Size-Selective Harvesting Effects on Reproductive Investment in Marine Medaka (Oryzias melastigma)
Abstract
1. Introduction
2. Materials and Methods
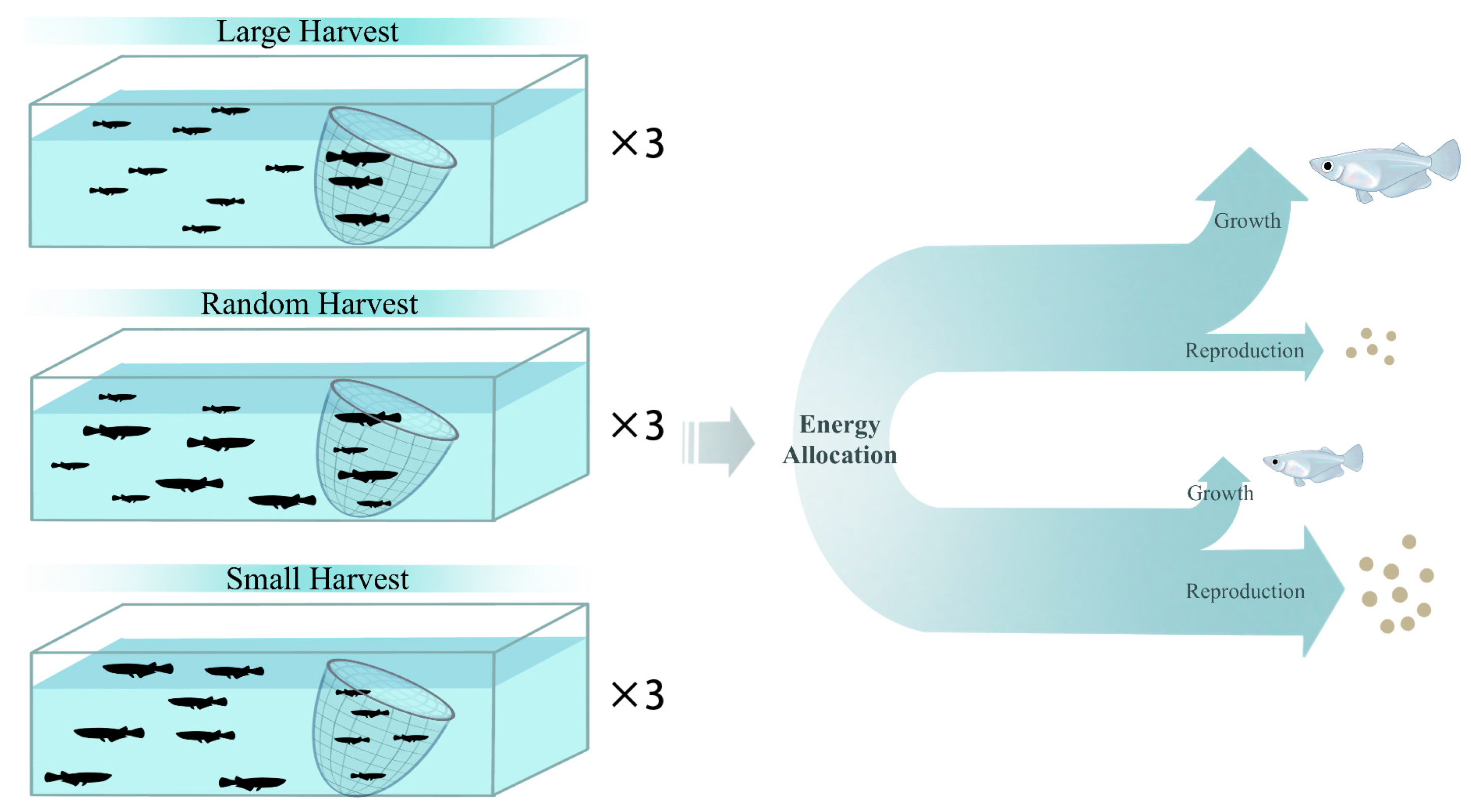
2.1. Experimental Design
2.2. Data Measurement
2.2.1. Body Size Measurement
2.2.2. Gonadal Weight
2.3. Calculation of Reproductive Investment
2.4. Maturity Probability
3. Results
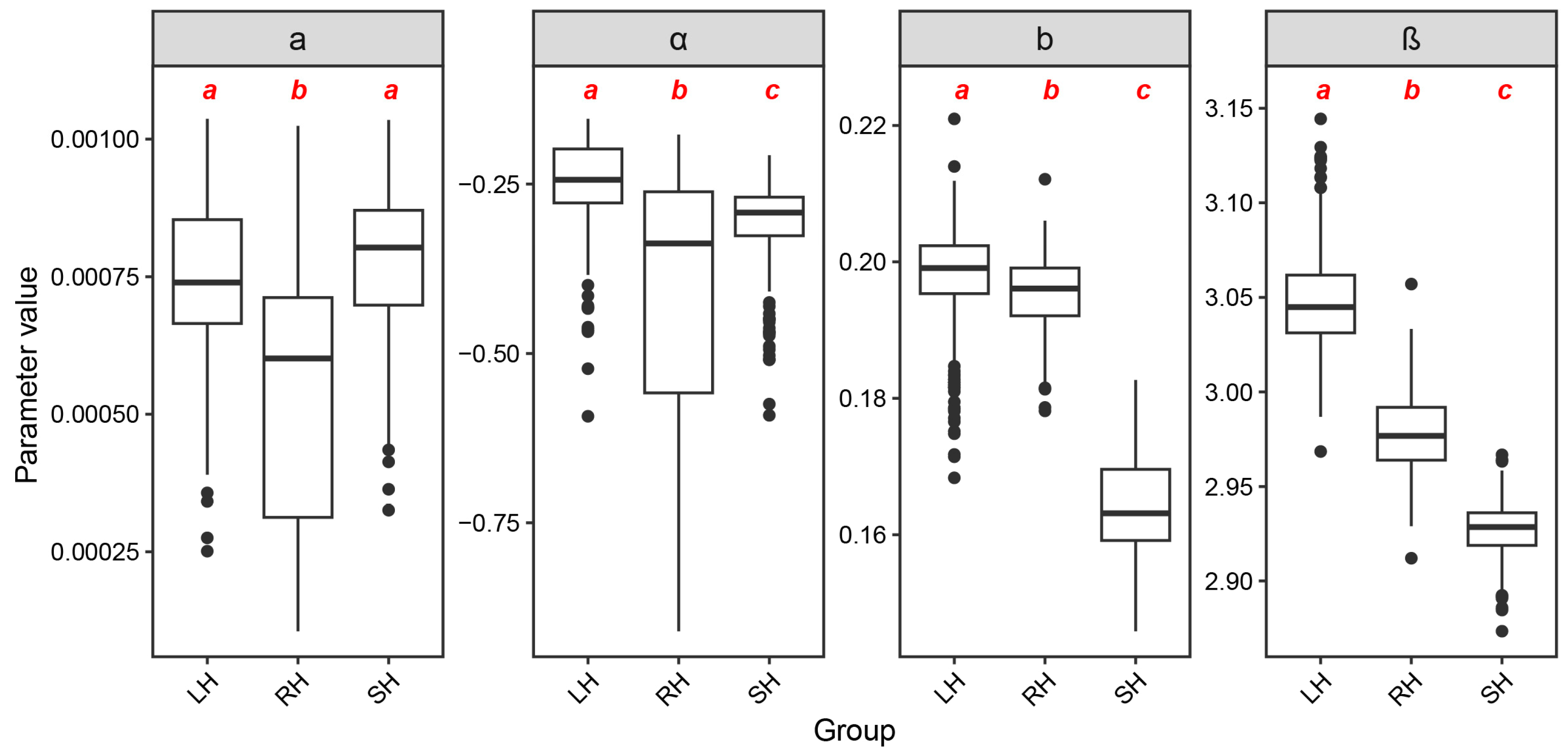
3.1. Parameter Estimation
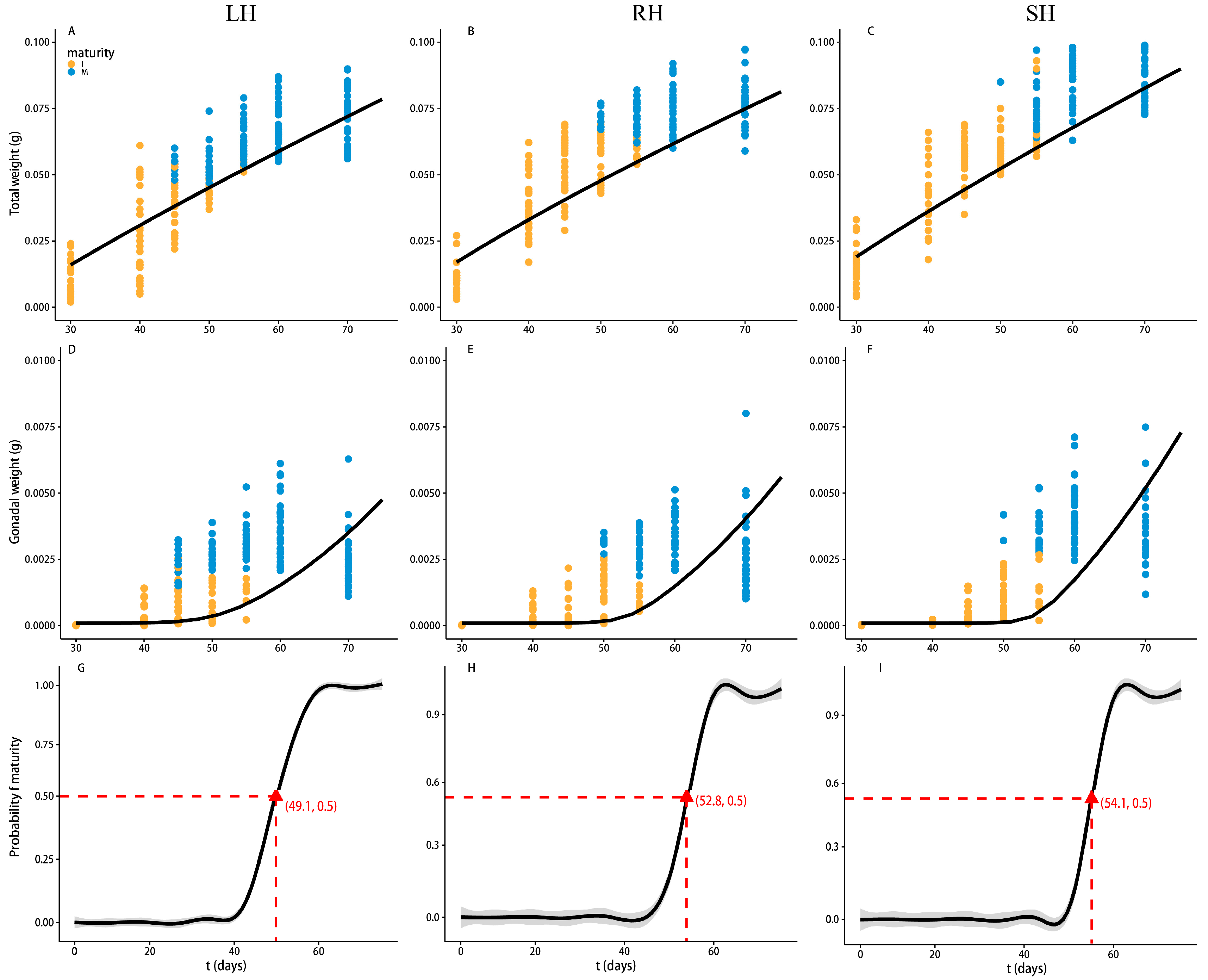
3.2. Relationship Between Length and Weight of Marine Medaka Under Different Harvesting Strategies
3.3. Trends in Growth and Reproduction of Marine Medaka Under Different Harvesting Strategies
3.4. Differences in Reproductive Investment in Marine Medaka Under Different Harvesting Strategies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zera, A.J.; Harshman, L.G. The Physiology of Life History Trade-Offs in Animals. Annu. Rev. Ecol. Syst. 2001, 32, 95–126. [Google Scholar] [CrossRef]
- McBride, R.S.; Somarakis, S.; Fitzhugh, G.R.; Albert, A.; Yaragina, N.A.; Wuenschel, M.J.; Alonso-Fernández, A.; Basilone, G. Energy Acquisition and Allocation to Egg Production in Relation to Fish Reproductive Strategies. Fish Fish. 2015, 16, 23–57. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy Homeostasis as an Integrative Tool for Assessing Limits of Environmental Stress Tolerance in Aquatic Invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Ganias, K.; Kjesbu, O.S.; Lowerre-Barbieri, S.; Fuentes-Pardo, A.P.; Andersson, L.; Brosset, P. Reproduction of Marine Fishes. In Ecology of Marine Fish; Elsevier: Amsterdam, The Netherlands, 2025; pp. 143–159. [Google Scholar]
- Kamler, E. Early Life History of Fish: An Energetics Approach; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-94-011-2324-2. [Google Scholar]
- Birnie-Gauvin, K.; Bordeleau, X.; Cooke, S.J.; Davidsen, J.G.; Eldøy, S.H.; Eliason, E.J.; Moore, A.; Aarestrup, K. Life-History Strategies in Salmonids: The Role of Physiology and Its Consequences. Biol. Rev. 2021, 96, 2304–2320. [Google Scholar] [CrossRef]
- Roff, D.A. An Allocation Model of Growth and Reproduction in Fish. Can. J. Fish. Aquat. Sci. 1983, 40, 1395–1404. [Google Scholar] [CrossRef]
