Cyprinid herpesvirus 2 ORF41 Protein Degrades Pyruvate Dehydrogenase (PDH)-E1β to Promote Viral Replication in Gibel Carp Brain (GiCB) Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Cells
2.2. Effects of ORF41 on CyHV-2 Replication and Pyruvate and Lactic Acid Metabolism
2.3. Western Blot
2.4. GST Pull-Down Assay
2.5. Protein Identification by Mass Spectrometry
2.6. Binding Mode and Interaction Analysis
2.7. Conformational Stability Analysis
2.8. Statistical Analysis
3. Results
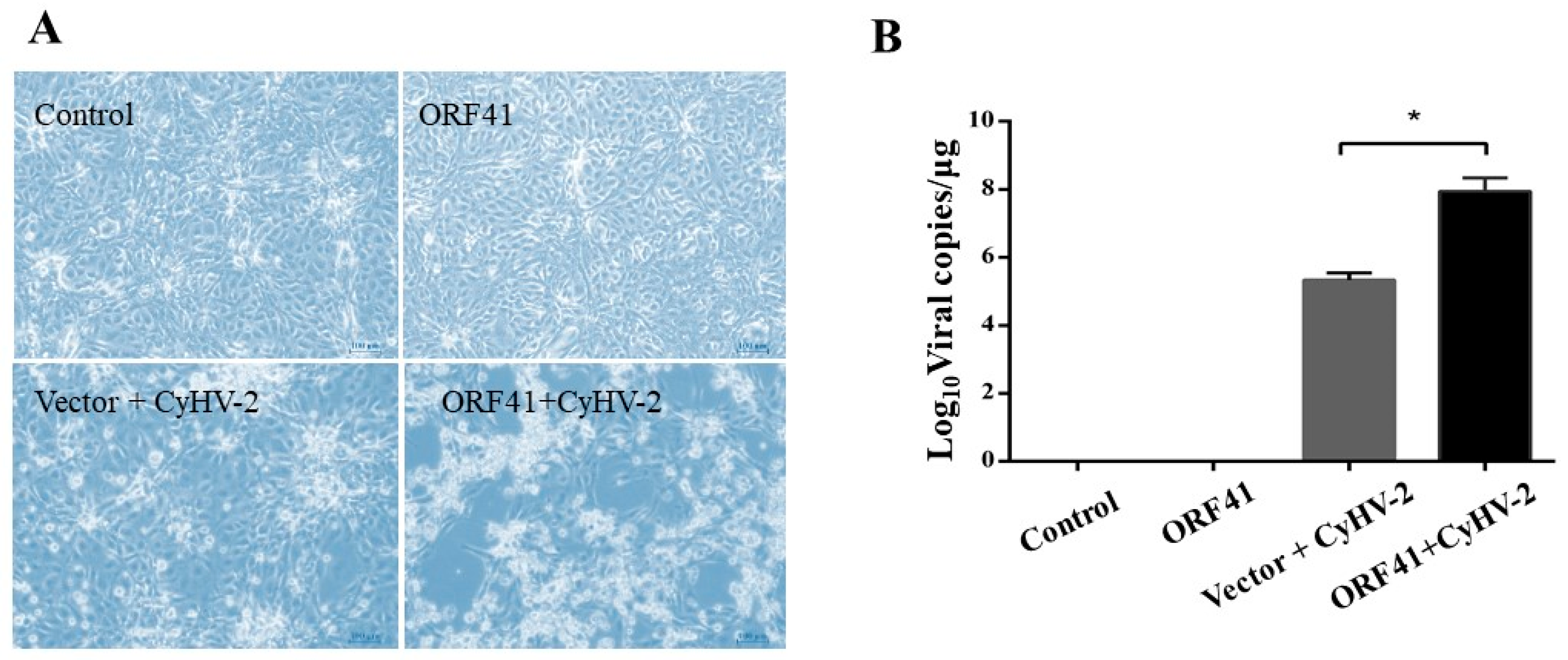
3.1. ORF41 Protein Promotes CyHV-2 Replication
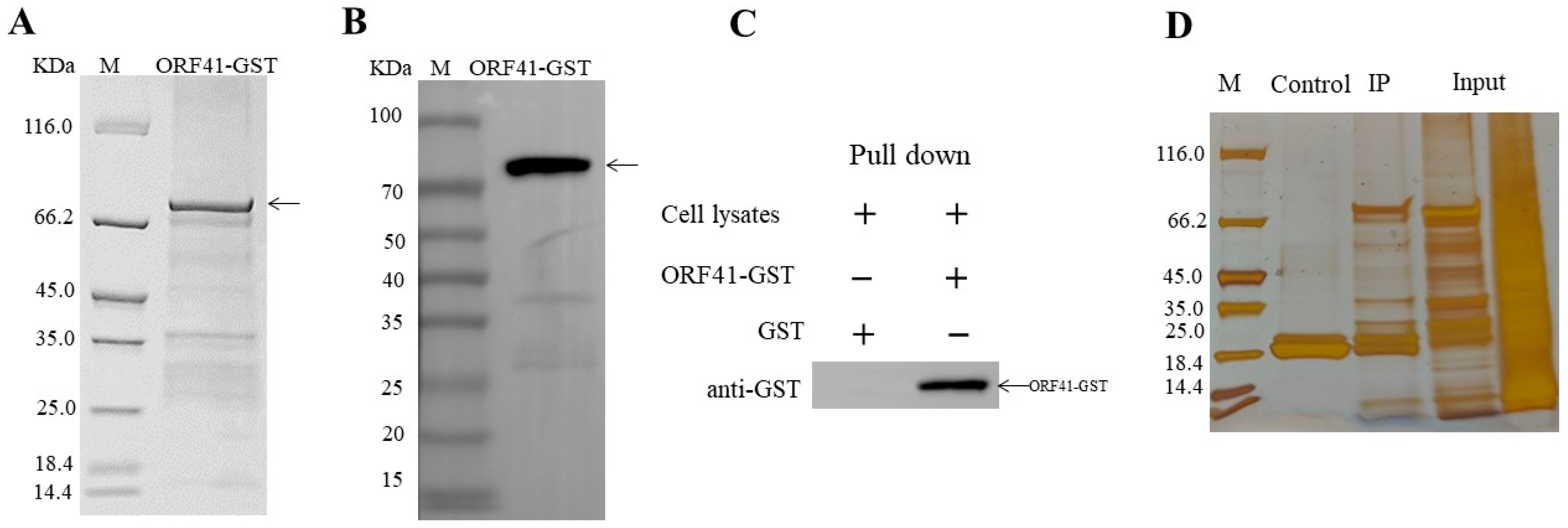
3.2. ORF41 Protein Interacts with PDH-E1β
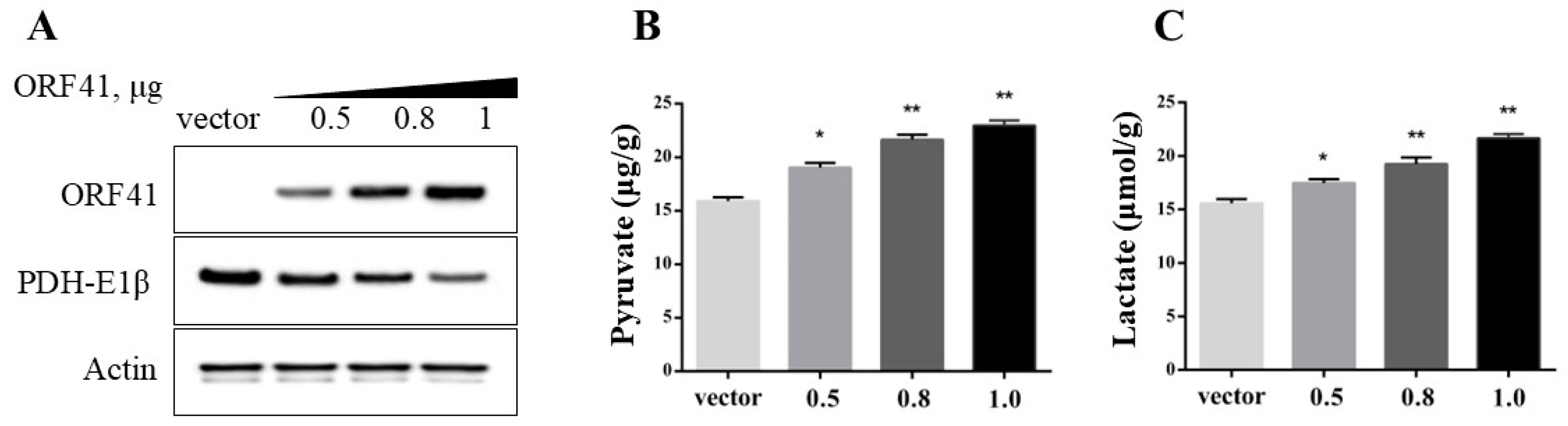
3.3. ORF41 Protein Decreases the Levels of PDH in GiCB Cells
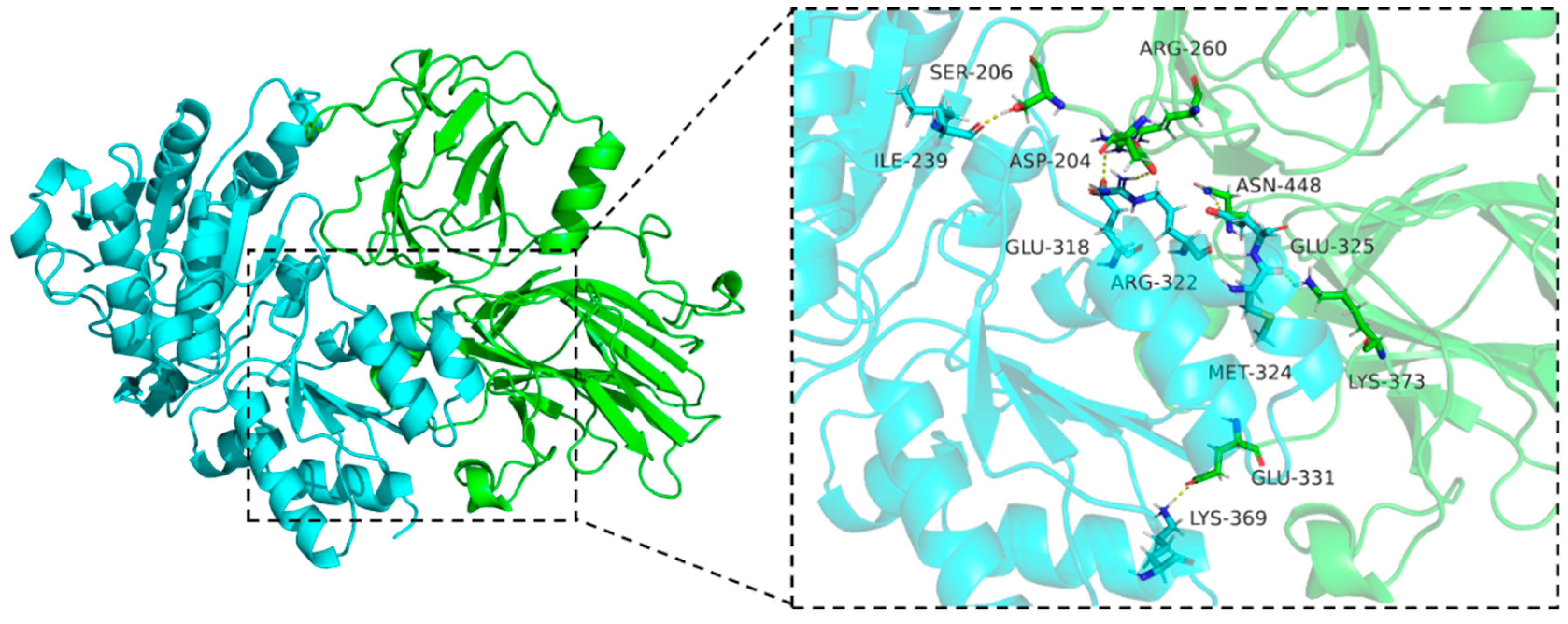
3.4. Molecular Docking
3.5. Molecular Dynamics Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Zeng, L.; Zhang, H.; Zhou, Y.; Ma, J.; Fan, Y. Cyprinid herpesvirus 2 infection emerged in cultured gibel carp, Carassius auratus gibelio in China. Vet. Microbiol. 2013, 166, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Dai, Y.P.; Tong, X.Y.; Zhang, Y.X.; Zhou, Y.; Cheng, J.L.; Jiang, Y.T.; Yang, R.L.; Wang, X.Y.; Cao, G.L.; et al. Circ-Udg Derived from Cyprinid Herpesvirus 2 Promotes Viral Replication. Microbiol. Spectr. 2022, 10, e00943-22. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, Y.; Xiao, S.; Li, P.; Lu, L.; Wang, H. Akt inhibitors prevent CyHV-2 infection in vitro. Fish Shellfish. Immunol. 2024, 154, 109940. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, J.; Liang, L.; Zheng, X.; Jia, P.; Shi, X.; Lan, W.; Xie, J.; Liu, H.; Xu, P. Mass mortality caused by Cyprinid Herpesvirus 2 (CyHV-2) in Prussian carp (Carassius gibelio) in China. Bull. Eur. Assoc. Fish Pathol. 2012, 32, 164–173. [Google Scholar]
- Ma, J.; Jiang, N.; LaPatra, S.E.; Jin, L.; Xu, J.; Fan, Y.; Zhou, Y.; Zeng, L. Establishment of a novel and highly permissive cell line for the efficient replication of cyprinid herpesvirus 2 (CyHV-2). Vet. Microbiol. 2015, 177, 315–325. [Google Scholar] [CrossRef]
- Tang, R.Z.; Lu, L.Q.; Wang, B.Y.; Yu, J.; Wang, H. Identification of the Immediate-Early Genes of Cyprinid Herpesvirus 2. Viruses 2020, 12, 994. [Google Scholar] [CrossRef]
- Polcicova, K.; Badurova, L.; Tomaskova, J. Metabolic reprogramming as a feast for virus replication. Acta Virol. 2020, 64, 201–215. [Google Scholar] [CrossRef]
- Su, G.; Liu, J.; Duan, C.; Fang, P.; Fang, L.; Zhou, Y.; Xiao, S. Enteric coronavirus PDCoV evokes a non-Warburg effect by hijacking pyruvic acid as a metabolic hub. Redox Biol. 2024, 71, 103112. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Goodwin, A.E.; Merry, G.E.; Sadler, J. Detection of the herpesviral hematopoietic necrosis disease agent (Cyprinid herpesvirus 2) in moribund and healthy goldfish: Validation of a quantitative PCR diagnostic method. Dis. Aquat. Org. 2006, 69, 137–143. [Google Scholar] [CrossRef]
