Sediment Deposition Impacts on Fish Migration in Vertical Slot Fishways
Abstract
1. Introduction
2. Materials and Methods
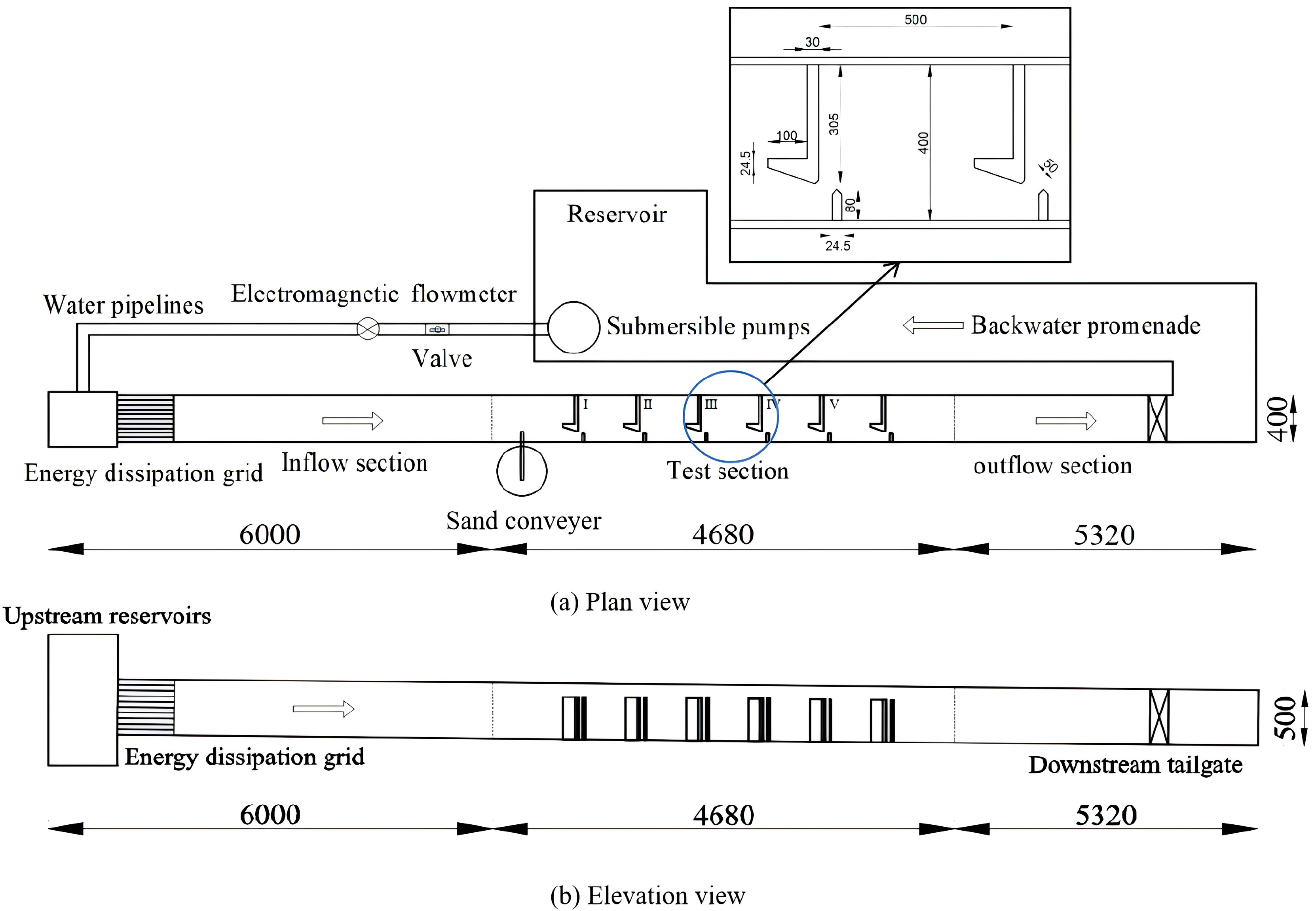
2.1. Experimental Setup
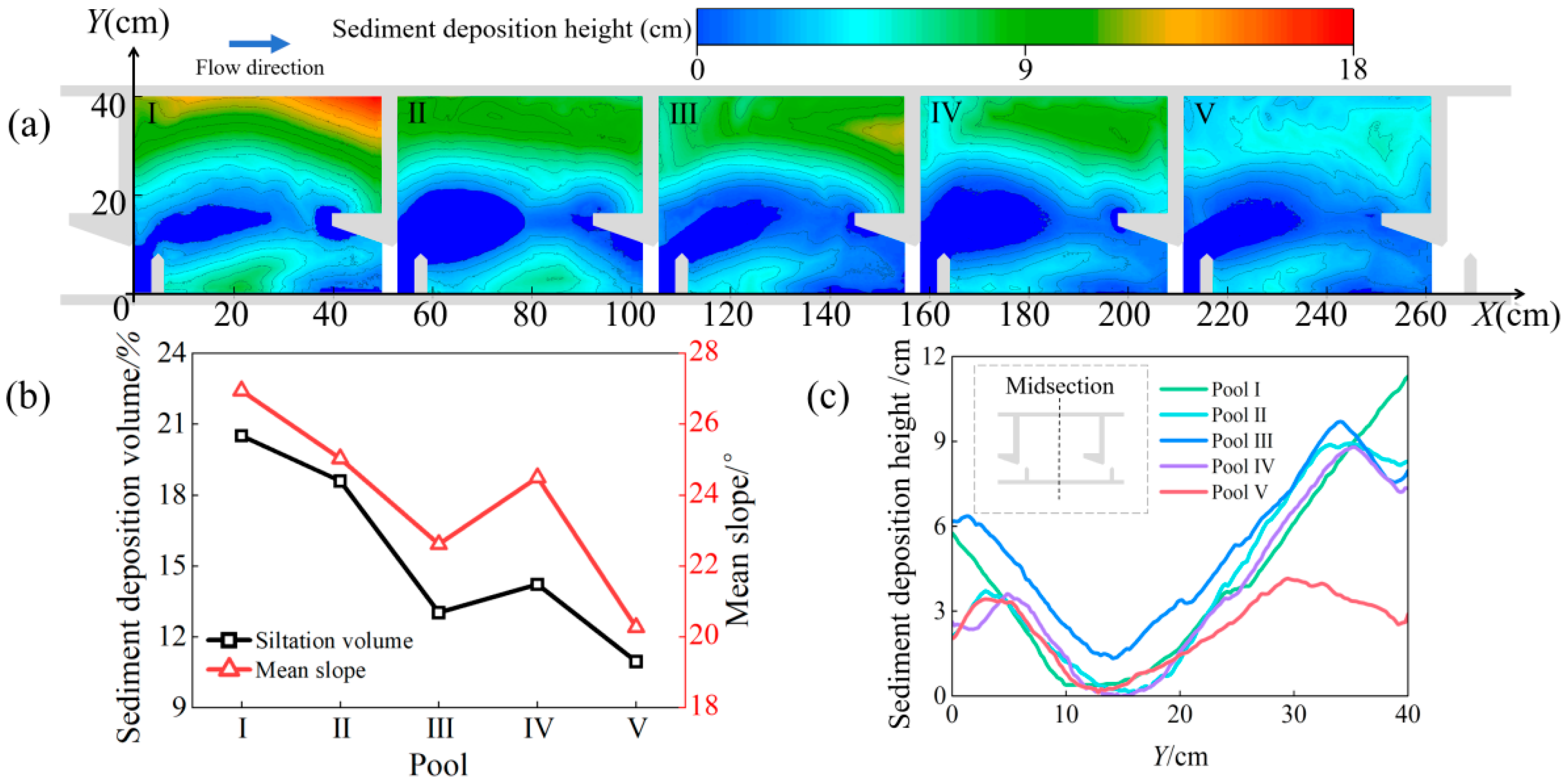
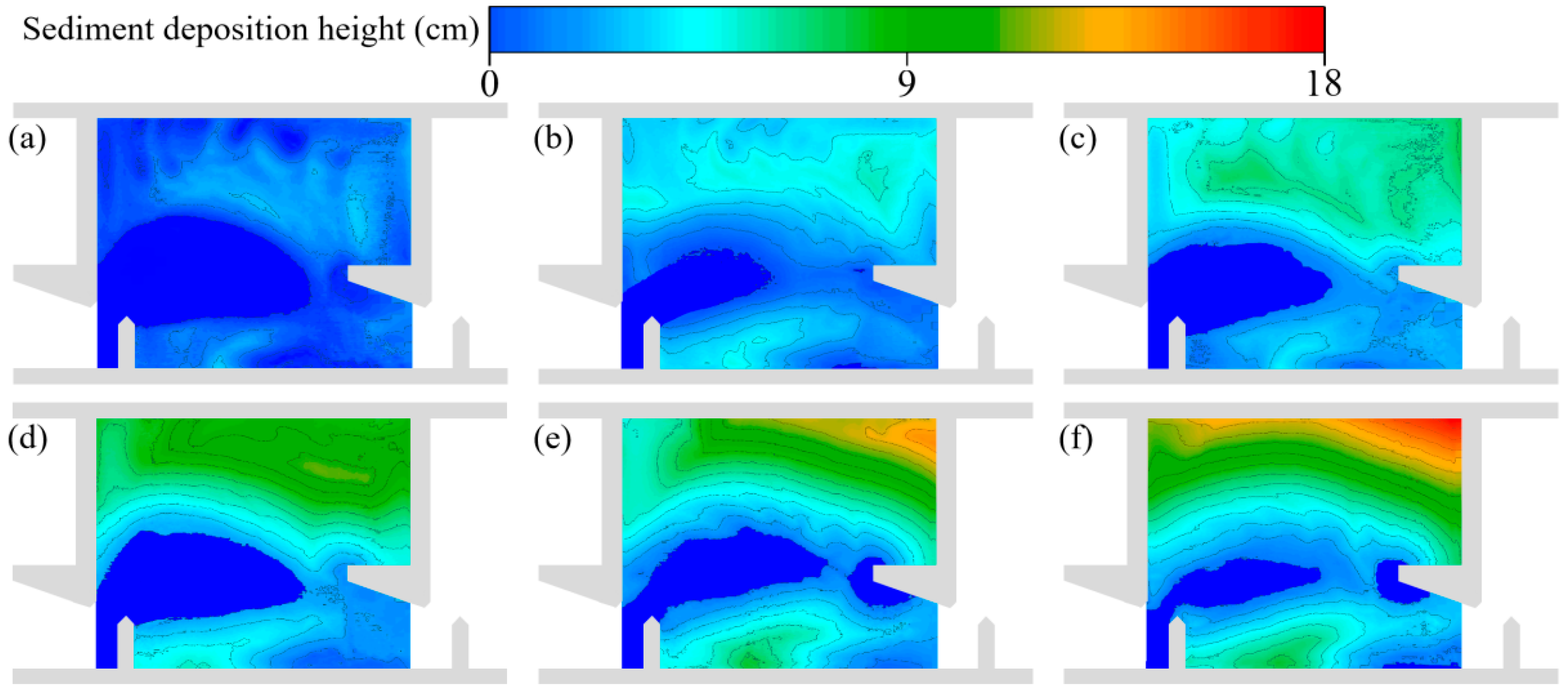
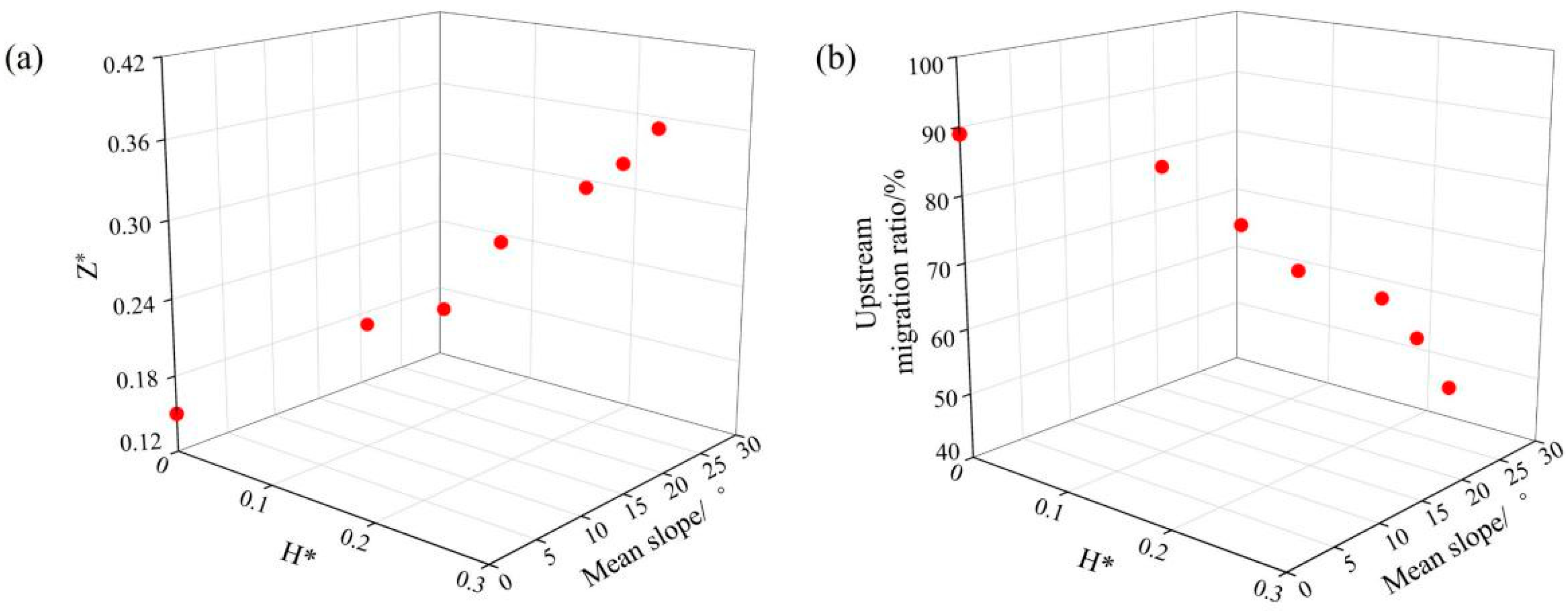
2.2. Sediment Deposition Experiment
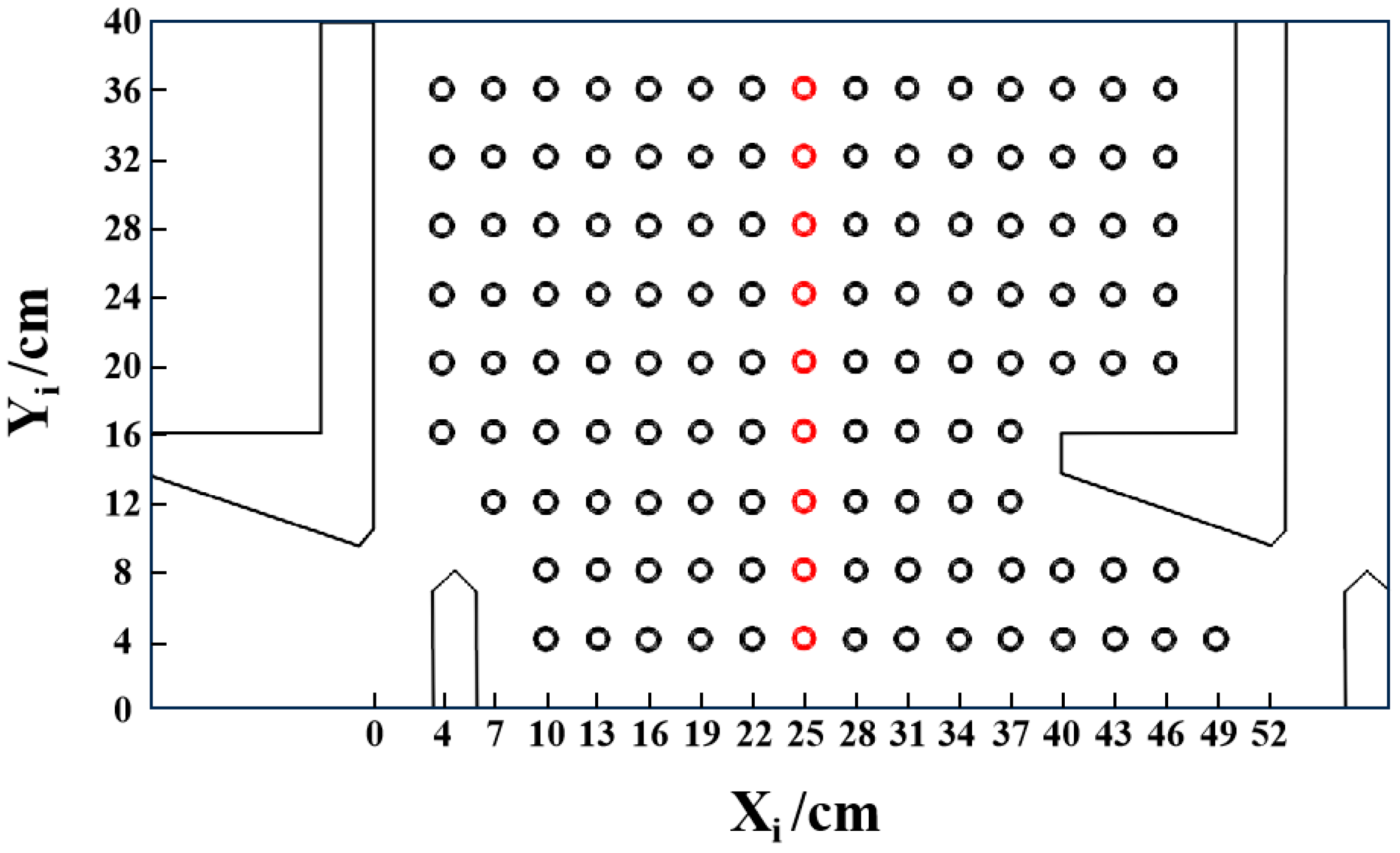
2.3. Fish Migration Experiment
2.4. Numerical Model
3. Results
3.1. Carp Migration Situation
3.1.1. Upstream Migration Ratio and Duration
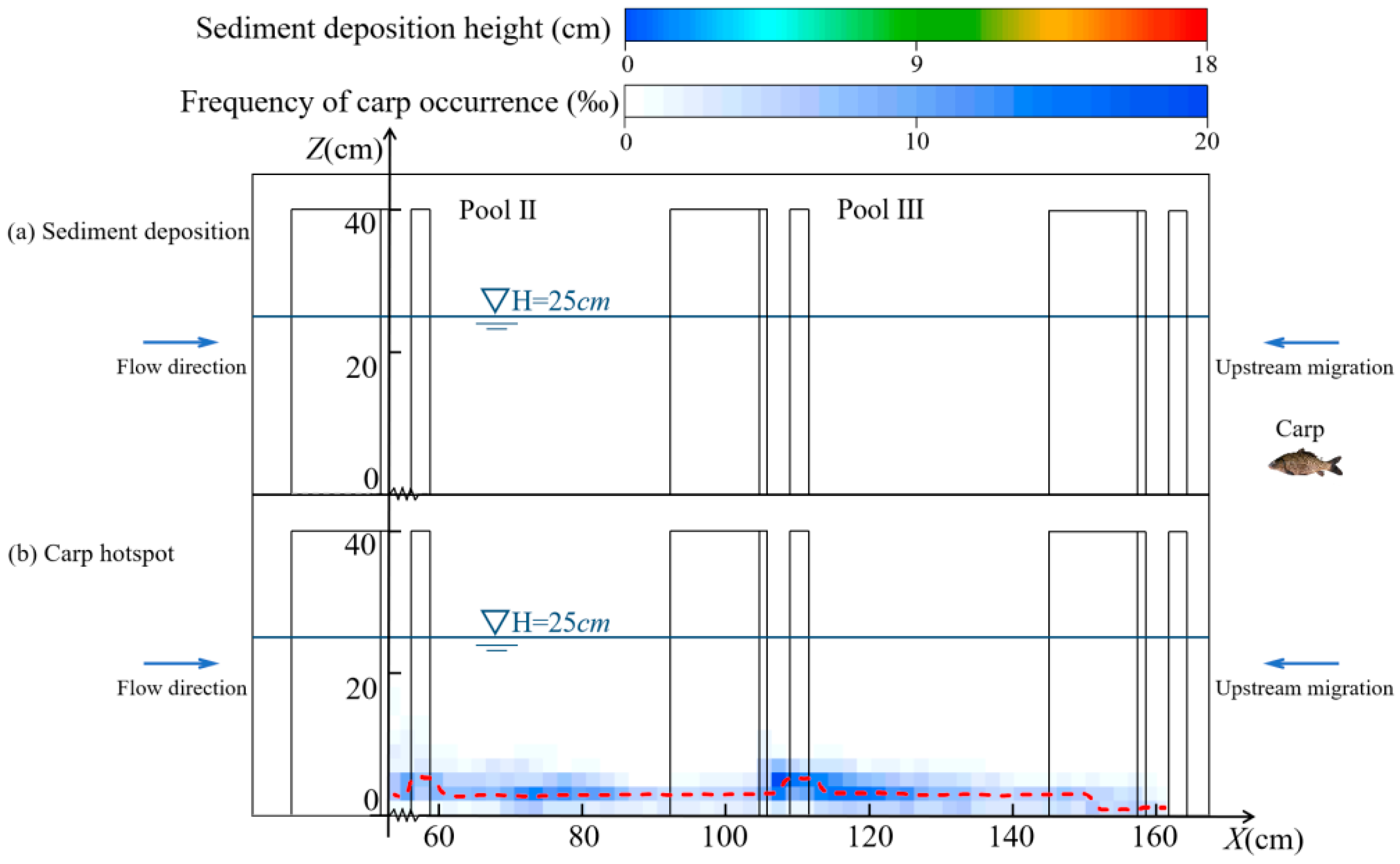
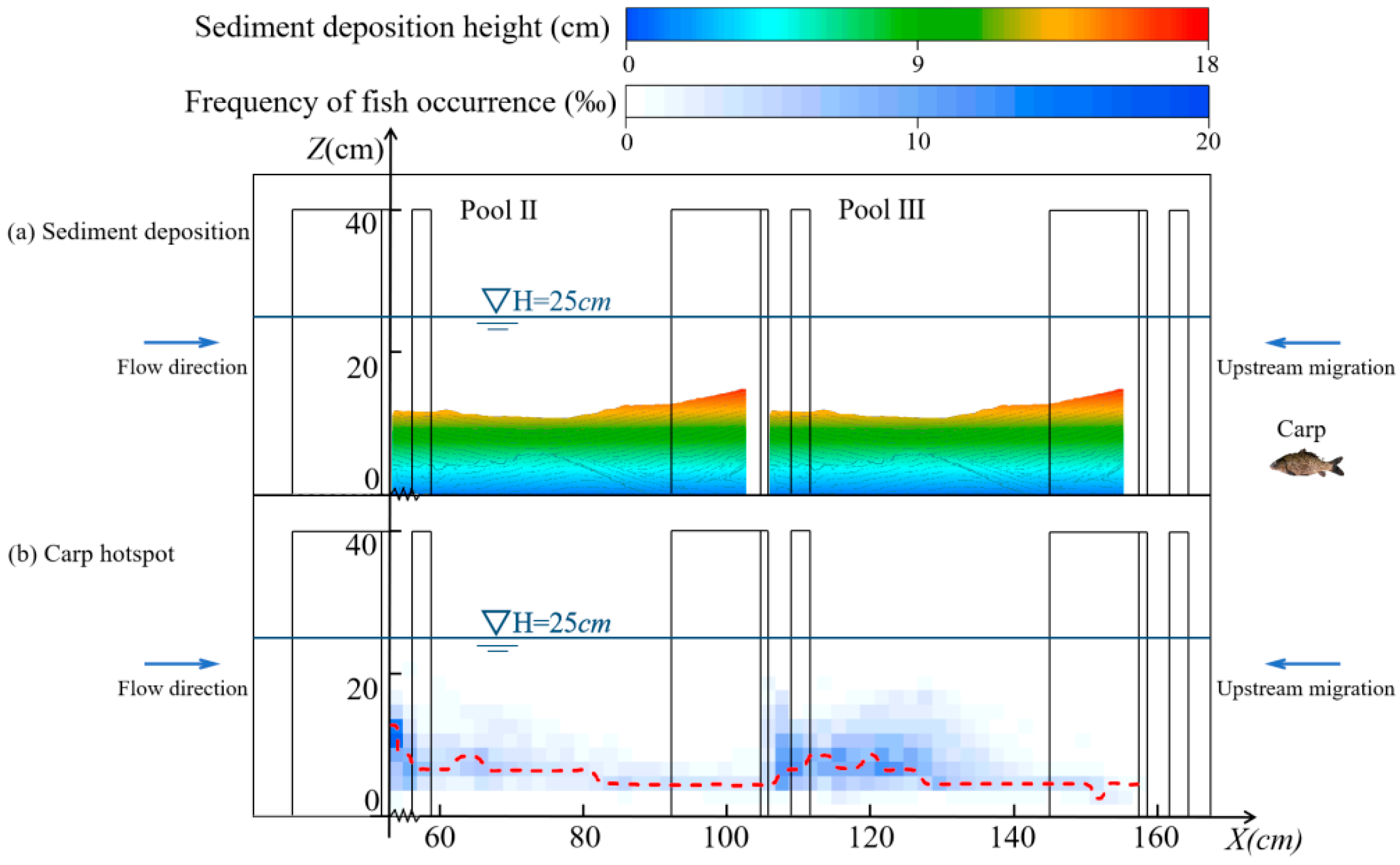
3.1.2. Migratory Characteristics in the Horizontal Direction
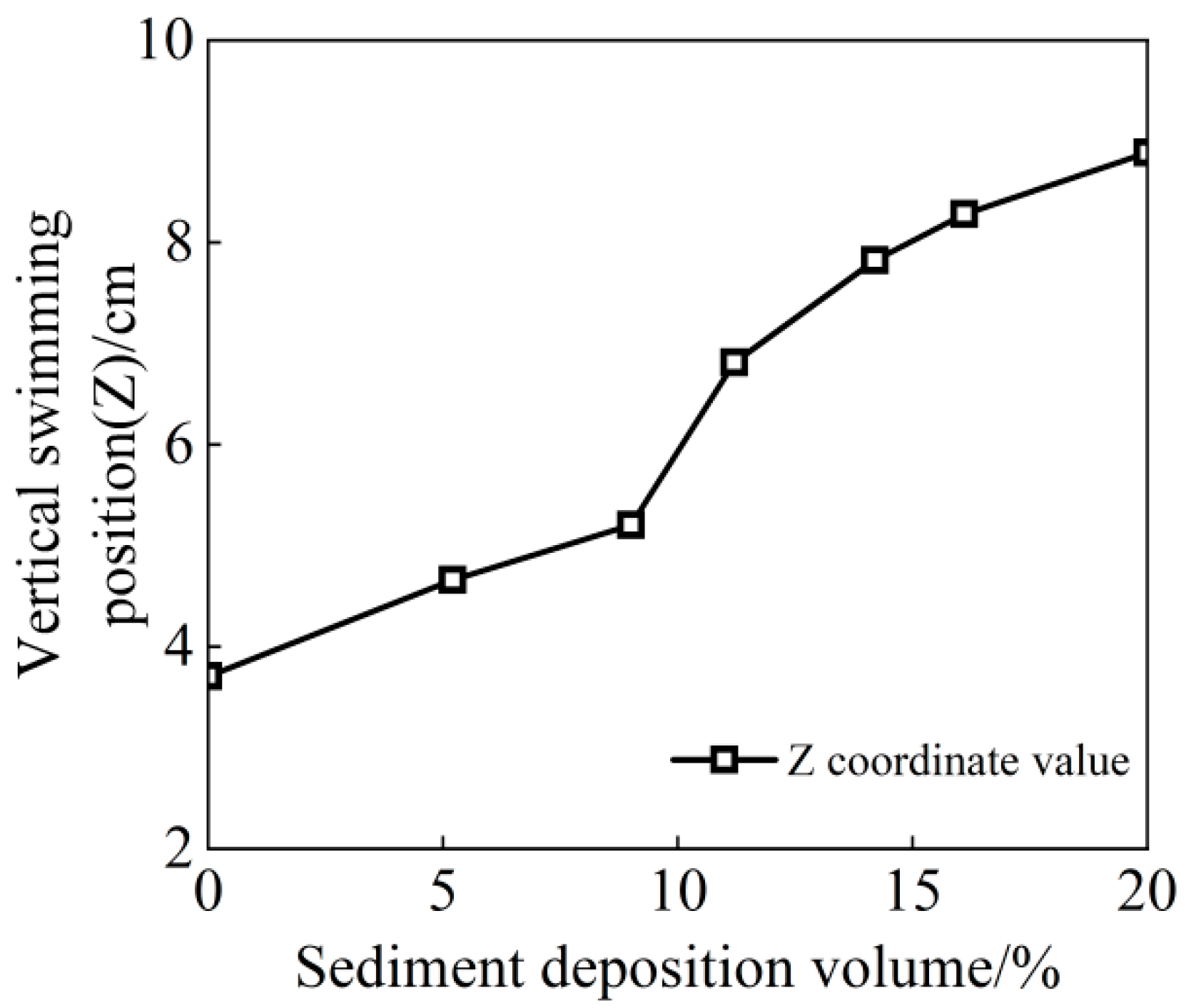
3.1.3. Migratory Characteristics in the Vertical Direction
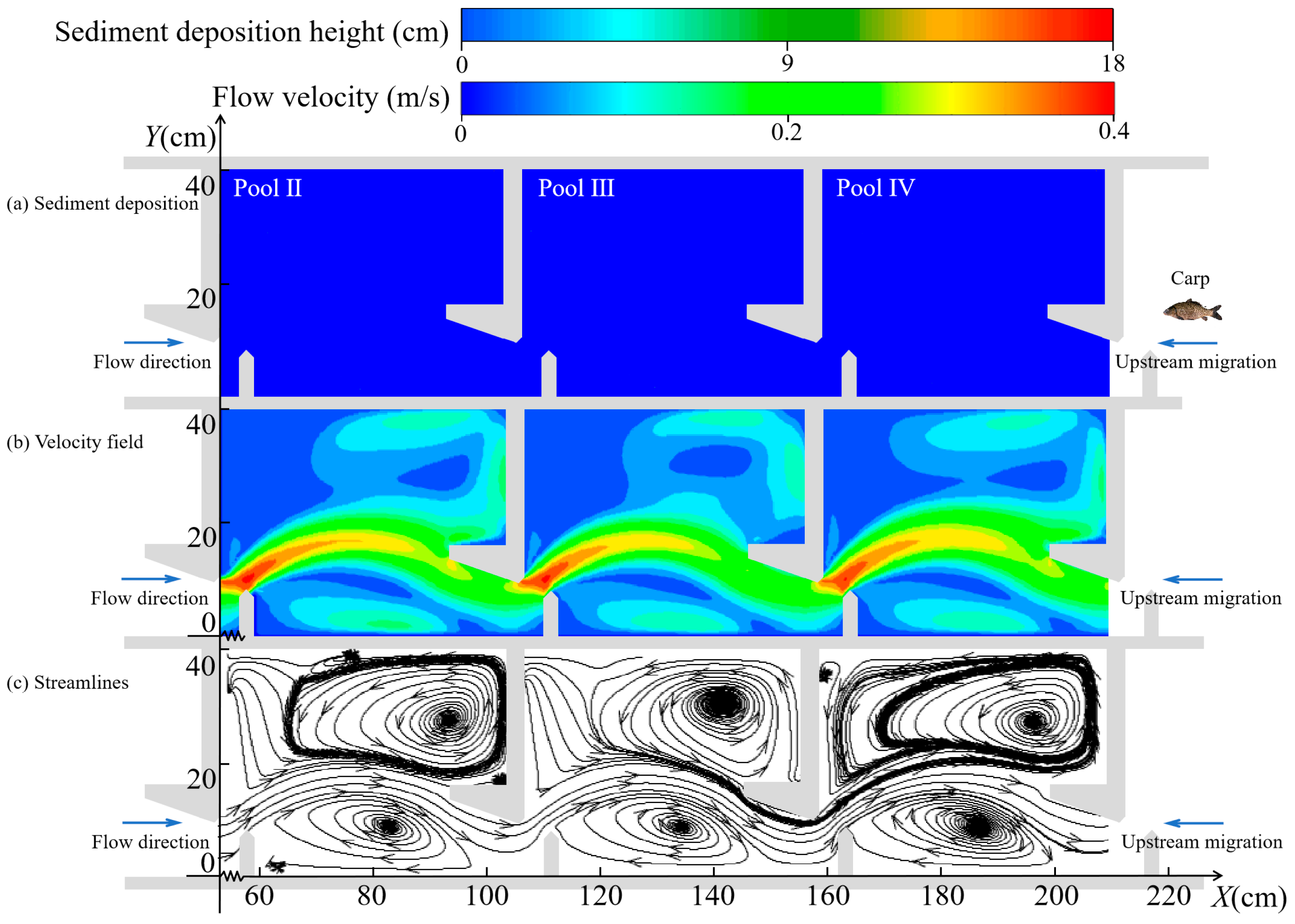
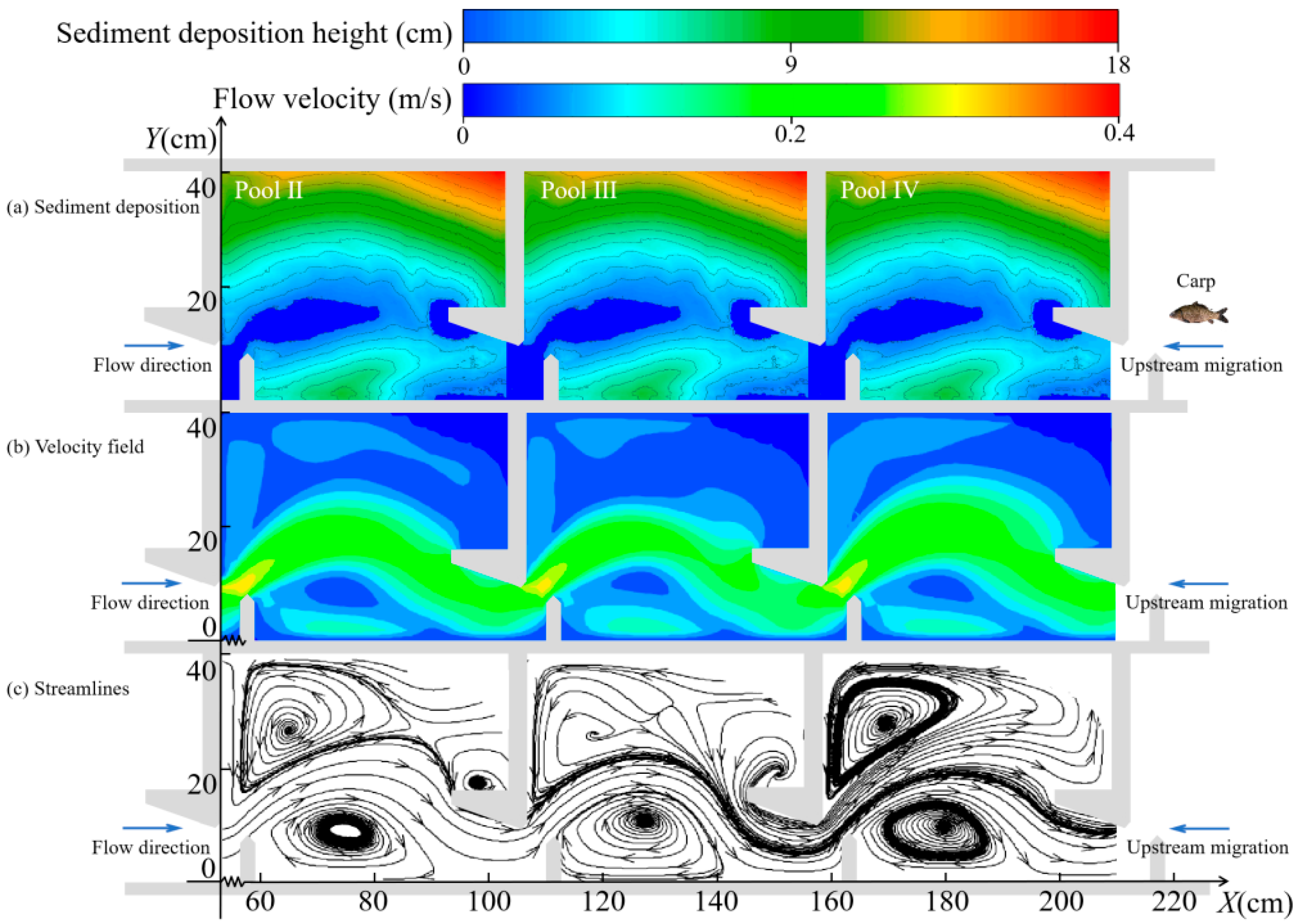
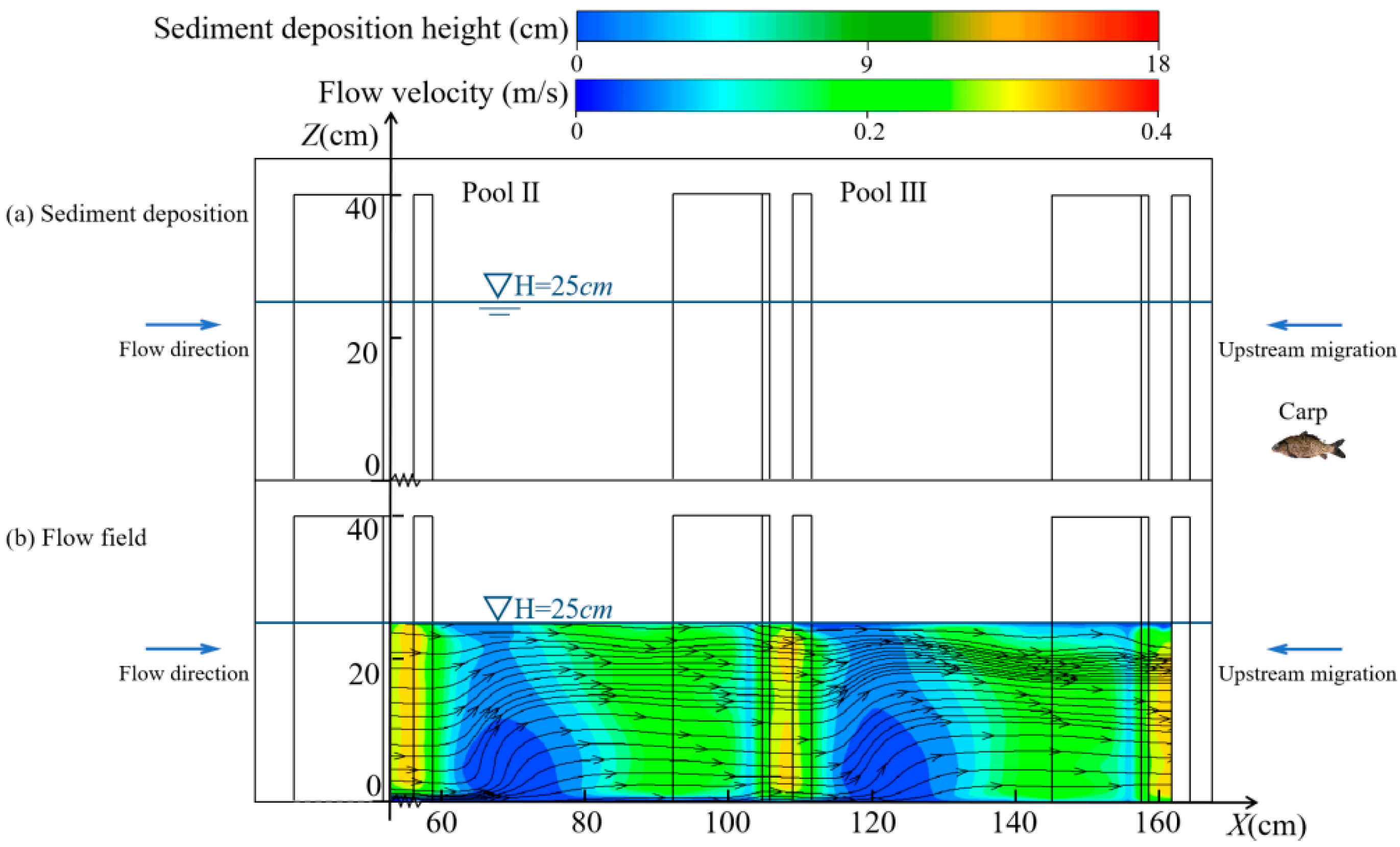
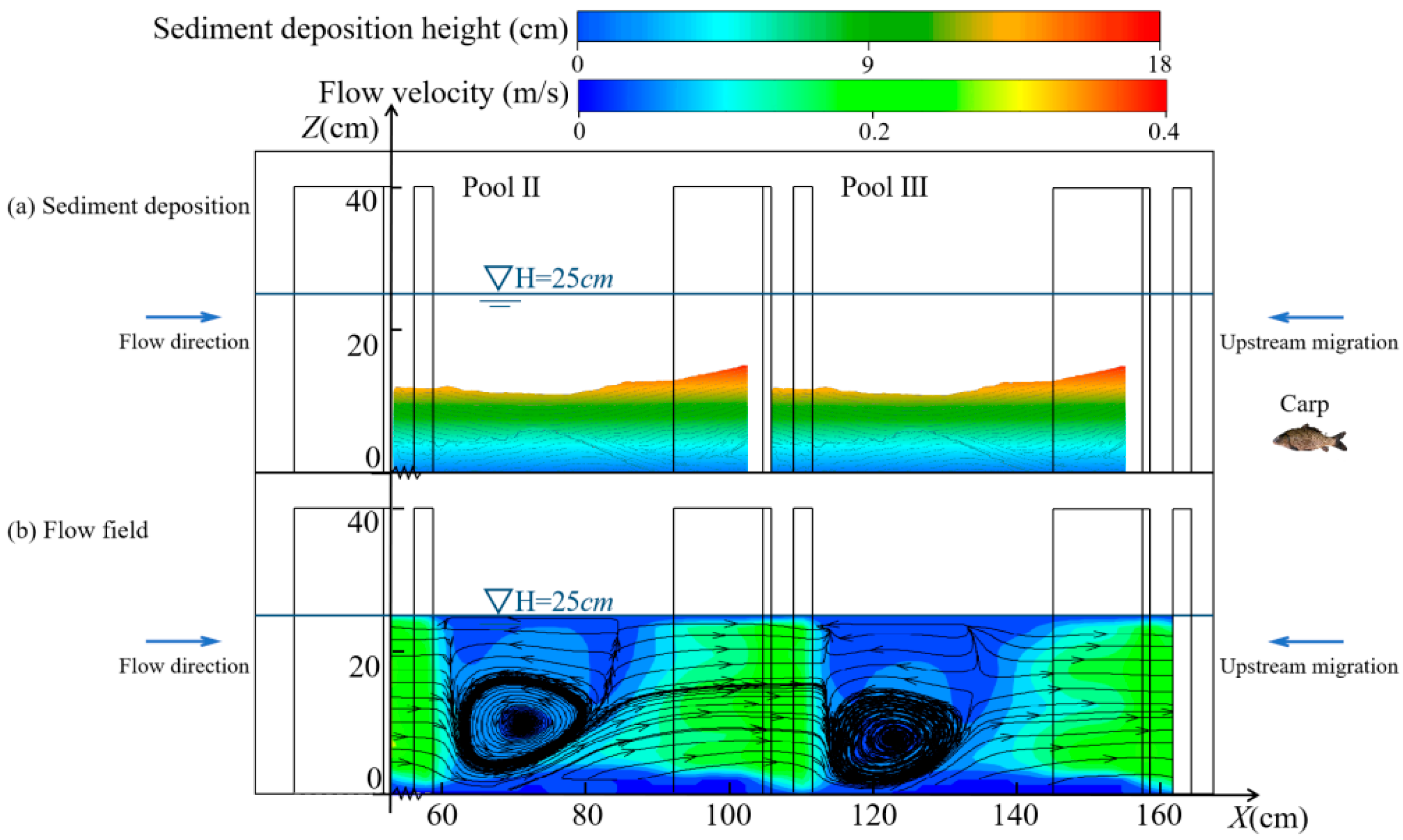
3.2. Variation in Hydraulic Characteristics
3.2.1. Changes in the Horizontal Direction
3.2.2. Changes in the Vertical Direction
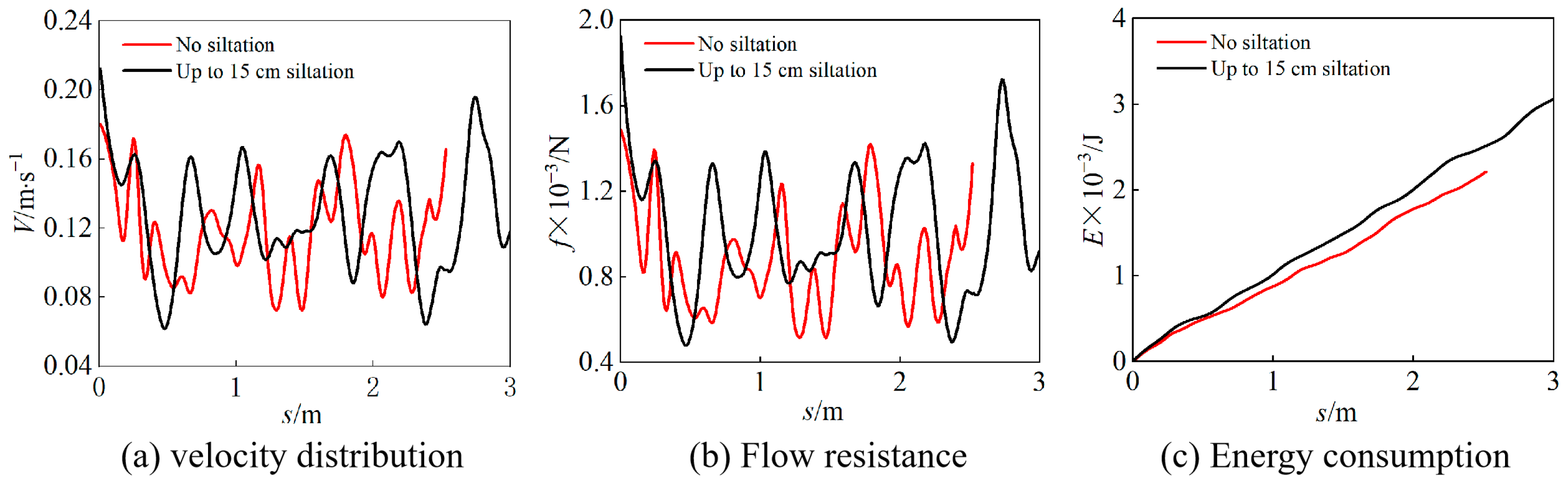
3.3. Energy Consumption of Carp Migration
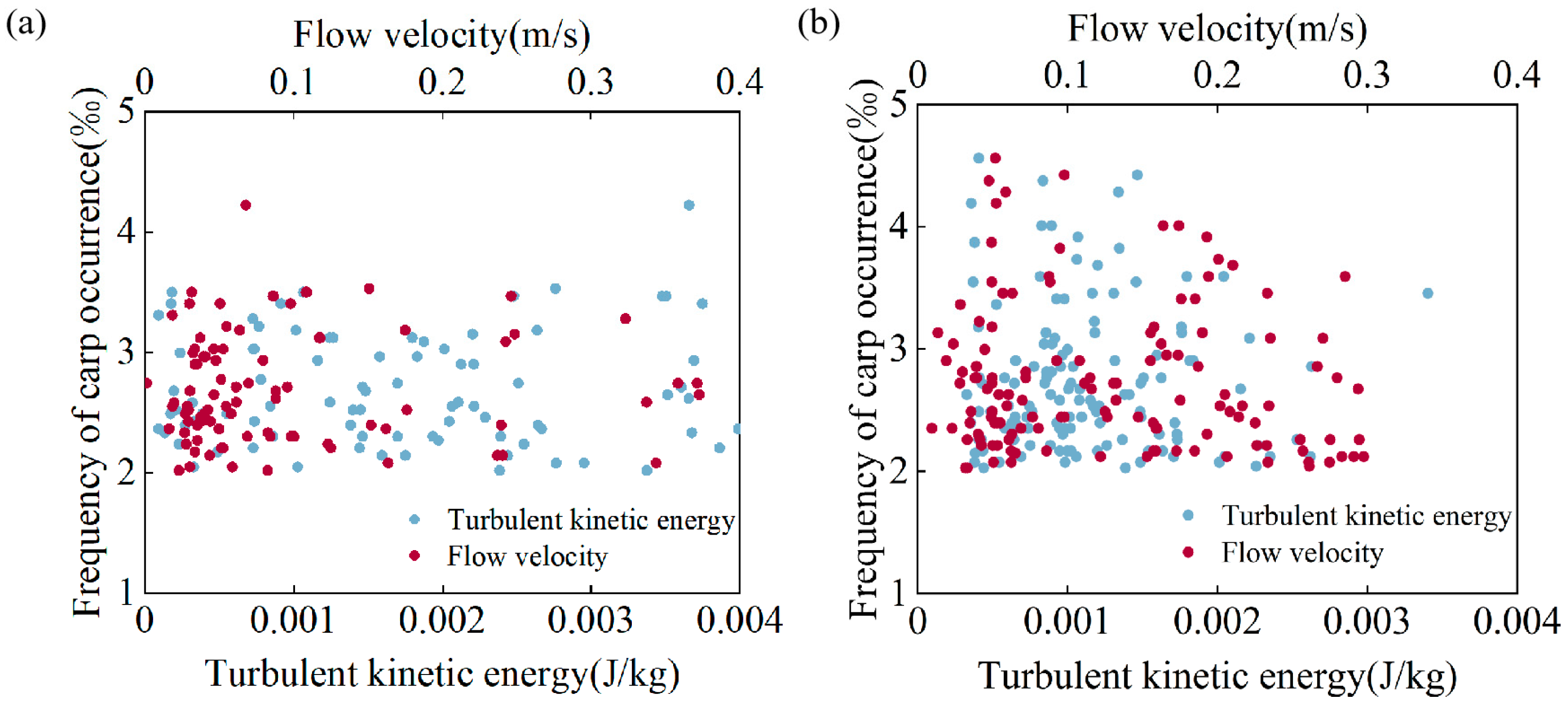
3.4. The Correlation Between the Upstream Trajectory and Hydraulic Factors