- Rijnsdorp, A.D.; van Damme, C.J.G.; Witthames, P.R. Ecology of Reproduction. In Flatfishes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 101–131. ISBN 978-1-118-50115-3. [Google Scholar]
- Niu, J.; Huss, M.; Vasemägi, A.; Gårdmark, A. Decades of Warming Alters Maturation and Reproductive Investment in Fish. Ecosphere 2023, 14, e4381. [Google Scholar] [CrossRef]
- Conroy, C.W.; Piccoli, P.M.; Secor, D.H. Carryover Effects of Early Growth and River Flow on Partial Migration in Striped Bass Morone Saxatilis. Mar. Ecol. Prog. Ser. 2015, 541, 179–194. [Google Scholar] [CrossRef]
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From Gametogenesis to Spawning: How Climate-Driven Warming Affects Teleost Reproductive Biology. J. Fish Biol. 2020, 97, 607–632. [Google Scholar] [CrossRef]
- Diaz, R.J.; Breitburg, D.L. The Hypoxic Environment. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 27, pp. 1–23. ISSN 1546-5098. [Google Scholar]
- Pollock, M.S.; Clarke, L.M.J.; Dubé, M.G. The Effects of Hypoxia on Fishes: From Ecological Relevance to Physiological Effects. Environ. Rev. 2007, 15, 1–14. [Google Scholar] [CrossRef]
- Calow, P.; Forbes, V.E. How Do Physiological Responses to Stress Translate into Ecological and Evolutionary Processes? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 11–16. [Google Scholar] [CrossRef]
- Kreiss, C.M.; Michael, K.; Lucassen, M.; Jutfelt, F.; Motyka, R.; Dupont, S.; Pörtner, H.-O. Ocean Warming and Acidification Modulate Energy Budget and Gill Ion Regulatory Mechanisms in Atlantic Cod (Gadus morhua). J. Comp. Physiol. B 2015, 185, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Melatunan, S.; Calosi, P.; Rundle, S.D.; Widdicombe, S.; Moody, A.J. Effects of Ocean Acidification and Elevated Temperature on Shell Plasticity and Its Energetic Basis in an Intertidal Gastropod. Mar. Ecol. Prog. Ser. 2013, 472, 155–168. [Google Scholar] [CrossRef]
- Holt, R.E.; Jørgensen, C. Climate Warming Causes Life-History Evolution in a Model for Atlantic Cod (Gadus morhua). Conserv. Physiol. 2014, 2, cou050. [Google Scholar] [CrossRef] [PubMed]
- Rooker, J.R.; Alvarado Bremer, J.R.; Block, B.A.; Dewar, H.; de Metrio, G.; Corriero, A.; Kraus, R.T.; Prince, E.D.; Rodríguez-Marín, E.; Secor, D.H. Life History and Stock Structure of Atlantic Bluefin Tuna (Thunnus thynnus). Rev. Fish. Sci. 2007, 15, 265–310. [Google Scholar] [CrossRef]
