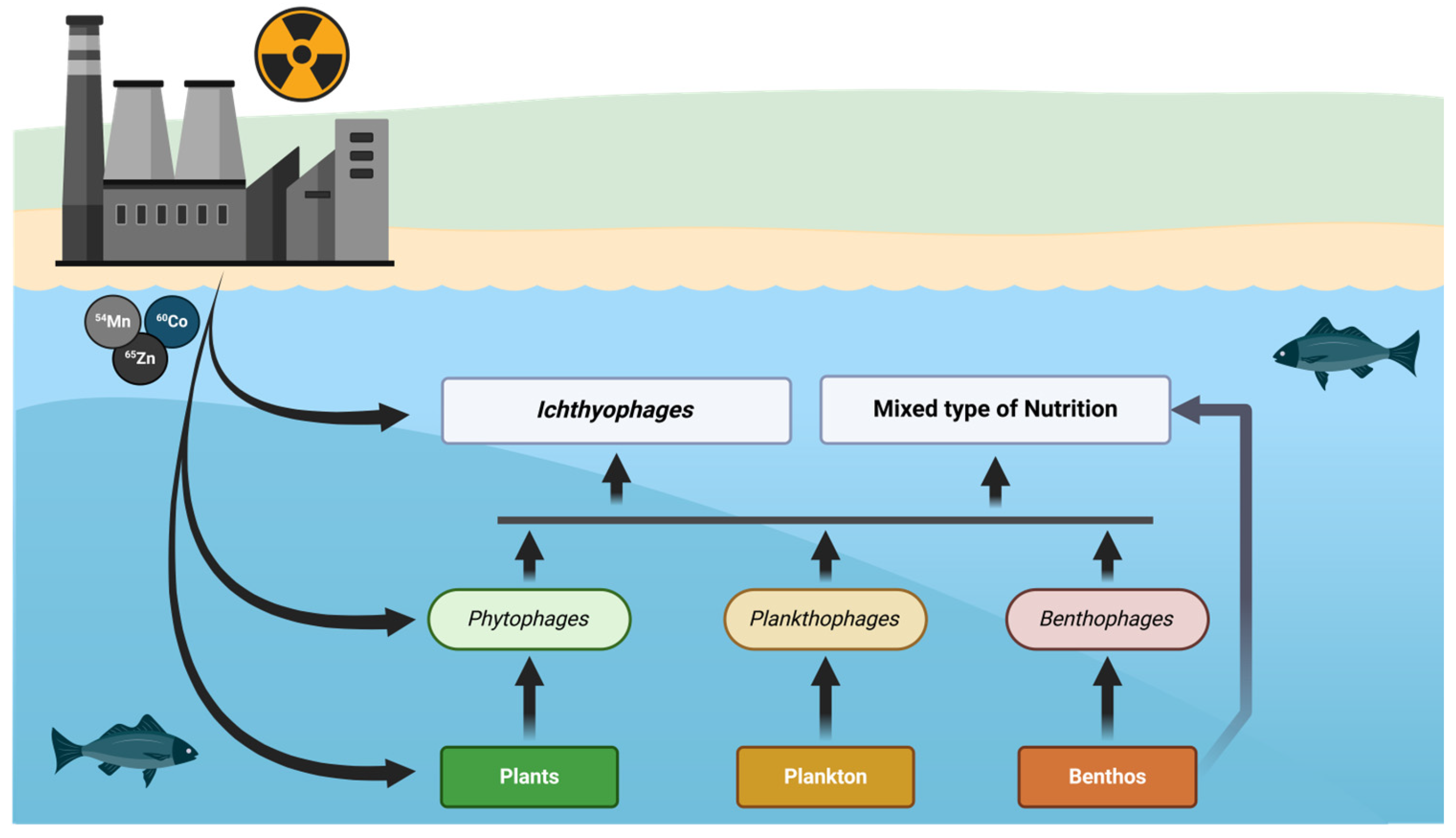
Table 4), which showed that concentrations of these radionuclides decrease with higher trophic levels (trophic transfer coefficients, TTF < 1). The scheme of
54Mn,
60Co, and
65Zn intake is shown in
Figure 4.
Radionuclides enter fish organisms through two main pathways: direct uptake from water and indirect intake via food sources (plankton, benthos, plants, and other fish). The efficiency and dominance of these pathways vary depending on the trophic level of the fish.
Radionuclides dissolved in water can be absorbed through the gills, skin, and gastrointestinal tract of fish. This pathway is particularly significant for cobalt (
60Co), as direct absorption from water is a key source of its accumulation [
11]. The gills, which have a large surface area and thin epithelial layers, serve as a primary site for the uptake of dissolved metals, including radionuclides.
Studies indicate that fish in contaminated waters exhibit high radioactivity levels in their gills and intestines, confirming the importance of waterborne radionuclide absorption [
16]. However, the extent of waterborne uptake varies among species and environmental conditions.
Food intake is the predominant pathway for the accumulation of
54Mn and
65Zn, while
60Co is absorbed both through food and directly from water [
10]. Different fish species, depending on their trophic level, exhibit variations in the efficiency of assimilation and accumulation of these radionuclides.
Fish are classified into different trophic levels based on their diet, which influences the pathways and magnitude of radionuclide accumulation.
Phytophage fish primarily consume aquatic plants, which can accumulate radionuclides from water and sediments.
65Zn tends to be more concentrated in plants compared to
54Mn and
60Co, making it the most prevalent radionuclide in this group [
3]. These fish accumulate radionuclides mainly through digestion, with the highest levels found in digestive organs and liver.
Planktophage acquire radionuclides by consuming plankton, which rapidly absorb contaminants from water.
54Mn and
65Zn are more effectively transferred through this pathway due to their biological functions in metabolic processes [
27]. These elements tend to accumulate in the gills and bones, whereas
60Co is less prevalent in this group since it does not strongly bind to plankton.
Benthophage fish consume benthic organisms, such as detritus feeders, mollusks, and invertebrates that live in contaminated sediments. This feeding behavior makes them particularly susceptible to
60Co and
65Zn accumulation, as these radionuclides bind strongly to sediment particles [
27]. They accumulate mainly in the liver, kidneys, and bones.
54Mn concentrations tend to be lower in benthophages compared to planktophages due to its preferential uptake by plankton [
11].
Fish with mixed feeding consume different types of animal food (benthic organisms and fish), allowing them to accumulate radionuclides from multiple sources. The relative levels of
54Mn,
60Co, and
65Zn in these fish depend on their predominant food source. Those with a higher intake of benthic organisms show increased
60Co accumulation [
27].
Ichthyophage fish, which feed on other fish, accumulate radionuclides primarily through trophic transfer. Unlike lower trophic levels, where direct waterborne uptake plays a larger role, predatory fish primarily accumulate
60Co,
65Zn, and
54Mn via their diet. However, trophic transfer coefficients (TTF) for these radionuclides are often <1, meaning that concentrations decrease as they move up the food chain [
3]. Consequently, predatory fish exhibit lower overall radionuclide levels than their prey, particularly for
54Mn, which is not efficiently biomagnified.
Because 54Mn, 60Co, and 65Zn have nearly disappeared from the environment due to their relatively short half-lives, many studies on these radionuclides have been conducted in laboratory settings since 1986. It should be noted that the Fukushima Dai-ichi nuclear accident in Japan in 2011 did not result in the release of these isotopes into the environment. Therefore, studies of the accumulation of radioactive isotopes of manganese, cobalt, and zinc by fish in natural conditions were not possible after this accident.
Recent decades of research largely confirm previously obtained data. Laboratory experiments with
Oncorhynchus mykiss (Walbaum, 1792) have demonstrated that different organs accumulate the radionuclide
54Mn at varying levels. The skin, gills, kidneys, liver, and intestines are the primary entry sites, while bones, the head, brain, and fins are receptor and storage sites. However, unlike the findings from other studies, this research identifies muscles as a significant tissue for storing radioactive manganese [
28].
Other studies have also suggested that muscles are a storage site for
54Mn,
60Co, and
65Zn in fish [
29]. Experiments on the distribution of these radionuclides, accumulated from seawater by
S. canicula,
Raja undulata (Lacepède, 1802),
Torpedo marmorata (Risso, 1810),
Scophthalmus maximus (Linnaeus, 1758),
Sparus aurata (Linnaeus, 1758), and
Dicentrarchus labrax (Linnaeus, 1758), revealed their heterogeneous distribution within the fish body. The kidneys were a secondary storage site for radionuclides for all these species, while the head, muscles, and skin were identified as the main sites of accumulation.
The accumulation of
60Co in different organs of
O. mykiss under laboratory conditions was also found to be uneven. The highest concentrations of this radionuclide were found in the gills, internal organs (swim bladder, heart, and spleen), and kidneys. By the end of the experiment, the kidneys had the highest concentrations of radio cobalt, followed by internal organs, the head, gills, and liver. The lowest concentration of
60Co was found in the muscles [
30], contrasting with previous findings.
Researchers have no consensus regarding the primary organ that stores
54Mn,
60Co, and
65Zn in fish. For example, Jeffree R.A. and co-workers [
31] suggest that the skin is the main reservoir for these radionuclides based on laboratory studies with
Scyliorhinus canicula (Linnaeus, 1758). In a laboratory study on
C. carpio, the highest concentrations of
65Zn were found in the gills, kidneys, internal organs, and eyes, as noted in [
32].
However, Jeng S.-S. and Lian J.-L. [
33] report that in laboratory studies on four fish species, neither the kidneys nor gills had as high specific radioactivity for
65Zn as the digestive tract, regardless of whether the zinc was ingested through food or water. Different fish species absorbed varying amounts of zinc from their diet, with the order of absorption being
C. carpio >
Oreochromis niloticus x
O. aureus >
Ctenopharyngodon idellus (Valenciennes, 1844) >
H. molitrix when adequate zinc concentrations were present in the diet and the water was uncontaminated.
The findings in [
34] confirm the results obtained in earlier studies of
54Mn,
60Co, and
65Zn accumulation by fish. Under laboratory conditions, the primary route of intake of these radionuclides into
Psetta maxima (Linnaeus, 1758) and
Scyliorhinus canicula (Linnaeus, 1758) was through food. Species differences were observed, particularly in the uptake of Co, which the authors attribute to differences in diet and physiological and ecological conditions.
Previously noted differences in the accumulation of manganese, cobalt, and zinc radioisotopes by different fish species are discussed in [
35]. As in the previous research, the studies were conducted with
P. maxima and
S. canicula. Radionuclides accumulated faster in
S. canicula than in
P. maxima. In
S. canicula, the highest levels of
54Mn were found in the kidneys, while the highest levels of
65Zn were in the digestive tract, although they also remained high in other organs and tissues. In
P. maxima, the highest concentrations of
65Zn and the second-highest concentrations of
54Mn and
57Co were found in the kidneys.
The absorption efficiency in juvenile
Menidia menidia (Linnaeus, 1758) fed copepods (
Acartia spp.) contaminated with radioactive cobalt and zinc isotopes under laboratory conditions was low, with
57Co at just 2% and
65Zn slightly higher at 6% [
36]. The authors suggest that the behavior of radiopharmaceuticals in fish fed artificial diets may not reflect their behavior in natural systems.
A significant result of studies on manganese, cobalt, and zinc accumulation in fish is that the concentrations of stable and radioactive elements differ within the fish. For example, the study described in [
6] indicates that
54Mn accumulates selectively compared to stable Mn. Mollusks of the
Pectinidae spp. also selectively accumulate
54Mn. Laboratory studies confirm field data on the differences in the accumulation of stable and radioactive isotopes of Zn and Mn by fish [
11].
Despite the significant differences in the concentrations of stable and radioactive isotopes of the same element in fish, as reported in various studies, the authors decided to present findings on the accumulation of stable manganese, cobalt, and zinc in fish.
Similar to the accumulation of radioactive isotopes of manganese, cobalt, and zinc, variations in the accumulation of their stable isotopes (Mn, Co, and Zn) are often associated with the trophic levels of aquatic organisms [
37]. Research presented in [
38] discusses the impact of the trophic level of marine animals on the accumulation of various elements. Studies conducted in Baffin Bay (Canada) revealed that Co and Mn do not exhibit biomagnification at each level of the ecological pyramid within the food chain of this ecosystem. However, stable Zn was found to biomagnify, increasing concentration with higher trophic levels.
Investigations carried out in the Olifants River, Mpumalanga (South Africa) with fish species
Oreochromis mossambicus (Peters, 1852) and
Clarias gariepinus (Burchell, 1822) demonstrated that muscle tissue consistently contained significantly lower levels of stable Zn than other tissues and organs, corroborating findings from studies on the accumulation of radioactive metal isotopes in fish. Gills and liver exhibited relatively high concentrations of Zn, with somewhat lower levels in the skin. The primary reason for species-specific differences in Zn bioaccumulation was attributed to variations in the dietary habits and behavior of the studied fish species [
4].
Canli M. and Atli G. [
39] reported that the average concentration of stable Zn in the muscles, gills, and liver of six fish species (
Sparus auratus (Linnaeus, 1758),
Atherina hepsetus (Linnaeus, 1758),
Mugil cephalus (Linnaeus, 1758),
Trigla cuculus (Linnaeus, 1758),
Sardina pilchardus (Walbaum, 1792), and
Scomberesox saurus (Walbaum, 1792)) from the northeast Mediterranean Sea, Karatas (Turkey), varied significantly. The authors suggested that these differences are due to ecological needs, swimming behavior, and metabolic activity among the different species. Zinc concentrations were highest in the liver and gills and lowest in the muscles of all studied fish species.
The relationship between the content of stable Zn and Mn isotopes in fish of different species and their diet was investigated by Malik and Maurya [
40]. Benthic fish (
Heteropneustes fossilis (Bloch, 1794)) in the Kali River (India) exhibited higher levels of concentration of zinc and manganese compared to surface-feeding fish (
Pethia ticto (Hamilton, 1822)). Seasonal variations in the accumulation of these metals were also observed, with minimal zinc and manganese accumulation during the rainy season and maximum levels in the summer.
The influence of species, sampling location, and season on the concentration of stable zinc in seven sampling stations distributed along the Ria de Aveiro (Portugal) in fish muscles is highlighted in [
41]. However, contrasting results were presented in [
42], where zinc levels in the muscles of two catfish species (
Channa punctata (Bloch, 1793) and
Aorichthys aor (Hamilton, 1822)) from the Ganges River at Allahabad (India) were found to be independent of the season. That study also noted that zinc concentrations in fish muscles were higher than the content of other heavy metals.
In
Pterois spp. fish caught in the Atlantic Ocean near the island of Cuba, the highest concentrations of stable Mn and Zn were found in the liver, compared to their levels in the kidneys and muscles. The highest levels of stable Co were measured in the kidneys, which the authors attributed to this organ’s excretory function. Although the level of Co in the kidneys was low, it was an order of magnitude higher than in the liver and muscles [
43].
In three fish species (
Solea vulgaris (Linnaeus, 1758),
Anguilla anguilla (Linnaeus, 1758), and
Liza aurata (Risso, 1810)) from water bodies in the Odiel estuary and two in the Bay of Cádiz on the southwestern Atlantic coast of Spain, significant levels of stable zinc were found in the liver. At the same time, manganese concentrations were minimal [
44]. The authors noted that Mn concentrations were higher in muscles than in the liver; however, this ratio varied among different fish species. This finding of higher manganese content in fish muscles contradicts numerous reports that this chemical element is minimally present in muscles.
Stable isotopes of Mn and Zn were measured in the fish
Dicentrarchus labrax (Linnaeus, 1758),
Liza ramada (Risso, 1827), and
A. anguilla from Lake Bafa (Turkey). The concentrations of these elements did not exceed 50 mg/kg for zinc [
45].
In laboratory studies of
Morone saxatilis (Walbaum, 1792), it was found that Zn distribution varied significantly within different organs of the fish. The element was primarily associated with the head (55–63%), with only 4% of Zn detected in the intestines [
46].
The ability of fish to regulate the levels of certain essential elements within their organisms is discussed in [
47]. Reinfelder et al. suggest that the concentrations of most trace elements in fish tissues are regulated through a feedback mechanism. According to the authors, the mechanism by which fish regulate the accumulation of trace elements likely involves concentration-dependent changes in intestinal absorption or adaptive modifications in excretory pathways. The degree of regulation may also vary across different tissues. This implies that the increase in element content within tissues is not necessarily proportional to its concentration in the diet. Observations on the accumulation of stable zinc in
O. mykiss during laboratory studies revealed that Zn levels in the blood plasma of this species were regulated within a narrow range. It was also found that Zn concentrations in muscle tissue remained relatively constant, regardless of its concentration in food and water.
Research on the accumulation of stable zinc in
Lutjanus argentimaculatus (Forsskål, 1775) under laboratory conditions indicates the existence of regulation mechanisms for Zn levels within fish [
48]. Dietary intake is crucial for maintaining metal levels in various fish tissues, though the authors stress that the absorption of metals from water cannot be ignored.
Experiments on the accumulation of stable zinc in two different fish species (
Colossoma macropomum (Cuvier, 1818) and
Trichogaster trichopterus var.
Marble (Pallas, 1770)) revealed that Zn concentrations in the fish were higher than in the water [
49]. This study also emphasized that different marine biota species have varying capacities for Zn accumulation.
6. Summary
The bioaccumulation of manganese (54Mn), cobalt (60Co), and zinc (65Zn) in different fish species and their redistribution within the food chain may contribute to long-term ecological disruptions. Changes in fish physiology, reproductive system, and survival rates could alter population dynamics, potentially affecting entire aquatic ecosystems. Understanding the accumulation of these radionuclides in fish is crucial for assessing their potential risks to aquatic organisms and human consumers.
6.1. Key Findings and Their Implications
Variability in Accumulation Across Organs and Tissues: The accumulation of 54Mn, 60Co, and 65Zn varies significantly among different fish organs and tissues. External organs such as gills and skin serve as primary entry points, while internal organs, particularly the kidneys and liver, often exhibit the highest concentrations of radionuclides. These differences are influenced by species-specific physiological and ecological factors.
Pathways of 54Mn, 60Co, and 65Zn Entry: Food intake is the primary pathway for radionuclide accumulation in fish, although direct absorption from water is also a contributing factor. This finding underscores the importance of studying trophic transfer processes and biomagnification potential within aquatic food chain.
Influence of Trophic Levels: The concentration of radionuclides generally decreases with increasing trophic levels, suggesting a dilution effect in the food chain. While higher trophic-level fish exhibit lower concentrations, potential risks to human consumers remain, particularly from species that show high bioaccumulation tendencies.
Radionuclide Concentrations in Muscle Tissue: Muscle tissue, the primary component consumed by humans, consistently exhibits the lowest radionuclide concentrations. Nevertheless, continued monitoring is essential to evaluate the long-term health risks associated with consuming fish from contaminated environments.
Potential for Physiological Regulation: Some studies indicate that fish have regulatory mechanisms to control concentrations of essential elements, including both stable and radioactive isotopes of manganese, cobalt, and zinc. Understanding these mechanisms could enhance predictive models for radionuclide distribution in aquatic organisms.
6.2. Limitations and Challenges
While significant progress has been made in understanding radionuclide accumulation in fish, several challenges complicate data interpretation and comparison:
Variability in Experimental Conditions: Differences in water chemistry, temperature, sediment composition, and ecological interactions across studies make direct comparisons challenging. Variations in experimental setups, such as exposure durations, radionuclide concentrations, and species selection, further contribute to inconsistencies in findings.
Challenges in Comparing Historical and Recent Data: Older studies often relied on different detection methods, making it difficult to directly compare historical and contemporary radionuclide concentrations. Advances in analytical techniques have improved sensitivity and accuracy, but discrepancies between past and present data persist.
Potential Biases in Literature: The reviewed studies exhibit variations in sample sizes, species selection, and data reporting methodologies. Additionally, studies where these radionuclides were not detected or detected at low concentrations, especially before the Chornobyl accident, were likely conducted during radioecological monitoring but never publicly reported. This could influence the perceived magnitude of radionuclide accumulation trends.
Knowledge Gaps in Long-Term Ecological Effects: Despite extensive research, the long-term ecological effects of short-lived radionuclides on fish health, reproductive system, and population dynamics remain poorly understood. Further investigation is needed to determine the potential chronic impacts of radionuclide exposure on aquatic ecosystems.
6.3. Future Research Directions
Given the remaining knowledge gaps, future research should focus on:
Trophic Transfer and Biomagnification Studies: Investigating how 54Mn, 60Co, and 65Zn transfer through aquatic food chain and assessing their biomagnification potential.
Long-Term Monitoring Programs: Establishing comprehensive, standardized monitoring programs to track changes in radionuclide concentrations over time and across different environmental conditions. Longitudinal studies assessing radionuclide concentrations in fish tissues and sediments can help determine trends and predict potential long-term consequences. Advanced bioindicators, such as species with higher accumulation tendencies, could be utilized for early detection of contamination events. Additionally, implementing standardized methodologies for sampling and analysis will improve data comparability across different regions and timeframes.
Multi-Stressor Assessments: Evaluating the combined effects of radionuclide exposure alongside other environmental stressors, such as pollution, climate change, and habitat degradation.
Improved Policy and Management Strategies: Strengthening regulations on radioactive discharge into aquatic environments and fostering collaboration between environmental scientists, policymakers, and fishery managers to mitigate contamination risks. The insights gained from this study could inform regulatory policies for environmental and public health protection. Authorities should establish permissible levels of these radionuclides in commercially available fish to mitigate human exposure risks. Strengthening regulations regarding radioactive waste disposal and nuclear facility management is crucial for preventing further contamination. Education and public awareness campaigns can also help communities make informed decisions regarding fish consumption in affected areas.
By expanding discussions on ecological consequences, health risks, and policy interventions, this research can significantly enhance its impact, contributing to more effective environmental protection and public safety measures.