3.5. Multivariable Linear Regression Model
4. Discussions
4.1. Changes in Hydraulic Characteristics of the Pools
4.2. Changes in Fish Migration Behavior
4.3. Correlation and Multiple Linear Regression Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADV | Acoustic Doppler Velocimetry |
| RANS | Reynolds-averaged Navier–Stokes |
| VSF | Virtual Switch Framework |
| TruVOF | True Volume of Fluid |
| GMRES | Generalized Minimal Residual |
| TKE | Turbulent Kinetic Energy |
| VIF | Variance Inflation Factors |
References
- Mao, X. Review of fishway research in China. Ecol. Eng. 2018, 115, 91–95. [Google Scholar] [CrossRef]
- He, F.; Zarfl, C.; Tockner, K.; Olden, J.D.; Campos, Z.; Muniz, F.; Svenning, J.-C.; Jähnig, S.C. Hydropower impacts on riverine biodiversity. Nat. Rev. Earth Environ. 2024, 5, 755–772. [Google Scholar] [CrossRef]
- Verhelst, P.; Reubens, J.; Buysse, D.; Goethals, P.; Van Wichelen, J.; Moens, T. Toward a roadmap for diadromous fish conservation: The Big Five considerations. Front. Ecol. Environ. 2021, 19, 396–403. [Google Scholar] [CrossRef]
- Li, M.; Shi, X.; Jin, Z.; Ke, S.; Lin, C.; An, R.; Li, J.; Katopodis, C.; Ne, L. Behaviour and ability of a cyprinid (Schizopygopsis younghusbandi) to cope with accelerating flows when migrating downstream. River Res. Appl. 2020, 37, 1168–1179. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Xu, J.; Zeng, G.; Sang, L.; Liu, Q.; Yin, Z.; Dai, J.; Yin, D.; Liang, J.; et al. Effects of dam construction on biodiversity: A review. J. Clean. Prod. 2019, 221, 480–489. [Google Scholar] [CrossRef]
- Yang, X.; Shi, H.; Liu, X.; Ou, Y.; Liu, X.; Li, M.; Yuan, Q. Effect of total dissolved gas supersaturation on the passage behavior of silver carp (Hypophthalmichthys molitrix) and ya-fish (Schizothorax prenanti) through an experimental vertical slot fishway. Ecotoxicol. Environ. Saf. 2024, 277, 116370. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, V.; Schmitt, R.J.P. Strategic restoration-development mitigates tradeoffs between hydropower and fish habitat fragmentation in the Mekong. One Earth 2024, 7, 1096–1107. [Google Scholar] [CrossRef]
- Katopodis, C.; Cai, L.; Johnson, D. Sturgeon survival: The role of swimming performance and fish passage research. Fish. Res. 2019, 212, 162–171. [Google Scholar] [CrossRef]
- Bravo Córdoba, F.; García-Vega, A.; Fuentes-Pérez, J.; Celestino, L.; Makrakis, S.; Sanz-Ronda, F. Bidirectional connectivity in fishways: A mitigation for impacts on fish migration of small hydropower facilities. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 549–565. [Google Scholar] [CrossRef]
