Molecular Phylogeny and Evolutionary History of the Genus Cyprinus (Teleostei: Cypriniformes)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Preparation, PCR Amplification, and Sequencing
2.3. Data Analyses
2.4. Divergence Time Estimation Analyses
3. Results
3.1. Sequences Characters
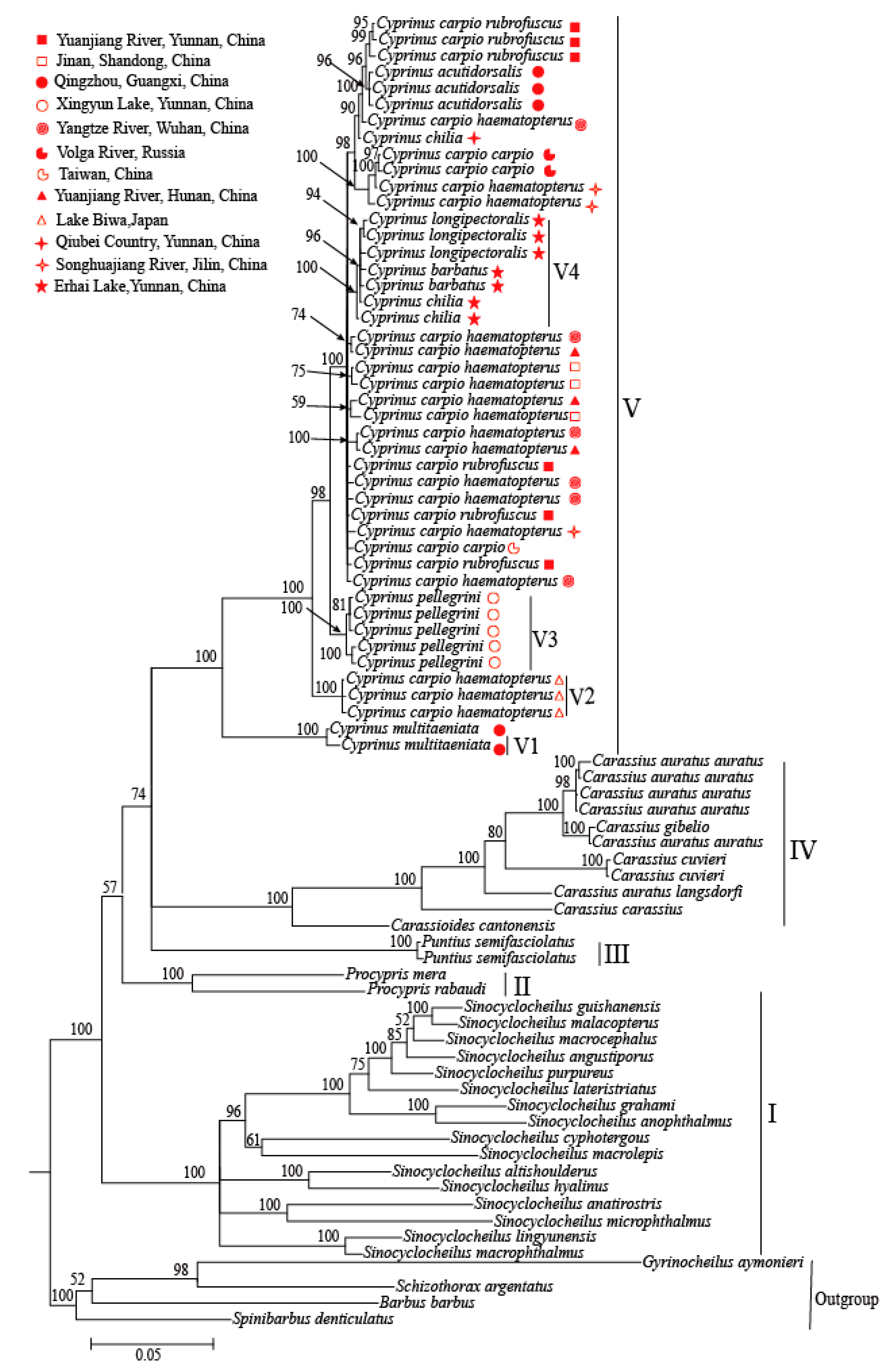
3.2. Phylogenetic Analyses
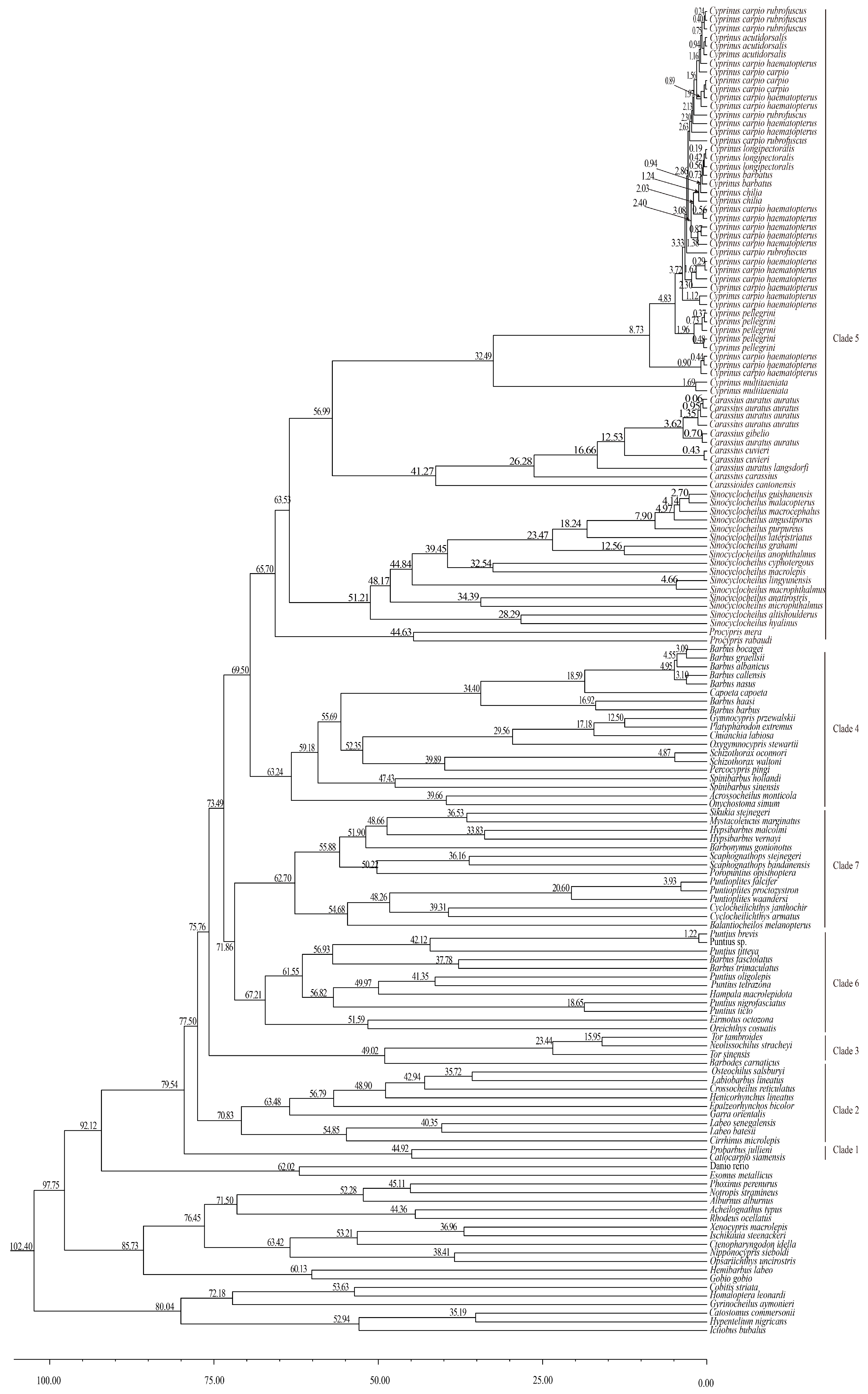
3.3. Divergence Time Estimation
4. Discussion
4.1. The Phylogeny and Geography Distribution of Cyprinus
4.2. Divergence Times in Cyprinus (Cyprinus)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavender, T.M.; Coburn, M.M. Phylogenetic relationships of North American Cyprinidae. In Systematics Historical Ecology and North American Freshwater Fishes; Mayden, R.L., Ed.; Stanford University Press: Stanford, CA, USA, 1992; pp. 293–327. [Google Scholar]
- Luo, Y.L.; Yue, P.Q. Cyprininae. In Fauna Sinica Osteichthyes: Cypriniformes III; Yue, P.Q., Ed.; Beijing Science Press: Beijing, China, 2000. [Google Scholar]
- Chen, X.L.; Huang, H.J. Cyprininae. In The Cyprinidae fishes of China, Part II; XW, W., Ed.; Science and Technology Press of Shanghai: Shanghai, China, 1977; pp. 395–438. [Google Scholar]
- Wang, Y.H. On the classification, distribution, origin and evolution of the fishes referred to the subfamily Cyprininae of China, with description of a new species. Acta Hydrobiol. Sin. 1979, 6, 419–438. [Google Scholar] [CrossRef]
- Zhou, W.; Chu, X.L. Systematic study of the genus Cyprinus (Pisces: Cyprinidae) in Yunnan, China. Zool. Res. 1986, 7, 297–310. [Google Scholar]
- Zhou, W. Phylogeny of the subfamily Cyprininae (Pisces: Cyprinidae). Acta Zootaxon. Sin. 1989, 14, 247–256. [Google Scholar]
- Chen, X.Y.; Yang, J.X. Cladistic analysis of the cyprinid subgenus Cyprinus (Mesocyprinus) Fang (Teleostei: Cyprinidae). Zool. Res. 2002, 23, 185–194. [Google Scholar]
- Cibert, C.; Fermon, Y.; Vallod, D.; Meunier, F.J. Morphological screening of carp Cyprinus carpio: Relationship between morphology and fillet yield. Aquat. Living Resour. 1999, 12, 1–10. [Google Scholar] [CrossRef]
- Khosravi, M.; Abdoli, A.; Tajbakhsh, F.; Ahmadzadeh, F.; Nemati, H.; Kiabi, B.H. An Effort toward Species Delimitation in the Genus Carassius (Cyprinidae) using Morphology and the Related Challenges: A Case Study from Inland Waters of Iran. J. Ichthyol. 2022, 62, 185–194. [Google Scholar] [CrossRef]