- Quince, C.; Abrams, P.A.; Shuter, B.J.; Lester, N.P. Biphasic Growth in Fish I: Theoretical Foundations. J. Theor. Biol. 2008, 254, 197–206. [Google Scholar] [CrossRef]
- Shimadzu, H.; Wang, H.-Y. Estimating Allometric Energy Allocation between Somatic and Gonadic Growth. Methods Ecol. Evol. 2022, 13, 407–418. [Google Scholar] [CrossRef]
- Folkvord, A.; Jørgensen, C.; Korsbrekke, K.; Nash, R.D.M.; Nilsen, T.; Skjæraasen, J.E. Trade-Offs between Growth and Reproduction in Wild Atlantic Cod. Can. J. Fish. Aquat. Sci. 2014, 71, 1106–1112. [Google Scholar] [CrossRef]
- Udupa, K.S.; Ganga, U.; Sobhana, K.S. Fishery and Biology of Cutlass Fishes from Indian Seas with Special Reference to the Largehead Hairtail Trichiurus Lepturus Linnaeus, 1758—A Review. Indian J. Fish. 2022, 69, 148–163. [Google Scholar] [CrossRef]
- Young, J.L.; Bornik, Z.B.; Marcotte, M.L.; Charlie, K.N.; Wagner, G.N.; Hinch, S.G.; Cooke, S.J. Integrating Physiology and Life History to Improve Fisheries Management and Conservation. Fish Fish. 2006, 7, 262–283. [Google Scholar] [CrossRef]
- Johnston, F.D.; Arlinghaus, R.; Dieckmann, U. Fish Life History, Angler Behaviour and Optimal Management of Recreational Fisheries. Fish Fish. 2013, 14, 554–579. [Google Scholar] [CrossRef]
- Rochet, M.-J. Short-Term Effects of Fishing on Life History Traits of Fishes. ICES J. Mar. Sci. 1998, 55, 371–391. [Google Scholar] [CrossRef]
- Heino, M.; Godø, O.R. Fisheries-Induced Selection Pressures in the Context of Sustainable Fisheries. Bull. Mar. Sci. 2002, 70, 639–656. [Google Scholar]
- Ohlberger, J.; Langangen, Ø.; Stige, L.C. Age Structure Affects Population Productivity in an Exploited Fish Species. Ecol. Appl. 2022, 32, e2614. [Google Scholar] [CrossRef] [PubMed]
- Fenberg, P.B.; Roy, K. Ecological and Evolutionary Consequences of Size-selective Harvesting: How Much Do We Know? Mol. Ecol. 2008, 17, 209–220. [Google Scholar] [CrossRef]
- Harvey, C.J.; Tolimieri, N.; Levin, P.S. Changes in Body Size, Abundance, and Energy Allocation in Rockfish Assemblages of the Northeast Pacific. Ecol. Appl. 2006, 16, 1502–1515. [Google Scholar] [CrossRef]
- Ricker, W.E. Changes in the Average Size and Average Age of Pacific Salmon. Can. J. Fish. Aquat. Sci. 1981, 38, 1636–1656. [Google Scholar] [CrossRef]