- Chorda, J.; Maubourguet, M.M.; Roux, H.; Larinier, M.; Tarrade, L.; David, L. Two-dimensional free surface flow numerical model for vertical slot fishways. J. Hydraul. Res. 2010, 48, 141–151. [Google Scholar] [CrossRef]
- Li, G.; Sun, S.; Liu, H.; Zheng, T. Schizothorax prenanti swimming behavior in response to different flow patterns in vertical slot fishways with different slot positions. Sci. Total Environ. 2021, 754, 142142. [Google Scholar] [CrossRef] [PubMed]
- Rajaratnam, N.; Vinne, G.; Katopodis, C. Hydraulics of Vertical Slot Fishways. J. Hydraul. Eng. 1986, 112, 909–927. [Google Scholar] [CrossRef]
- Marriner, B.A.; Baki, A.B.M.; Zhu, D.Z.; Cooke, S.J.; Katopodis, C. The hydraulics of a vertical slot fishway: A case study on the multi-species Vianney-Legendre fishway in Quebec, Canada. Ecol. Eng. 2016, 90, 190–202. [Google Scholar] [CrossRef]
- Rajaratnam, N.; Katopodis, C.; Solanki, S. New design for vertical slot fishways. Can. J. Civ. Eng. 2011, 19, 402–414. [Google Scholar] [CrossRef]
- Khan, L. A three-dimensional computational fluid dynamics (CFD) model analysis of free surface hydrodynamics and fish passage energetics in a vertical-slot fishway. N. Am. J. Fish. Manag. 2006, 26, 255–267. [Google Scholar] [CrossRef]