- Dong, C.; Xu, J.; Wang, B.; Feng, J.; Jeney, Z.; Sun, X.; Xu, P. Phylogeny and Evolution of Multiple Common Carp (Cyprinus carpio L.) Populations Clarified by Phylogenetic Analysis Based on Complete Mitochondrial Genomes. Mar. Biotechnol. 2015, 17, 565–575. [Google Scholar] [CrossRef]
- Davis, K.M.; Dixon, P.I.; Harris, J.H. Allozyme and mitochondrial DNA analysis of carp, Cyprinus carpio L., from south-eastern Australia. Mar. Freshw. Res. 1999, 50, 253–260. [Google Scholar] [CrossRef]
- Kohlmann, K.; Kersten, P. Genetic variability of German and foreign common carp (Cyprinus carpio L.) populations. Aquaculture 1999, 173, 435–445. [Google Scholar] [CrossRef]
- David, L.; Rajasekaran, P.; Fang, J.; Hillel, J.; Lavi, U. Polymorphism in ornamental and common carp strains (Cyprinus carpio L.) as revealed by AFLP analysis and a new set of microsatellite markers. Mol. Genet. Genom. 2001, 266, 353–362. [Google Scholar] [CrossRef]
- Desvignes, J.F.; Laroche, J.; Durand, J.D.; Bouvet, Y. Genetic variability in reared stocks of common carp (Cyprinus carpio L.) based on allozymes and microsatellites. Aquaculture 2001, 194, 291–301. [Google Scholar] [CrossRef]
- Froufe, E.; Magyary, I.; Lehoczky, I.; Weiss, S. MtDNA sequence data supports an Asian ancestry and single introduction of the common carp into the Danube Basin. J. Fish. Biol. 2002, 61, 301–304. [Google Scholar] [CrossRef]
- Zhou, J.F.; Wu, Q.J.; Wang, Z.; Ye, Y.Z. Molecular phylogeny of three subspecies of common carp Cyprinus carpio, based on sequence analysis of cytochrome b and control region of mtDNA. J. Zool. Syst. Evol. Res. 2004, 42, 266–269. [Google Scholar] [CrossRef]
- Zhou, J.F.; Wu, Q.J.; Ye, Y.Z.; Tong, J.G. Genetic divergence between Cyprinus carpio carpio and Cyprinus carpio haematopterus as assessed by mitochondrial DNA analysis, with emphasis on origin of European domestic carp. Genetica 2003, 119, 93–97. [Google Scholar] [CrossRef]
- Kohlmann, K.; Gross, R.; Murakaeva, A.; Kersten, P. Genetic variability and structure of common carp (Cyprinus carpio) populations throughout the distribution range inferred from allozyme, microsatellite and mitochondrial DNA markers. Aquat. Living Resour. 2003, 16, 421–431. [Google Scholar] [CrossRef]
- Kohlmann, K.; Kersten, P.; Flajšhans, M. Microsatellite-based genetic variability and differentiation of domesticated, wild and feral common carp (Cyprinus carpio L.) populations. Aquaculture 2005, 247, 253–266. [Google Scholar] [CrossRef]
- Mabuchi, K.; Senou, H.; Suzuki, T.; Nishida, M. Discovery of an ancient lineage of Cyprinus carpio from Lake Biwa, central Japan, based on mtDNA sequence data, with reference to possible multiple origins of koi. J. Fish. Biol. 2005, 66, 1516–1528. [Google Scholar] [CrossRef]
- Mabuchi, K.; Miya, M.; Senou, H.; Suzuki, T.; Nishida, M. Complete mitochondrial DNA sequence of the Lake Biwa wild strain of common carp (Cyprinus carpio L.): Further evidence for an ancient origin. Aquaculture 2006, 257, 68–77. [Google Scholar] [CrossRef]
- Mabuchi, K.; Senou, H.; Nishida, M. Mitochondrial DNA analysis reveals cryptic large-scale invasion of non-native genotypes of common carp (Cyprinus carpio) in Japan. Mol. Ecol. 2008, 17, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Memiş, D.; Kohlmann, K. Genetic characterization of wild common carp (Cyprinus carpio L.) from Turkey. Aquaculture 2006, 258, 257–262. [Google Scholar] [CrossRef]
- Thai, B.T.; Burridge, C.P.; Pham, T.A.; Austin, C.M. Using mitochondrial nucleotide sequences to investigate diversity and genealogical relationships within common carp (Cyprinus carpio L.). Anim. Genet. 2005, 36, 23–28. [Google Scholar] [CrossRef]
- Thai, B.T.; Pham, T.A.; Austin, C.M. Genetic diversity of common carp in Vietnam using direct sequencing and SSCP analysis of the mitochondrial DNA control region. Aquaculture 2006, 258, 228–240. [Google Scholar] [CrossRef]
- Liao, X.L.; Yu, X.M.; Tong, J.G. Genetic diversity of common carp from two largest Chinese lakes and the Yangtze River revealed by microsatellite markers. Hydrobiologia 2006, 568, 445–453. [Google Scholar] [CrossRef]
- Tong, J.G.; Wu, Q.J. Sequence conservation on segments of mitochondrial 16SrRNA and cytochrome b in strains of common carp (Cyprinus carpio L. Var.). Acta Hydrobiol. Sin. 2001, 25, 50–53. [Google Scholar] [CrossRef]
- Zheng, B.R.; Zhang, Y.P.; Zan, R.G. A primary detection of close genetic similarity of four Cyprinus species in Erhai Lake. Hereditas 2001, 23, 544–546. [Google Scholar]
- Zheng, B.R.; Zhang, Y.P.; Xiao, C.J.; Xiao, H.; Zan, R.G. The genetic evidence for sympatric speciation pattern of Cyprinus from Erhai Lake. Acta Genet. Sin. 2004, 31, 976–982. [Google Scholar]
- Wang, J.; Wu, X.; Chen, Z.M.; Yue, Z.P.; Chen, S.; Xiao, H. Molecular phylogeny of European and African Barbus and their West Asian relatives in the Cyprininae (Teleostei: Cypriniformes) and orogenesis of the Qinghai-Tibetan Plateau. Chin. Sci. Bull. 2013, 58, 3738–3746. [Google Scholar] [CrossRef]
- Yang, L.; Mayden, R.L.; Sado, T.; He, S.; Saitoh, K.; Miya, M. Molecular phylogeny of the fishes traditionally referred to Cyprinini sensu stricto (Teleostei: Cypriniformes). Zool. Scr. 2010, 39, 527–550. [Google Scholar] [CrossRef]
- Mayden, R.L.; Chen, W.J.; Bart, H.L.; Doosey, M.H.; Simons, A.M.; Tang, K.L.; Wood, R.M.; Agnew, M.K.; Yang, L.; Hirt, M.V. Reconstructing the phylogenetic relationships of the earth’s most diverse clade of freshwater fishes-order Cypriniformes (Actinopterygii: Ostariophysi): A case study using multiple nuclear loci and the mitochondrial genome. Mol. Phylogenet. Evol. 2009, 51, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Zardoya, R.; Doadrio, I. Phylogenetic relationships of Iberian Cyprinids: Systematic and biogeographical implications. Proc. Biol. Sci. 1998, 265, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Zardoya, R.; Economidis, P.S.; Doadrio, I. Phylogenetic relationships of Greek cyprinidae: Molecular evidence for at least two origins of the Greek cyprinid fauna. Mol. Phylogenet. Evol. 1999, 13, 122–131. [Google Scholar] [CrossRef]
- Tsigenopoulos, C.S.; Berrebi, P. Molecular phylogeny of north mediterranean freshwater barbs (Genus Barbus: Cyprinidae) inferred from cytochrome b sequences: Biogeographic and systematic implications. Mol. Phylogenet. Evol. 2000, 14, 165–179. [Google Scholar] [CrossRef]
- Durand, J.D.; Tsigenopoulos, C.S.; Unlü, E.; Berrebi, P. Phylogeny and biogeography of the family Cyprinidae in the Middle East inferred from cytochrome b DNA—Evolutionary significance of this region. Mol. Phylogenet. Evol. 2002, 22, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Tsigenopoulos, C.S.; Ráb, P.; Naran, D.; Berrebi, P. Multiple origins of polyploidy in the phylogeny of southern African barbs (Cyprinidae) as inferred from mtDNA markers. Heredity 2002, 88, 466–473. [Google Scholar] [CrossRef]
- He, D.K.; Feng, C.Y.; Ming, C.Z. Molecular phylogeny of the specialized schizothoracine fishes (Teleostei: Cyprinidae),with their implications for the uplift of the Qinghai-Tibetan Plateau. Chin. Sci. Bull. 2004, 49, 39–48. [Google Scholar] [CrossRef]
- He, D.K.; Chen, Y.F. Molecular phylogeny and biogeography of the highly specialized grade schizothoracine fishes (Teleostei: Cyprinidae) inferred from cytochrome b sequences. Chin. Sci. Bull. 2007, 52, 777–788. [Google Scholar] [CrossRef]
- Rüber, L.; Kottelat, M.; Tan, H.H.; Ng, P.K.; Britz, R. Evolution of miniaturization and the phylogenetic position of Paedocypris, comprising the world’s smallest vertebrate. BMC Evol. Biol. 2007, 7, 38. [Google Scholar] [CrossRef]
- Levin, B.A.; Freyhof, J.; Lajbner, Z.; Perea, S.; Abdoli, A.; Gaffaroğlu, M.; Özuluğ, M.; Rubenyan, H.R.; Salnikov, V.B.; Doadrio, I. Phylogenetic relationships of the algae scraping cyprinid genus Capoeta (Teleostei: Cyprinidae). Mol. Phylogenet. Evol. 2012, 62, 542–549. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, S.Y.; Liu, Z.M.; Zhang, R.D.; Li, W.X.; Zan, R.G.; Zhang, Y.P. Molecular phylogeny of Sinocyclocheilus (Cypriniformes: Cyprinidae) inferred from mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2005, 36, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.H.; Zhang, Y.P.; Liu, H.Z. Molecular systematics of Xenocyprinae (Teleostei: Cyprinidae): Taxonomy, biogeography, and coevolution of a special group restricted in East Asia. Mol. Phylogenet. Evol. 2001, 18, 163–173. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Xia, X.; Xie, Z. DAMBE: Software package for data analysis in molecular biology and evolution. J. Hered. 2001, 92, 371–373. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Near, T.J.; Eytan, R.I.; Dornburg, A.; Kuhn, K.L.; Moore, J.A.; Davis, M.P.; Wainwright, P.C.; Friedman, M.; Smith, W.L. Resolution of ray-finned fish phylogeny and timing of diversification. Proc. Natl. Acad. Sci. USA 2012, 109, 13698–13703. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.Y. Molecular Phylogeny and Biogeography of Percocypris (Cyprinidae, Teleostei). PLoS ONE 2013, 8, e61827. [Google Scholar] [CrossRef]
- Chen, G.J.; Chang, M.M.; Liu, H.Z. Revision of Cyprinus maomingensis Liu 1957 and the first discovery of Procypris-hke cyprinid (Teleostei, Pisces) from the late Eocene of South China. Sci. China Earth Sci. 2015, 58, 1123–1132. [Google Scholar] [CrossRef]
- Chen, G.J.; Chang, M.M. A new early cyprinin from Oligocene of South China. Sci. China Earth Sci. 2011, 54, 481–492. [Google Scholar] [CrossRef]
- Zardoya, R.; Doadrio, I. Molecular evidence on the evolutionary and biogeographical patterns of European cyprinids. J. Mol. Evol. 1999, 49, 227–237. [Google Scholar] [CrossRef]
- Zardoya, R.; Meyer, A. Phylogenetic performance of mitochondrial protein-coding genes in resolving relationships among vertebrates. Mol. Biol. Evol. 1996, 13, 933–942. [Google Scholar] [CrossRef]
- Li, Z.Q.; Guo, B.C.; Li, J.B.; He, S.P.; Chen, Y.Y. Bayesian mixed models and divergence time estimation of Chinese cavefishes (Cyprinidae: Sinocyclocheilus). Chin. Sci. Bull. 2008, 53, 2342–2352. [Google Scholar] [CrossRef]
- Duan, Y.X. The Evolution of Erhai Lake; Shen, R.X., Ed.; Yunnan National Publishing House: Kunming, China, 1989; pp. 212–222. [Google Scholar]
- Li, H. A Reall of Aquatic Vegetation of Erhai Lake; Shen, R.X., Ed.; Yunnan National Publishing House: Kunming, China, 1989; pp. 31–44. [Google Scholar]
- Gao, L.C.; Zhuang, D.D. A preliminary study on the evolution and differentiation of fish fauna in relation to orisination and dynamices of Erhai Lake. In Scientific Works on Erhai Lake in Yunnan; Press of Yunnan Province: Kunming, China, 1989; pp. 277–283. [Google Scholar]
- Nakajima, T. Pliocene cyprinid pharyngeal teeth from Japan and East Asia Neogene cyprinid zoogeography. In Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes; The Ichthyological Society of Japan: Tokyo, Japan, 1986. [Google Scholar]
- Zhang, M.X.; Zhao, Y.J.; Yao, C.; Liu, X.; Huang, S.M.; Li, H.T.; Dai, Z.L.; Wu, X.W.; Ling, F.K. Genetic Structure of Common Carp Populations of Cyprinus carpio yuankiang and Cyprinus carpio chilia Based on Mitochondrial Cyt b Gene and D-loop Region Sequences. Chin. J. Fish. 2024, 37, 13–21. [Google Scholar]
- Ren, L.; Tu, X.; Luo, M.; Liu, Q.; Cui, J.; Gao, X.; Zhang, H.; Tai, Y.; Zeng, Y.; Li, M.; et al. Genomes reveal pervasive distant hybridization in nature among cyprinid fishes. GigaScience 2025, 14, giae117. [Google Scholar] [CrossRef]
- Kenji, S.; Tetsuya, S.; Doosey, M.H.; Bart, J.H.L.; Inoue, J.G.; Mutsumi, N.; Mayden, R.L.; Masaki, M. Evidence from mitochondrial genomics supports the lower Mesozoic of South Asia as the time and place of basal divergence of cypriniform fishes (Actinopterygii: Ostariophysi). Zool. J. Linn. Soc. 2011, 161, 633–662. [Google Scholar]
- Bowen, W.; Bass, A.L.; Rocha, L.A.; Grant, W.S.; Robertson, D.R. Phylogeography of the trumpetfishes (Aulostomus): Ring species complex on a global scale. Evolution 2001, 55, 1029–1039. [Google Scholar] [CrossRef]
- Bermingham, E.; Mccafferty, S.S.; Martin, A.P. Fish biogeography and molecular clocks: Perspectives from the panamanian isthmus. In Molecular Systematics of Fishes; Kocher, T.D., Stepien, C.A., Eds.; Academic Press: New York, NY, USA, 1997; pp. 113–128. [Google Scholar]
- Yao, D.L.; Wang, C.; Zhou, A.G.; Xie, S.L.; Zou, J.X. Phylogenetic analysis of Cyprinus acutidorsalis Wang in Cypriniformes based on the Cyt b Marker. Guangdong Agric. Sci. 2014, 41, 114–118. [Google Scholar]
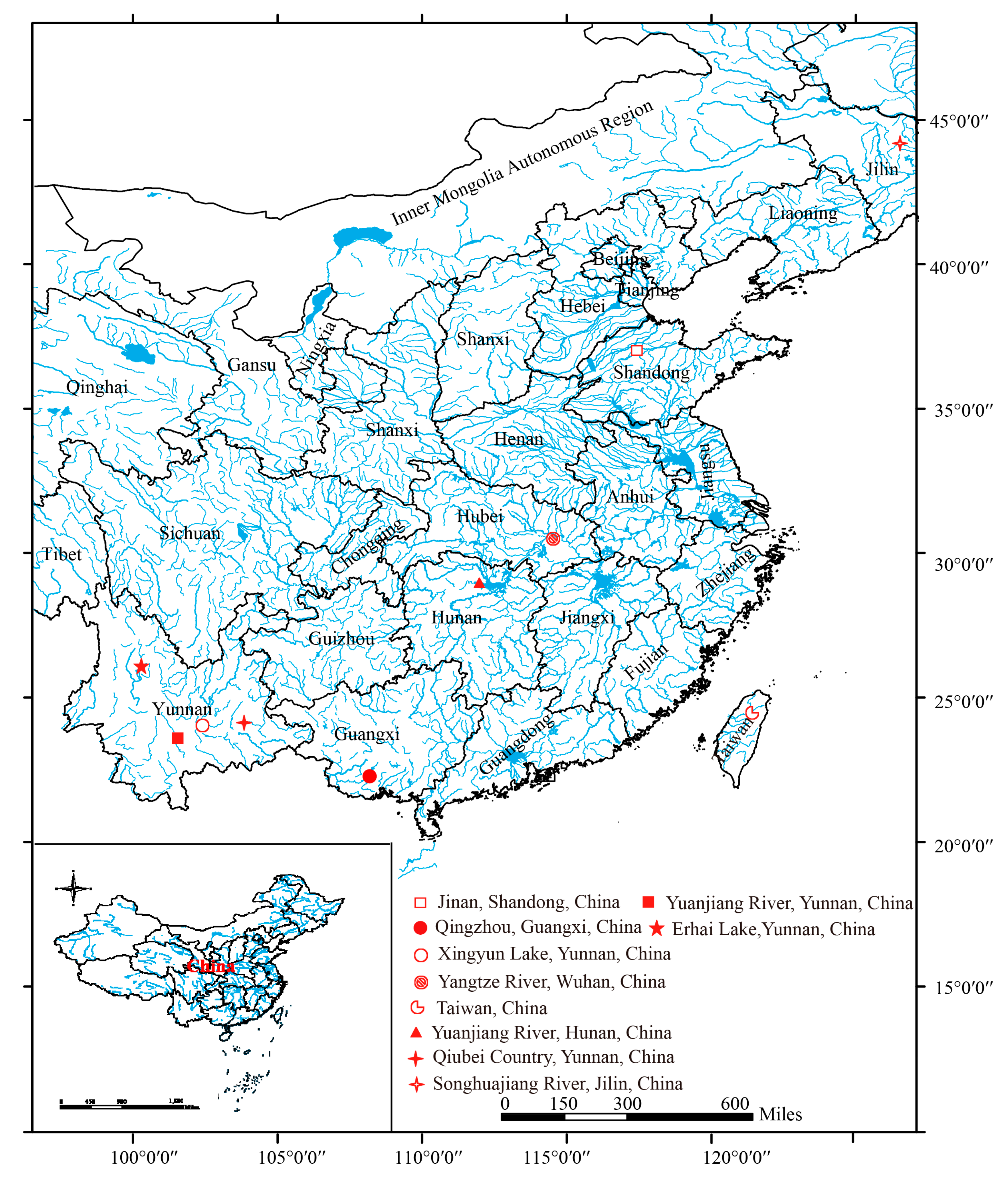
| Species | Locality | N |
|---|---|---|
| Cyprinus pellegrini | Xingyun Lake, Yunnan, China | 5 |
| Cyprinus longipectoralis | Erhai Lake, Yunnan, China | 3 |
| Cyprinus barbatus | Erhai Lake, Yunnan, China | 2 |
| Cyprinus chilia | Erhai Lake, Yunnan, China | 2 |
| Cyprinus chilia | Qiubei Country, Yunnan, China | 1 |
| Cyprinus carpio rubrofuscus | Yuanjiang River, Yunnan, China | 4 |
| Cyprinus carpio haematopterus | Yuanjiang River, Hunan, China | 3 |
| Cyprinus carpio haematopterus | Jinan, Shandong, China | 3 |
| Cyprinus carpio haematopterus | Yangtze River, Wuhan, China | 4 |
| Cyprinus carpio haematopterus | Songhuajiang River, Jilin, China | 3 |
| Cyprinus acutidorsalis | Qingzhou, Guangxi, China | 3 |
| Carassius auratus auratus | Dianchi Lake, Yunnan, China | 2 |
| Carassius auratus auratus | Shili Country, Yunnan, China | 1 |
| Carassius auratus auratus | Dolsko, Slovenija, Europe | 1 |
| Species | Locality | Accession No. |
|---|---|---|
| Cyprinus carpio carpio | Volga River, Russia | AY347294, AY347295 |
| Cyprinus carpio haematopterus | Lake Biwa, Japan | AB158803, AB158804. AB158805 |
| Cyprinus carpio haematopterus | Yangtze River, Wuhan, China | AY347281, AY347291 |
| Cyprinus carpio rubrofuscus | Yuanjiang River, Yunnan, China | AY347280, AY347290 |
| Cyprinus carpio carpio | Taiwan, China | X61010 |
| Cyprinus multitaeniata | Qinzhou, Guangxi, China | KC696556, KC696557 |
| Carassioides cantonensis | Qinzhou, Guangxi, China | KC696558 |
| Carassius auratus auratus | - | AB111951 |
| Carassius auratus langsdorfi | - | AB006953 |
| Carassius carassius | - | GU135602 |
| Carassius cuvieri | - | AB045144 |
| Carassius cuvieri | - | AP011237 |
| Carassius gibelio | - | GU138989 |
| Procypris mera | Xijiang, Guangxi, China | KC696555 |
| Procypris rabaudi | Mudong, Chongqing, China | NC_011192 |
| Puntius semifasciolatus | Guangxi, China | AY856116 |
| Puntius semifasciolatus | Luoping, Yunnan, China | KC696521 |
| Sinocyclocheilus altishoulderus | Mashan County, Guangxi | FJ984568 |
| Sinocyclocheilus anatirostris | Leye County, Guangxi | AY854708 |
| Sinocyclocheilus angustiporus | Luxi County, Yunnan | AY854702 |
| Sinocyclocheilus anophthalmus | Jiuxiang, Yiliang County, Yunnan | AY854715 |
| Sinocyclocheilus cyphotergous | Luodian County, Guizhou | AY854711 |
| Sinocyclocheilus grahami | Qinglongsi, Kunming, Yunnan | GQ148557 |
| Sinocyclocheilus guishanensis | Guishan, Shilin County, Yunnan | AY854722 |
| Sinocyclocheilus hyalinus | Alugudong, Luxi County, Yunnan | AY854721 |
| Sinocyclocheilus lateristriatus | Luliang County, Guangxi | AY854703 |
| Sinocyclocheilus lingyunensis | Shading, Linyun County, Guangxi | AY854691 |
| Sinocyclocheilus macrocephalus | Heilongtan, Shilin County, Yunnan | AY854683 |
| Sinocyclocheilus macrolepis | Nandan County, Guangxi | AY854729 |
| Sinocyclocheilus macrophthalmus | Xiaao, Duan County, Guangxi | HM536792 |
| Sinocyclocheilus malacopterus | Jinji, Luoping County, Yunnan | AY854697 |
| Sinocyclocheilus microphthalmus | Shading, Linyun County, Guangxi | AY854690 |
| Sinocyclocheilus purpureus | Yanshan County, Yunnan | EU366189 |
| Outgroup | ||
| Barbus barbus | Europe, France | AB238965 |
| Gyrinocheilus aymonieri | Southeast Asia | NC008672 |
| Schizothorax argentatus | Li River, Kazakhstan | AY954269 |
| Spinibarbus denticulatus | Fuxian Lake, Yunnan, China | KC696533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xiao, H.; Yue, Z.; Wu, X.; Zan, R.; Chen, S. Molecular Phylogeny and Evolutionary History of the Genus Cyprinus (Teleostei: Cypriniformes). Fishes 2025, 10, 121. https://doi.org/10.3390/fishes10030121
Chen Y, Xiao H, Yue Z, Wu X, Zan R, Chen S. Molecular Phylogeny and Evolutionary History of the Genus Cyprinus (Teleostei: Cypriniformes). Fishes. 2025; 10(3):121. https://doi.org/10.3390/fishes10030121
Chicago/Turabian StyleChen, Yanyan, Heng Xiao, Zhaoping Yue, Xiaoyun Wu, Ruiguang Zan, and Shanyuan Chen. 2025. "Molecular Phylogeny and Evolutionary History of the Genus Cyprinus (Teleostei: Cypriniformes)" Fishes 10, no. 3: 121. https://doi.org/10.3390/fishes10030121
APA StyleChen, Y., Xiao, H., Yue, Z., Wu, X., Zan, R., & Chen, S. (2025). Molecular Phylogeny and Evolutionary History of the Genus Cyprinus (Teleostei: Cypriniformes). Fishes, 10(3), 121. https://doi.org/10.3390/fishes10030121