- Xi, X.; Sun, P.; Sun, R.; Tian, Y.; Heino, M. Size-Selective Harvesting Alters Biological Traits of Marine Medaka (Oryzias melastigma). Fish. Res. 2023, 266, 106775. [Google Scholar] [CrossRef]
- Zwanenburg, K.C.T. The Effects of Fishing on Demersal Fish Communities of the Scotian Shelf. ICES J. Mar. Sci. 2000, 57, 503–509. [Google Scholar] [CrossRef]
- Pellowe, K.E.; Leslie, H.M. Size-Selective Fishing Leads to Trade-Offs between Fishery Productivity and Reproductive Capacity. Ecosphere 2020, 11, e03071. [Google Scholar] [CrossRef]
- Uusi-Heikkilä, S.; Whiteley, A.R.; Kuparinen, A.; Matsumura, S.; Venturelli, P.A.; Wolter, C.; Slate, J.; Primmer, C.R.; Meinelt, T.; Killen, S.S.; et al. The Evolutionary Legacy of Size-Selective Harvesting Extends from Genes to Populations. Evol. Appl. 2015, 8, 597–620. [Google Scholar] [CrossRef]
- Jørgensen, C.; Ernande, B.; Fiksen, Ø. Size-selective Fishing Gear and Life History Evolution in the Northeast Arctic Cod. Evol. Appl. 2009, 2, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, J.A. Life History Consequences of Overexploitation to Population Recovery in Northwest Atlantic Cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 2005, 62, 824–832. [Google Scholar] [CrossRef]
- Stearns, S.C. Trade-Offs in Life-History Evolution. Funct. Ecol. 1989, 3, 259–268. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Rose, K.A. Why Do Most Fish Produce so Many Tiny Offspring? Am. Nat. 1993, 142, 585–603. [Google Scholar] [CrossRef]
- Yuan, R.; Hascup, E.; Hascup, K.; Bartke, A. Relationships among Development, Growth, Body Size, Reproduction, Aging, and Longevity—Trade-Offs and Pace-Of-Life. Biochemistry 2023, 88, 1692–1703. [Google Scholar] [CrossRef]
- Giesel, J.T. Reproductive Strategies as Adaptations to Life in Temporally Heterogeneous Environments. Annu. Rev. Ecol. Syst. 1976, 7, 57–79. [Google Scholar] [CrossRef]
- Conover, D.O.; Munch, S.B. Sustaining Fisheries Yields over Evolutionary Time Scales. Science 2002, 297, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Conover, D.O.; Present, T.M.C. Countergradient Variation in Growth Rate: Compensation for Length of the Growing Season among Atlantic Silversides from Different Latitudes. Oecologia 1990, 83, 316–324. [Google Scholar] [CrossRef]