- Sanagiotto, D.; Rossi, J.; Lauffer, L.; Bravo, J. Three-dimensional numerical simulation of flow in vertical slot fishways: Validation of the model and characterization of the flow. RBRH 2019, 24, e20. [Google Scholar] [CrossRef]
- Liao, L.; Chen, M.; An, R.; Li, J.; Tang, X.; Yan, Z. Identifying three-dimensional swimming corridors for fish to match their swimming characteristics under different hydropower plant operations: Optimization of entrance location for fish-passing facilities. Sci. Total Environ. 2022, 822, 153599. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liao, L.; Chen, M.; Li, J.; An, R. Relation between fish morphological differentiation and pressure drag difference. Ecol. Indic. 2023, 156, 111071. [Google Scholar] [CrossRef]
- Liao, L.; Li, J.; Chen, M.; An, R. Effects of hydraulic cues in barrier environments on fish navigation downstream of dams. J. Environ. Manag. 2024, 365, 121495. [Google Scholar] [CrossRef]
- Katopodis, C.; Williams, J.G. The development of fish passage research in a historical context. Ecol. Eng. 2012, 48, 8–18. [Google Scholar] [CrossRef]
- Li, G.; Sun, S.; Zhang, C.; Liu, H.; Zheng, T. Evaluation of flow patterns in vertical slot fishways with different slot positions based on a comparison passage experiment for juvenile grass carp. Ecol. Eng. 2019, 133, 148–159. [Google Scholar] [CrossRef]
- Plesiński, K.; Bylak, A.; Radecki-Pawlik, A.; Mikołajczyk, T.; Kukuła, K. Possibilities of fish passage through the block ramp: Model-based estimation of permeability. Sci. Total Environ. 2018, 631–632, 1201–1211. [Google Scholar] [CrossRef]
- Schenk, L.; Bragg, H. Sediment transport, turbidity, and dissolved oxygen responses to annual streambed drawdowns for downstream fish passage in a flood control reservoir. J. Environ. Manag. 2021, 295, 113068. [Google Scholar] [CrossRef]
- Pennock, C.; Bender, D.; Hofmeier, J.; Travis, J.; Waters, R.; Weaver, V.; Gido, K. Can fishways mitigate fragmentation effects on Great Plains fish communities? Can. J. Fish. Aquat. Sci. 2017, 75, 121–130. [Google Scholar] [CrossRef]
- Chen, S.C.; Wang, S.C.; Tfwala, S.S. Hydraulics driven upstream migration of Taiwanese indigenous fishes in a fish-bone-type fishway. Ecol. Eng. 2017, 108, 179–193. [Google Scholar] [CrossRef]
- Guo, J.; Rui, J.L. Question and Suggestion on Fishway Construction in China: Lesson Learned from the Operation of Yangtang Lock Fishway. Water Power 2010, 36, 8–10+19. [Google Scholar] [CrossRef]
- Wu, X.C.; Shi, J.Q. Construction and management of fish passage on Shaliu River adjacent to Qinghai Lake based on ecological restoration. Trans. Chin. Soc. Agric. Eng. 2014, 30, 130–136. [Google Scholar] [CrossRef]
- Chang, W.Y.; Lien, H.C.; Lee, L.C.; Tsai, W.F.; Lai, J.S.; Tan, Y.C.; Guo, W.D. Simulations of 3D flow and sediment transport in a fishway. In Proceedings of the 19th IASTED International Conference on Modelling and Simulation, Québec City, QC, Canada, 26–28 May 2008; ACTA Press: Anaheim, CA, USA, 2008; pp. 187–192. Available online: https://dl.acm.org/doi/10.5555/1722520.1722556 (accessed on 7 October 2025).
- Mumot, J.; Tymiński, T. Hydraulic research of sediment transport in the vertical slot fish passes. J. Ecol. Eng. 2016, 17, 143–148. [Google Scholar] [CrossRef]
- Tymiński, T.; Xia, J.-X.; Mumot, J.; Karpowicz, D. Sedimentation of load in a step-pool rock ramp fishway with biotechnical embedded elements. Meteorol. Hydrol. Water Manag. 2017, 5, 35–42. [Google Scholar] [CrossRef]
- Yasuda, Y.; Ohnishi, T.; Ohtsu, I. Flow Characteristics of Pool-Type Fishway with Trapezoidal Section. In Advances in Water Resources & Hydraulic Engineering, Proceedings of the 16th IAHR-APD Congress and 3rd Symposium of IAHR-ISHS, Nanjing, China, 20–23 October 2008; Altinakar, M., Kokpinar, M.A., Darama, Y., Yegen, I., Harman, I.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 345–352. [Google Scholar] [CrossRef]
- Cong, J.; Cui, D.; Yu, F.; Wang, J.Y.; Xie, L.Q. Hydromechanical analysis of desilting mechanism of ecological arc-shaped baffle fishway. Adv. Mater. Res. 2012, 511, 154–158. [Google Scholar] [CrossRef]
- Duguay, J.; Foster, B.; Lacey, J.; Castro-Santos, T. Sediment infilling benefits rainbow trout passage in a baffled channel. Ecol. Eng. 2018, 125, 38–49. [Google Scholar] [CrossRef]
- Hu, Q.; Miao, J.S.; Li, S.Z.; Liu, C.L.; Su, X.H. Study on the Growth Characteristics of Carp in Jili Lake. Xinjiang Farm Res. Sci. Technol. 2018, 41, 28–30. [Google Scholar]
- Katopodis, C.; Gervais, R. Fish Swimming Performance Database and Analyses; Canadian Science Advisory Secretariat: Ottawa, ON, Canada, 2016. [Google Scholar]
- Beamish, F.W.H. 2—Swimming Capacity. Fish Physiol. 1978, 7, 101–187. [Google Scholar] [CrossRef]
- Tan, J.; Liu, Z.; Wang, Y.; Wang, Y.; Ke, S.; Shi, X. Analysis of Movements and Behavior of Bighead Carps (Hypophthalmichthys nobilis) Considering Fish Passage Energetics in an Experimental Vertical Slot Fishway. Animals 2022, 12, 1725. [Google Scholar] [CrossRef]
- Snyder, R.J. Physiological Ecology of Pacific Salmon. Trans. Am. Fish. