Effects of Water Temperature, Light Intensities and Photoperiod on the Survival and Growth of Juvenile Schizothorax irregularis and Diptychus maculates
Abstract
1. Introduction
2. Materials and Methods
2.1. General System Design
2.2. Experiment 1: Water Temperature
2.3. Experiment 2: Photoperiod
2.4. Experiment 3: Light Intensity
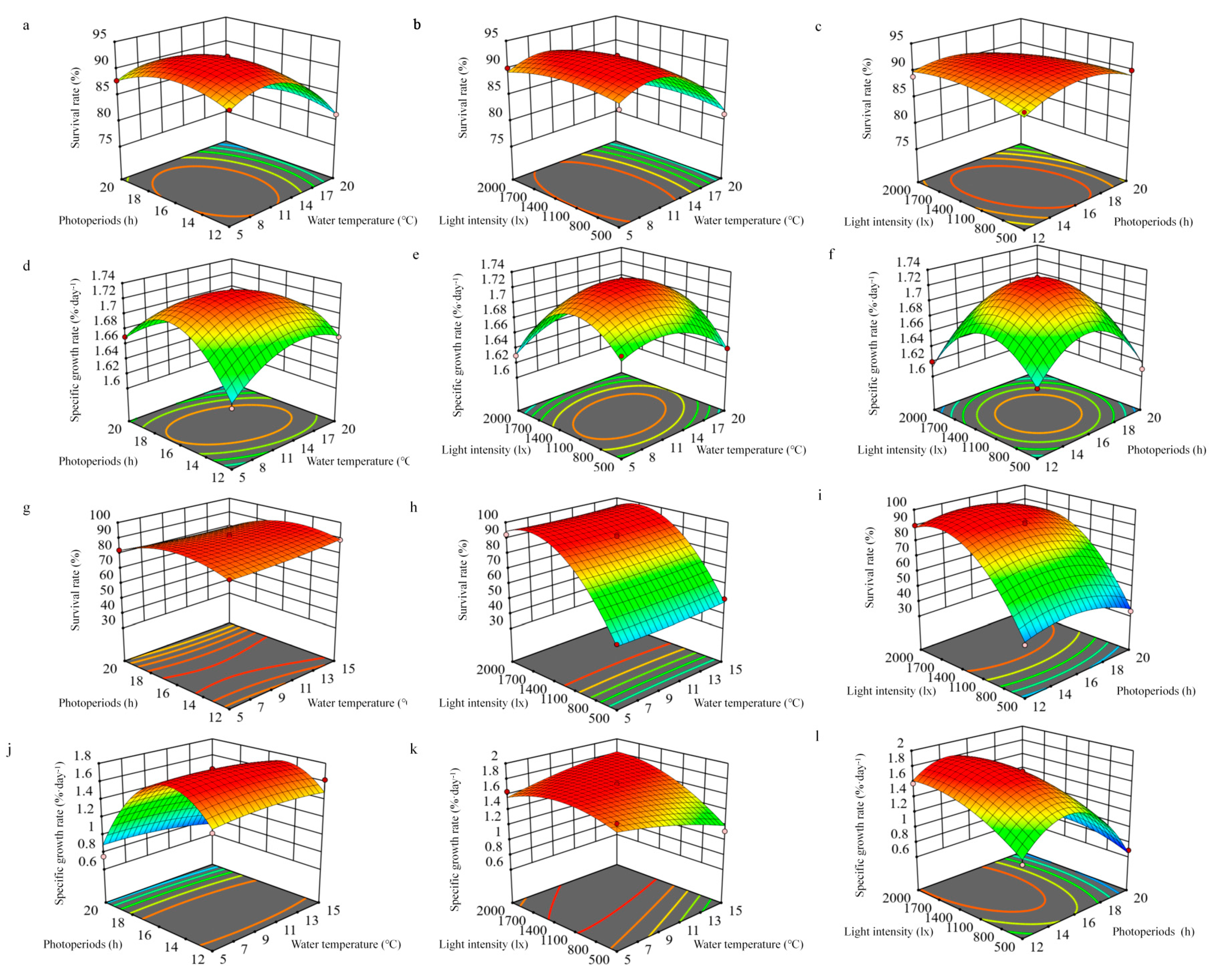
2.5. Experiment 4: Combined Effects of Water Temperature, Photoperiod, and Light Intensity
2.6. Data Collection
3. Results
3.1. Experiment 1: Water Temperature, Photoperiod, and Light Intensity
3.2. Experiment 2: Photoperiod
3.3. Experiment 3: Light Intensity
3.4. Experiment 4: Combined Effects of Water Temperature, Photoperiod, and Light Intensity
4. Discussion
4.1. Water Temperature
4.2. Photoperiod
4.3. Light Intensity
4.4. The Synergistic Effect of Temperature, Photoperiod, and Light Intensity
- (1)
- Limitations of the experimental period: The research period of this study was set to 80 days, which may not be sufficient for the study of long-term growth and development processes. Fishes may respond differently to environmental factors at different growth stages. A relatively short experimental period may not be able to fully capture these changes, and key information may be overlooked, thus affecting the accurate judgment of their growth and survival laws.