- Yamahira, K.; Conover, D.O. Intra- vs. interspecific latitudinal variation in growth: Adaptation to temperature or seasonality? Ecology 2002, 83, 1252–1262. [Google Scholar] [CrossRef]
- Uusi-Heikkilä, S.; Sävilammi, T.; Leder, E.; Arlinghaus, R.; Primmer, C.R. Rapid, Broad-scale Gene Expression Evolution in Experimentally Harvested Fish Populations. Mol. Ecol. 2017, 26, 3954–3967. [Google Scholar] [CrossRef]
- van Wijk, S.J.; Taylor, M.I.; Creer, S.; Dreyer, C.; Rodrigues, F.M.; Ramnarine, I.W.; van Oosterhout, C.; Carvalho, G.R. Experimental Harvesting of Fish Populations Drives Genetically Based Shifts in Body Size and Maturation. Front. Ecol. Environ. 2013, 11, 181–187. [Google Scholar] [CrossRef]
- Inoue, K.; Takei, Y. Asian Medaka Fishes Offer New Models for Studying Mechanisms of Seawater Adaptation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 136, 635–645. [Google Scholar] [CrossRef]
- Iwamatsu, T. Stages of Normal Development in the Medaka Oryzias latipes. Mech. Dev. 2004, 121, 605–618. [Google Scholar] [CrossRef]
- Im, J.H.; Gil, H.W.; Lee, T.H.; Kong, H.J.; Ahn, C.M.; Kim, B.S.; Kim, D.S.; Zhang, C.I.; Park, I.-S. Morphometric Characteristics and Fin Dimorphism between Male and Female on the Marine Medaka, Oryzias Dancena. Dev. Reprod. 2016, 20, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Amaral, I.P.G.; Johnston, I.A. Experimental Selection for Body Size at Age Modifies Early Life-History Traits and Muscle Gene Expression in Adult Zebrafish. J. Exp. Biol. 2012, 215, 3895–3904. [Google Scholar] [CrossRef] [PubMed]
- Therkildsen, N.O.; Wilder, A.P.; Conover, D.O.; Munch, S.B.; Baumann, H.; Palumbi, S.R. Contrasting Genomic Shifts Underlie Parallel Phenotypic Evolution in Response to Fishing. Science 2019, 365, 487–490. [Google Scholar] [CrossRef]
- Cleveland, W.S.; Devlin, S.J. Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Speakman, J.R. The Physiological Costs of Reproduction in Small Mammals. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 375–398. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R. Generalized Additive Models: Some Applications. J. Am. Stat. Assoc. 1987, 82, 371–386. [Google Scholar] [CrossRef]
- Uusi-Heikkilä, S. Implications of Size-Selective Fisheries on Sexual Selection. Evol. Appl. 2020, 13, 1487–1500. [Google Scholar] [CrossRef]
- Petit-Marty, N.; Liu, M.; Tan, I.Z.; Chung, A.; Terrasa, B.; Guijarro, B.; Ordines, F.; Ramírez-Amaro, S.; Massutí, E.; Schunter, C. Declining Population Sizes and Loss of Genetic Diversity in Commercial Fishes: A Simple Method for a First Diagnostic. Front. Mar. Sci. 2022, 9, 872537. [Google Scholar] [CrossRef]
- Castillo, P.R.; Ñiquen, M.; Cruz, L.L.; Guevara-Carrasco, R.; Cuadros, G.; Castillo, P.R.; Ñiquen, M.; Cruz, L.L.; Guevara-Carrasco, R.; Cuadros, G. Migration Behavior of Anchoveta (Engraulis ringens) in the Northern Humboldt Current System between September 2019 and September 2020. Lat. Am. J. Aquat. Res. 2021, 49, 702–716. [Google Scholar] [CrossRef]
- Grant, W.; Bowen, B. Shallow Population Histories in Deep Evolutionary Lineages of Marine Fishes: Insights from Sardines and Anchovies and Lessons for Conservation. J. Hered. 1998, 89, 415–426. [Google Scholar] [CrossRef]
- Hilborn, R.; Amoroso, R.O.; Anderson, C.M.; Baum, J.K.; Branch, T.A.; Costello, C.; de Moor, C.L.; Faraj, A.; Hively, D.; Jensen, O.P.; et al. Effective Fisheries Management Instrumental in Improving Fish Stock Status. Proc. Natl. Acad. Sci. USA 2020, 117, 2218–2224. [Google Scholar] [CrossRef]
- Trochta, J.T.; Branch, T.A.; Shelton, A.O.; Hay, D.E. The Highs and Lows of Herring: A Meta-Analysis of Patterns and Factors in Herring Collapse and Recovery. Fish Fish. 2020, 21, 639–662. [Google Scholar] [CrossRef]
- Walker, B.H.; Abel, N.; Anderies, J.M.; Ryan, P. Resilience, Adaptability, and Transformability in the Goulburn-Broken Catchment, Australia. Ecol. Soc. 2009, 14, 12. [Google Scholar] [CrossRef]
- Burnet, A.M.R. Studies on the Ecology of the New Zealand Long-Finned Eel, Anguilla Dieffenbachii Gray. Mar. Freshw. Res. 1952, 3, 32–63. [Google Scholar] [CrossRef]
- Stuart, R.E.; Closs, G.P.; Lokman, P.M.; Jellyman, D. The Influence of Environmental Cues on the Activity of Silver Eels (Anguilla dieffenbachii) in Lake Manapouri, New Zealand. N. Z. J. Mar. Freshw. Res. 2019, 53, 169–181. [Google Scholar] [CrossRef]
- Jørgensen, T. Long-Term Changes in Age at Sexual Maturity of Northeast Arctic Cod (Gadus morhua L.). ICES J. Mar. Sci. 1990, 46, 235–248. [Google Scholar] [CrossRef]
- Köster, F.W.; Trippel, E.A.; Tomkiewicz, J. Linking Size and Age at Sexual Maturation to Body Growth, Productivity and Recruitment of Atlantic Cod Stocks Spanning the North Atlantic. Fish. Res. 2013, 138, 52–61. [Google Scholar] [CrossRef]
- Berkeley, S.A.; Hixon, M.A.; Larson, R.J.; Love, M.S. Fisheries Sustainability via Protection of Age Structure and Spatial Distribution of Fish Populations. Fisheries 2004, 29, 23–32. [Google Scholar] [CrossRef]
- Heino, M.; Pauli, B.D.; Dieckmann, U. Fisheries-Induced Evolution. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 461–480. [Google Scholar] [CrossRef]
- Du, J.; Tian, H.; Xiang, Z.; Zhao, K.; Yu, L.; Duan, X.; Chen, D.; Xu, J.; Liu, M. Impact of the Fishing Ban on Fish Diversity and Population Structure in the Middle Reaches of the Yangtze River, China. Front. Environ. Sci. 2025, 12, 1530716. [Google Scholar] [CrossRef]
- Sloat, M.R.; Reeves, G.H. Individual Condition, Standard Metabolic Rate, and Rearing Temperature Influence Steelhead and Rainbow Trout (Oncorhynchus mykiss) Life Histories. Can. J. Fish. Aquat. Sci. 2014, 71, 491–501. [Google Scholar] [CrossRef]
- Stearns, S.C. Life-History Tactics: A Review of the Ideas. Q. Rev. Biol. 1976, 51, 3–47. [Google Scholar] [CrossRef]
- Blažek, R.; Polačik, M.; Reichard, M. Rapid Growth, Early Maturation and Short Generation Time in African Annual Fishes. EvoDevo 2013, 4, 24. [Google Scholar] [CrossRef]
- Sadovy, Y. The Threat of Fishing to Highly Fecund Fishes. J. Fish Biol. 2001, 59, 90–108. [Google Scholar] [CrossRef]
- Enberg, K.; Jørgensen, C.; Dunlop, E.S.; Varpe, Ø.; Boukal, D.S.; Baulier, L.; Eliassen, S.; Heino, M. Fishing-Induced Evolution of Growth: Concepts, Mechanisms and the Empirical Evidence. Mar. Ecol. 2012, 33, 1–25. [Google Scholar] [CrossRef]
- Jørgensen, C.; Holt, R.E. Natural Mortality: Its Ecology, How It Shapes Fish Life Histories, and Why It May Be Increased by Fishing. J. Sea Res. 2013, 75, 8–18. [Google Scholar] [CrossRef]
- Brown, S.C.; Giuliano, A.M.; Versak, B.A. Female Age at Maturity and Fecundity in Atlantic Striped Bass. Mar. Coast. Fish. 2024, 16, e10280. [Google Scholar] [CrossRef]
- Ferreri, R.; Genovese, S.; Barra, M.; Biagiotti, I.; Bourdeix, J.-H.; de Felice, A.; Gasparevic, D.; Hattab, T.; Iglesias, M.; Juretic, T.; et al. Variability in Size at Maturity of the European Anchovy (Engraulis encrasicolus) in the Mediterranean Sea. Mediterr. Mar. Sci. 2021, 22, 858–870. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, Y.; Yang, L.; Yan, L.; Zhang, Y.; Xu, M.; Tang, J.; Zhou, Y.; Hu, F.; Cheng, J. Incorporating Egg-Transporting Pathways into Conservation Plans of Spawning Areas: An Example of Small Yellow Croaker (Larimichthys polyactis) in the East China Sea Zone. Front. Mar. Sci. 2022, 9, 941411. [Google Scholar] [CrossRef]
- Yan, L.; Jiang, Y.; Xu, Q.; Ding, G.; Chen, X.; Liu, M. Reproductive Dynamics of the Large Yellow Croaker Larimichthys crocea (Sciaenidae), A Commercially Important Fishery Species in China. Front. Mar. Sci. 2022, 9, 868580. [Google Scholar] [CrossRef]
- Shen, G.; Heino, M. An Overview of Marine Fisheries Management in China. Mar. Policy 2014, 44, 265–272. [Google Scholar] [CrossRef]
- Sun, M.; Li, Y.; Chen, Y. Unveiling Unselective Fishing in China: A Nationwide Meta-Analysis of Multispecies Fisheries. Fish Fish. 2023, 24, 142–158. [Google Scholar] [CrossRef]
- Newman, S.J.; Brown, J.I.; Fairclough, D.V.; Wise, B.S.; Bellchambers, L.M.; Molony, B.W.; Lenanton, R.C.J.; Jackson, G.; Smith, K.A.; Gaughan, D.J.; et al. A Risk Assessment and Prioritisation Approach to the Selection of Indicator Species for the Assessment of Multi-Species, Multi-Gear, Multi-Sector Fishery Resources. Mar. Policy 2018, 88, 11–22. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, G.; Liu, G.; Sun, X.; Deng, W.; Lv, F.; Tian, Y.; Sun, P. Size-Selective Harvesting Effects on Reproductive Investment in Marine Medaka (Oryzias melastigma). Fishes 2025, 10, 112. https://doi.org/10.3390/fishes10030112
Gan G, Liu G, Sun X, Deng W, Lv F, Tian Y, Sun P. Size-Selective Harvesting Effects on Reproductive Investment in Marine Medaka (Oryzias melastigma). Fishes. 2025; 10(3):112. https://doi.org/10.3390/fishes10030112
Chicago/Turabian StyleGan, Guochen, Guankui Liu, Xinyao Sun, Wenbo Deng, Fengming Lv, Yongjun Tian, and Peng Sun. 2025. "Size-Selective Harvesting Effects on Reproductive Investment in Marine Medaka (Oryzias melastigma)" Fishes 10, no. 3: 112. https://doi.org/10.3390/fishes10030112
APA StyleGan, G., Liu, G., Sun, X., Deng, W., Lv, F., Tian, Y., & Sun, P. (2025). Size-Selective Harvesting Effects on Reproductive Investment in Marine Medaka (Oryzias melastigma). Fishes, 10(3), 112. https://doi.org/10.3390/fishes10030112