- Sun, J.J.; Lan, J.F.; Zhao, X.F.; Vasta, G.R.; Wang, J.X. Binding of a C-type lectin’s coiled-coil domain to the Domeless receptor directly activates the JAK/STAT pathway in the shrimp immune response to bacterial infection. PLoS Pathog. 2017, 13, e1006626. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.W.; Ding, Z.Y.; Zhang, Y.; Pang, B.H.; Gui, L.; Liu, Y.; Lu, L.Q.; Roengjitd, P.; Wang, H. Establishment and Application of a Rapid Detection Method of Cyprinid Herpesvirus 2 (CyHV-2) in Aquacultural Waters by Using a Novel One-Pot RAA-CRISPR/Cas12a Combined Fe-Iron Flocculation Technology. J. Fish Dis. 2025, e14089. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.J.; Chen, Q.K.; Ren, Y.L.; Zhang, Y.; Lu, L.Q.; Gui, L.; Xu, D. The identification of viral ribonucleotide reductase encoded by ORF23 and ORF141 genes and effect on CyHV-2 replication. Front. Microbiol. 2023, 14, 1154840. [Google Scholar] [CrossRef]
- Li, R.Q.; Chen, C.; He, J.; Zhang, L.L.; Zhang, L.; Guo, Y.Y.; Zhang, W.T.; Tan, K.; Huang, J.H. E3 ligase ASB8 promotes porcine reproductive and respiratory syndrome virus proliferation by stabilizing the viral Nsp1α protein and degrading host IKKβ kinase. Virology 2019, 532, 55–68. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, F.; Liu, J.; Xu, Y.; Miao, Y.; Yuan, Y.; Chen, X.; Zhang, H.-G.; Wang, J.; Zheng, H.; et al. HSV-1-encoded ICP0 degrades the host deubiquitinase BRCC36 to antagonize interferon antiviral response. Mol. Immunol. 2021, 135, 28–35. [Google Scholar] [CrossRef]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The Pyruvate Dehydrogenase Complexes: Structure-based Function and Regulation. J. Biol. Chem. 2014, 289, 16615–16623. [Google Scholar] [CrossRef]
- Selvamani, S.P.; Khan, A.; Tay, E.S.E.; Garvey, M.; Ajoyan, H.; Diefenbach, E.; Gloss, B.S.; Tu, T.; George, J.; Douglas, M.W. Hepatitis B Virus and Hepatitis C Virus Affect Mitochondrial Function Through Different Metabolic Pathways, Explaining Virus-Specific Clinical Features of Chronic Hepatitis. J. Infect. Dis. 2024, 230, 1012–1022. [Google Scholar] [CrossRef]
- Vastag, L.; Koyuncu, E.; Grady, S.L.; Shenk, T.E.; Rabinowitz, J.D. Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism. PLoS Pathog. 2011, 7, e1002124. [Google Scholar] [CrossRef]
- Huang, P.; Wang, X.; Lei, M.Y.; Ma, Y.; Chen, H.L.; Sun, J.; Hu, Y.Z.; Shi, J.D. Metabolomics Profiles Reveal New Insights of Herpes Simplex Virus Type 1 Infection. Int. J. Mol. Sci. 2023, 24, 1521. [Google Scholar] [CrossRef] [PubMed]
- Delisle, L.; Fuhrmann, M.; Quéré, C.; Pauletto, M.; Pichereau, V.; Pernet, F.; Corporeau, C. The Voltage-Dependent Anion Channel (VDAC) of Pacific Oysters Crassostrea gigas Is Upaccumulated During Infection by the Ostreid Herpesvirus-1 (OsHV-1): An Indicator of the Warburg Effect. Mar. Biotechnol. 2018, 20, 87–97. [Google Scholar] [CrossRef] [PubMed]

| No | Description | MW (kDa) | Score | Sequence Coverage (%) |
|---|---|---|---|---|
| 1 | Pyruvate dehydrogenase E1 component subunit beta | 39.3 | 63.6 | 17.1 |
| 2 | Peptidyl-prolyl cis-trans isomerase | 26.4 | 36.6 | 11.9 |
| 3 | PRDX3 Thioredoxin-dependent peroxide reductase | 27.2 | 31.3 | 7.5 |
| 4 | Protein-L-isoaspartate O-methyltransferase | 26.4 | 20.1 | 8.8 |
| 5 | 2′,3′-cyclic-nucleotide 3′-phosphodiesterase | 23.9 | 19.3 | 9.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, M.; Xu, C.; Huang, Z.; Meng, Y.; Jiang, N.; Fan, Y.; Zhou, Y. Cyprinid herpesvirus 2 ORF41 Protein Degrades Pyruvate Dehydrogenase (PDH)-E1β to Promote Viral Replication in Gibel Carp Brain (GiCB) Cells. Fishes 2025, 10, 107. https://doi.org/10.3390/fishes10030107
Xue M, Xu C, Huang Z, Meng Y, Jiang N, Fan Y, Zhou Y. Cyprinid herpesvirus 2 ORF41 Protein Degrades Pyruvate Dehydrogenase (PDH)-E1β to Promote Viral Replication in Gibel Carp Brain (GiCB) Cells. Fishes. 2025; 10(3):107. https://doi.org/10.3390/fishes10030107
Chicago/Turabian StyleXue, Mingyang, Chen Xu, Zhenyu Huang, Yan Meng, Nan Jiang, Yuding Fan, and Yong Zhou. 2025. "Cyprinid herpesvirus 2 ORF41 Protein Degrades Pyruvate Dehydrogenase (PDH)-E1β to Promote Viral Replication in Gibel Carp Brain (GiCB) Cells" Fishes 10, no. 3: 107. https://doi.org/10.3390/fishes10030107
APA StyleXue, M., Xu, C., Huang, Z., Meng, Y., Jiang, N., Fan, Y., & Zhou, Y. (2025). Cyprinid herpesvirus 2 ORF41 Protein Degrades Pyruvate Dehydrogenase (PDH)-E1β to Promote Viral Replication in Gibel Carp Brain (GiCB) Cells. Fishes, 10(3), 107. https://doi.org/10.3390/fishes10030107