- (2)
- Simplification of experimental conditions: In the experiment, only the main environmental factors such as water temperature, photoperiod, and light intensity were controlled. However, the actual natural environment is extremely complex and contains the interactions of multiple environmental factors, such as water flow velocity, dynamic changes in dissolved oxygen in the water, the content of various minerals and trace elements in the water, and the water’s microbial community. Since these factors were not considered in the study, there may be a deviation between the experimental results and the actual situation in the natural environment.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Familia | Species | Optimal Survival Temperature | Optimal Growth Temperature |
|---|---|---|---|
| Cyprinidae | Schizothorax biddulphi [24] | 5–25 °C | 15 °C |
| Schizothorax lissolabiatus [22] | 7–26 °C | - | |
| Schizothorax prenanti [23] | 5–27 °C | - | |
| Schizothorax oconnori [12] | 15.15–17.24 °C | - | |
| Bothidae | Pseudopleuronectes yokohamae [11] | - | 20 °C |
| Clupeidae | Sillago robusta [20] | 25–28 °C | - |
| Mugilidae | Chelon labrosus [21] | - | 22 °C |
Appendix B
| Group | Coded Value | SR (%) | SGR (% Day−1) | ||
|---|---|---|---|---|---|
| W (°C) | I (h) | L (lx) | |||
| Schizothorax irregularis | |||||
| 1 | 0(12.5) | 0(16) | 0(1250) | 92.22 | 1.73 |
| 2 | 1(20) | 0(16) | 1(2000) | 82.22 | 1.64 |
| 3 | 0(12.5) | 1(20) | 1(2000) | 85.56 | 1.61 |
| 4 | 0(12.5) | 1(20) | −1(500) | 88.89 | 1.61 |
| 5 | 1(20) | −1(12) | 0(1250) | 81.11 | 1.67 |
| 6 | 0(12.5) | 0(16) | 0(1250) | 92.22 | 1.73 |
| 7 | −1(5) | 1(20) | 0(1250) | 87.78 | 1.67 |
| 8 | −1(5) | 0(16) | 1(2000) | 90.00 | 1.63 |
| 9 | −1(5) | −1(12) | 0(1250) | 88.89 | 1.63 |
| 10 | 1(20) | 0(16) | −1(500) | 81.11 | 1.64 |
| 11 | 0(12.5) | −1(12) | 1(2000) | 88.89 | 1.62 |
| 12 | 1(20) | 1(20) | 0(1250) | 78.89 | 1.62 |
| 13 | 0(12.5) | 0(16) | 0(1250) | 92.22 | 1.73 |
| 14 | 0(12.5) | 0(16) | 0(1250) | 92.22 | 1.73 |
| 15 | 0(12.5) | 0(16) | 0(1250) | 92.22 | 1.73 |
| 16 | 0(12.5) | −1(12) | −1(500) | 88.89 | 1.64 |
| 17 | −1(5) | 0(16) | −1(500) | 88.89 | 1.68 |
| Diptychus maculates | |||||
| 1 | 0(10) | 0(16) | 0(1250) | 92.22 | 1.74 |
| 2 | 1(15) | −1(12) | 0(1250) | 88.89 | 1.62 |
| 3 | 1(15) | 1(20) | 0(1250) | 78.89 | 0.78 |
| 4 | 1(15) | 0(16) | −1(500) | 50.00 | 1.12 |
| 5 | 0(10) | 0(16) | 0(1250) | 91.11 | 1.74 |
| 6 | 0(10) | −1(12) | −1(500) | 40.00 | 1.02 |
| 7 | 1(15) | 0(16) | 1(2000) | 93.33 | 1.72 |
| 8 | −1(5) | −1(12) | 0(1250) | 85.56 | 1.43 |
| 9 | 0(10) | 0(16) | 0(1250) | 91.11 | 1.73 |
| 10 | −1(5) | 0(16) | 1(2000) | 92.22 | 1.64 |
| 11 | 0(10) | 0(16) | 0(1250) | 90.00 | 1.73 |
| 12 | −1(5) | 0(16) | −1(500) | 47.78 | 1.68 |
| 13 | 0(10) | −1(12) | 1(2000) | 90.00 | 1.58 |
| 14 | −1(5) | 1(20) | 0(1250) | 82.22 | 0.75 |
| 15 | 0(10) | 1(20) | 1(2000) | 84.44 | 0.81 |
| 16 | 0(10) | 1(20) | −1(500) | 3.33 | 0.69 |
| 17 | 0(10) | 0(16) | 0(1250) | 90.00 | 1.74 |
References
- Miller, T.J.; Crowder, L.B.; Rice, J.A.; Marschall, E.A. Larval size and recruitment mechanisms in fishes: Toward a conceptual framework. Can. J. Fish. Aquat. Sci. 1988, 45, 1657–1670. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, L.; Zhou, Y.; Wang, C.; Lv, W.; Li, L.; He, S. Hb adaptation to hypoxia in high-altitude fishes: Fresh evidence from schizothoracinae fishes in the Qinghai-Tibetan Plateau. Int. J. Biol. Macromol. 2021, 185, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.W.; Guo, Y.; Zhang, R.M. Fauna composition and distribution of aboriginal fish in the Tarim River of Xinjiang Uygur Autonomous Region. J. Fish. China 2009, 33, 949–956. [Google Scholar]
- Han, J.J.; Chen, P.; Qi, F. Situations of Fish Stocks in Weigan River in Xinjiang. Fish. Sci. 2022, 41, 92–101. [Google Scholar]
- Guo, Y. Xinjiang Fish Journal; Xinjiang Science and Technology Press: Urumqi, China, 2012; pp. 118–127. [Google Scholar]
- Li, G.G.; Feng, C.G.; Tang, T.Y. Survey of native fish resources in inland river system in Xinjiang. J. Gansu Agric. Univ. 2017, 52, 22–27. [Google Scholar]
- Arken, K.; Hao, C.-L.; Guo, A.-M.; Zhang, W.-R.; Rong, M.-J.; Kamal, W.; Tian, S.-L.; Kadir, M.; Yue, C. A new species of Paradiplozoon (Monogenea: Diplozoidae), a gill parasite of the Schizothorax fish (Cyprinidae: Schizothoracinae) from the Yarkand River, Xinjiang, China. Acta Parasitol. 2022, 67, 330–339. [Google Scholar] [CrossRef]
- Yanagitsuru, Y.R.; Main, M.A.; Lewis, L.S.; Hobbs, J.A.; Hung, T.-C.; Connon, R.E.; Fangue, N.A. Effects of temperature on hatching and growth performance of embryos and yolk-sac larvae of a threatened estuarine fish: Longfin smelt (Spirinchus thaleichthys). Aquaculture 2021, 537, 736502. [Google Scholar] [CrossRef]
- Yesser, A.; Al-Faiz, N.; Al-Qatrani, L.; Ali, A. Effects of Temperature and Salinity on the Growth Performance and Survival of Blue Tilapia Oreochromis Aureus (Steindachner, 1864). Basic Appl. Sci.-Sci. J. King Faisal Univ. 2021, 22, 11699. [Google Scholar] [CrossRef]
- Kusakabe, K.; Hata, M.; Shoji, J.; Hori, M.; Tomiyama, T. Effects of water temperature on feeding and growth of juvenile marbled flounder Pseudopleuronectes yokohamae under laboratory conditions: Evaluation by group-and individual-based methods. Fish. Sci. 2017, 83, 215–219. [Google Scholar] [CrossRef]
- Fonds, M.; Cronie, R.; Vethaak, A.D.; Van der Puyl, P. Metabolism, food consumption and growth of plaice (Pleuronectes platessa) and flounder (Platichthys flesus) in relation to fish size and temperature. Neth. J. Sea Res. 1992, 29, 127–143. [Google Scholar] [CrossRef]
- Zeng, B.H.; Zhou, J.S.; Wang, W.L. Effects of water temperature on mortality, feeding and growth of Schizothorax o'connori. Freshw. Fish. 2018, 48, 77–82. [Google Scholar]
- Villamizar, N.; Blanco-Vives, B.; Migaud, H.; Davie, A.; Carboni, S.; Sanchez-Vazquez, F.J. Effects of light during early larval development of some aquacultured teleosts: A review. Aquaculture 2011, 315, 86–94. [Google Scholar] [CrossRef]
- Han, J.; Wang, M.L.; Cao, S.N. Effects of light color, light intensity and photoperiod on growth and feeding of juvenile turbot (Scophthalmus maximus). J. Dalian Ocean Univ. 2023, 38, 787–794. [Google Scholar]
- Ma, Z.; Zhang, J.; Zhang, X. Effects of temperature and photoperiod on growth, physiological, and behavioral performance in steelhead trout (Oncorhynchus mykiss) under indoor aquaculture condition. Front. Mar. Sci. 2023, 10, 1114662. [Google Scholar] [CrossRef]
- Cuvier-Péres, A.; Jourdan, S.; Fontaine, P.; Kestemont, P. Effects of light intensity on animal husbandry and digestive enzyme activities in sea bass Dicentrachus labrax post-larvae. Aquaculture 2001, 202, 317–328. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Fan, K. Effects of illumination intensities on growth, digestive and metabolic enzyme activities and antioxidant capacities of juvenile Takifugu rubripes. Aquaculture 2022, 548, 737594. [Google Scholar] [CrossRef]
- Lohne, P.; Imsland, A.K.; Larsen, S.; Foss, A.; Pittman, K. Interactive effect of photoperiod and temperature on the growth rates, muscle growth and feed intake in juvenile Atlantic halibut. Aquac. Res. 2012, 43, 187–197. [Google Scholar] [CrossRef]
- Chatterjee, N.; Pal, A.K.; Manush, S.M.; Das, T.; Mukherjee, S.C. Thermal tolerance and oxygen consumption of Labeo rohita and Cyprinus carpio early fingerlings acclimated to three different temperatures. J. Therm. Biol. 2004, 29, 265–270. [Google Scholar] [CrossRef]
- Tan, M.; Hall, K.C.; Litchfield, S. Water temperature affects somatic growth, body condition and oxygen and carbon otolith isotopes of stout whiting (Sillago robusta). Sci. Total Environ. 2024, 945, 174058. [Google Scholar] [CrossRef]
- Sanz-Latorre, M.; Soto, M.; Izagirre, U.; Lekube, X. Effects of water temperature on growth, health and digestive processes of the thicklip grey mullet Chelon labrosus. Aquaculture 2025, 595, 741537. [Google Scholar] [CrossRef]
- Jin, F.P.; Li, G.H.; Gao, H.T. Tolerance Test of Juvenile Schizothorax lissolabiatus to Temperature, Salinity and pH. Fish. Sci. Technol. Inf. 2016, 43, 303–307. [Google Scholar]
- Wu, Q.; Cai, L.M.; Lu, J.P. Studies on the Tolerence Ability of Young Schizothorax prenanti to Variance of the Water Temperature and Dissolved Oxygen. J. Sichuan Inst. Anim. Husb. Vet. Med. 2001, 3, 20–22. [Google Scholar]
- Zhao, N.H.; Zhao, H.; Qiang, Z. Effects of water temperature, photoperiod and light intensity on survival, feeding and growth of Schizothorax biddulphi juveniles and their tolerance of salinity and alkalinity. South China Fish. Sci. 2021, 17, 54–63. [Google Scholar]
- Pereira-Davison, E.; Callan, C.K. Effects of photoperiod, light intensity, turbidity and prey density on feed incidence and survival in first feeding yellow tang (Zebrasoma flavescens)(Bennett). Aquac. Res. 2017, 49, 890–899. [Google Scholar] [CrossRef]
- Ma, H.; Wei, P.; Li, X.; Liu, S.; Tian, Y.; Zhang, Q.; Liu, Y. Effects of photoperiod on growth, digestive, metabolic and non-special immunity enzymes of Takifugu rubripes larvae. Aquaculture 2021, 542, 736–840. [Google Scholar] [CrossRef]
- Andrade, C.A.P.; Brazão, I.P.G.; Nogueira, N.; Ferreira, M.P.; Dillinger, T.; Dinis, M.T.; Narciso, L. Red porgy (Pagrus pagrus) larval feeding performance and behavior at the onset of exogenous feeding. J. Exp. Mar. Biol. Ecol. 2011, 407, 377–381. [Google Scholar] [CrossRef]
- Downing, G.; Litvak, M.K. The effect of photoperiod, tank colour and light intensity on growth of larval haddock. Aquac. Int. 1999, 7, 369–382. [Google Scholar] [CrossRef]
- Fielder, D.S.; Bardsley, W.J.; Allan, G.L.; Pankhurst, P.M. Effect of photoperiod on growth and survival of snapper Pagrus auratus larvae. Aquaculture 2002, 211, 135–150. [Google Scholar] [CrossRef]
- Martinez-Cardenas, L.; Purser, G.J. Effect of stocking density and photoperiod on growth and survival in cultured early juvenile pot-bellied seahorses Hippocampus abdominalis Lesson, 1827. Aquac. Res. 2012, 43, 1536–1549. [Google Scholar] [CrossRef]
- Dharma, T.S.; Setiadi, A.; Hutapea, J.H. The nursery of yellowfin tuna seeds (Thunnus albacares) by increasing light intensity in the rear control system. Earth Environ. Sci. 2023, 1137, 012026. [Google Scholar] [CrossRef]
- Di, Z.; Li, K.; Li, T. Effects of light intensity and photoperiod on the growth performance of juvenile Murray cods (Maccullochella peelii) in recirculating aquaculture system (RAS). Aquac. Fish. 2023, 8, 274–279. [Google Scholar] [CrossRef]
- Yoseda, K.; Yamamoto, K.; Asami, K.; Chimura, M.; Hashimoto, K.; Kosaka, S. Influence of light intensity on feeding, growth, and early survival of leopard coral grouper (Plectropomus leopardus) larvae under mass-scale rearing conditions. Aquaculture 2008, 279, 55–62. [Google Scholar] [CrossRef]
- Degidio, J.-M.L. Assessment of the Milletseed Butterflyfish, Chaetodon miliaris, as a Model Species for Marine Ornamental Aquaculture and an Evaluation of Early Culture Parameters. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2014. [Google Scholar]
- Copeland, K.A.; Watanabe, W.O. Light intensity effects on early life stages of black sea bass, Centropristis striata (Linnaeus 1758). Aquac. Res. 2006, 37, 1458–1463. [Google Scholar] [CrossRef]




| Term | Coefficient | d.f. | SE | 95% CI | |
|---|---|---|---|---|---|
| Low | High | ||||
| Schizothorax irregularis | |||||
| Intercept | 92.22 | 1 | 0.4309 | 91.2 | 93.24 |
| W | −4.03 | 1 | 0.3406 | −4.83 | −3.22 |
| I | −0.7775 | 1 | 0.3406 | −1.58 | 0.0279 |
| L | −0.3338 | 1 | 0.3406 | −1.14 | 0.4717 |
| WI | −0.2775 | 1 | 0.4817 | −1.42 | 0.8616 |
| WL | 0 | 1 | 0.4817 | −1.14 | 1.14 |
| IL | −1.22 | 1 | 0.4817 | −2.36 | −0.0834 |
| W2 | −5.33 | 1 | 0.4695 | −6.44 | −4.22 |
| I2 | −2.72 | 1 | 0.4695 | −3.83 | −1.61 |
| L2 | −1.33 | 1 | 0.4695 | −2.44 | −0.2223 |
| Diptychus maculates | |||||
| Intercept | 90.89 | 1 | 0.6974 | −1.23 | 2.07 |
| W | 0.4162 | 1 | 0.6974 | −4.85 | −1.55 |
| I | −3.20 | 1 | 0.6974 | 21.96 | 25.26 |
| L | 23.61 | 1 | 0.9862 | −4.00 | 0.6671 |
| WI | −1.66 | 1 | 0.9862 | −2.61 | 2.05 |
| WL | −0.2775 | 1 | 0.9862 | −2.05 | 2.61 |
| IL | 0.2775 | 1 | 0.9613 | −1.33 | 3.22 |
| W2 | 0.9460 | 1 | 0.9613 | −10.22 | −5.67 |
| I2 | −7.94 | 1 | 0.9613 | −23.27 | −18.73 |
| L2 | −21.00 | 1 | 0.6974 | −1.23 | 2.07 |
| Term | Coefficient | d.f. | SE | 95% CI | |
|---|---|---|---|---|---|
| Low | High | ||||
| Schizothorax irregularis | |||||
| Intercept | 1.73 | 1 | 0.0028 | 1.72 | 1.74 |
| W | −0.005 | 1 | 0.0022 | −0.0102 | 0.0002 |
| I | −0.0062 | 1 | 0.0022 | −0.0115 | −0.001 |
| L | −0.0087 | 1 | 0.0022 | −0.014 | −0.0035 |
| WI | −0.0225 | 1 | 0.0031 | −0.0299 | −0.0151 |
| WL | 0.0125 | 1 | 0.0031 | 0.0051 | 0.0199 |
| IL | 0.005 | 1 | 0.0031 | −0.0024 | 0.0124 |
| W2 | −0.0275 | 1 | 0.0031 | −0.0347 | −0.0203 |
| I2 | −0.055 | 1 | 0.0031 | −0.0622 | −0.0478 |
| L2 | −0.055 | 1 | 0.0031 | −0.0622 | −0.0478 |
| Diptychus maculates | |||||
| Intercept | 1.74 | 1 | 0.0482 | 1.62 | 1.85 |
| W | −0.0325 | 1 | 0.0381 | −0.1227 | 0.0577 |
| I | −0.3312 | 1 | 0.0381 | −0.4214 | −0.2411 |
| L | 0.1513 | 1 | 0.0381 | 0.0611 | 0.2414 |
| WI | −0.0400 | 1 | 0.0539 | −0.1675 | 0.0875 |
| WL | 0.1600 | 1 | 0.0539 | 0.0325 | 0.2875 |
| IL | −0.1025 | 1 | 0.0539 | −0.2300 | 0.0250 |
| W2 | −0.0418 | 1 | 0.0526 | −0.1660 | 0.0825 |
| I2 | −0.5493 | 1 | 0.0526 | −0.6735 | −0.4250 |
| L2 | −0.1542 | 1 | 0.0526 | −0.2785 | −0.0300 |
| Source | SS | d.f. | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Schizothorax irregularis | |||||
| Model | 313.02 | 9 | 34.78 | 37.47 | <0.0001 |
| W | 129.85 | 1 | 129.85 | 139.89 | <0.0001 |
| I | 4.84 | 1 | 4.84 | 5.21 | 0.0564 |
| L | 0.8911 | 1 | 0.8911 | 0.9601 | 0.3598 |
| WI | 0.3080 | 1 | 0.3080 | 0.3319 | 0.5826 |
| WL | 0.000 0 | 1 | 0.0000 | 0.0000 | 1.0000 |
| IL | 5.98 | 1 | 5.98 | 6.44 | 0.0388 |
| W2 | 119.73 | 1 | 119.73 | 128.99 | <0.0001 |
| I2 | 31.15 | 1 | 31.15 | 33.56 | 0.0007 |
| L2 | 7.48 | 1 | 7.48 | 8.05 | 0.0251 |
| Diptychus maculates | |||||
| Model | 6759.00 | 9 | 751.00 | 193.03 | <0.0001 |
| W | 1.39 | 1 | 1.39 | 0.3563 | 0.5694 |
| I | 81.73 | 1 | 81.73 | 21.01 | 0.0025 |
| L | 4459.46 | 1 | 4459.46 | 1146.20 | <0.0001 |
| WI | 11.09 | 1 | 11.09 | 2.85 | 0.1352 |
| WL | 0.308 0 | 1 | 0.3080 | 0.0792 | 0.7866 |
| IL | 0.308 0 | 1 | 0.3080 | 0.0792 | 0.7866 |
| W2 | 3.77 | 1 | 3.77 | 0.9685 | 0.3578 |
| I2 | 265.7 1 | 1 | 265.71 | 68.30 | <0.0001 |
| L2 | 1857.1 1 | 1 | 1857.11 | 477.33 | <0.0001 |
| Source | SS | d.f. | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Schizothorax irregularis | |||||
| Model | 0.0355 | 9 | 0.0039 | 100.54 | <0.0001 |
| W | 0.0002 | 1 | 0.0002 | 5.09 | 0.0587 |
| I | 0.0003 | 1 | 0.0003 | 7.95 | 0.0258 |
| L | 0.0006 | 1 | 0.0006 | 15.59 | 0.0055 |
| WI | 0.002 | 1 | 0.002 | 51.55 | 0.0002 |
| WL | 0.0006 | 1 | 0.0006 | 15.91 | 0.0053 |
| IL | 0.0001 | 1 | 0.0001 | 2.55 | 0.1546 |
| W2 | 0.0032 | 1 | 0.0032 | 81.05 | <0.0001 |
| I2 | 0.0127 | 1 | 0.0127 | 324.21 | <0.0001 |
| L2 | 0.0127 | 1 | 0.0127 | 324.21 | <0.0001 |
| Diptychus maculates | |||||
| Model | 2.66 | 9 | 0.2955 | 25.41 | 0.0002 |
| W | 0.0085 | 1 | 0.0085 | 0.7267 | 0.4222 |
| I | 0.8778 | 1 | 0.8778 | 75.49 | <0.0001 |
| L | 0.1830 | 1 | 0.1830 | 15.74 | 0.0054 |
| WI | 0.0064 | 1 | 0.0064 | 0.5504 | 0.4823 |
| WL | 0.1024 | 1 | 0.1024 | 8.81 | 0.0209 |
| IL | 0.0420 | 1 | 0.0420 | 3.61 | 0.0990 |
| W2 | 0.0073 | 1 | 0.0073 | 0.6312 | 0.4530 |
| I2 | 1.27 | 1 | 1.27 | 109.24 | <0.0001 |
| L2 | 0.1002 | 1 | 0.1002 | 8.62 | 0.0219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Z.; Hao, H.; Zhao, H.; Zhao, N.; Li, L.; Qiang, Z.; Hamid, S.M.; Wei, J. Effects of Water Temperature, Light Intensities and Photoperiod on the Survival and Growth of Juvenile Schizothorax irregularis and Diptychus maculates. Fishes 2025, 10, 122. https://doi.org/10.3390/fishes10030122
Nie Z, Hao H, Zhao H, Zhao N, Li L, Qiang Z, Hamid SM, Wei J. Effects of Water Temperature, Light Intensities and Photoperiod on the Survival and Growth of Juvenile Schizothorax irregularis and Diptychus maculates. Fishes. 2025; 10(3):122. https://doi.org/10.3390/fishes10030122
Chicago/Turabian StyleNie, Zhulan, Huimin Hao, He Zhao, Nianhua Zhao, Li Li, Zhuang Qiang, Syeda Maira Hamid, and Jie Wei. 2025. "Effects of Water Temperature, Light Intensities and Photoperiod on the Survival and Growth of Juvenile Schizothorax irregularis and Diptychus maculates" Fishes 10, no. 3: 122. https://doi.org/10.3390/fishes10030122
APA StyleNie, Z., Hao, H., Zhao, H., Zhao, N., Li, L., Qiang, Z., Hamid, S. M., & Wei, J. (2025). Effects of Water Temperature, Light Intensities and Photoperiod on the Survival and Growth of Juvenile Schizothorax irregularis and Diptychus maculates. Fishes, 10(3), 122. https://doi.org/10.3390/fishes10030122






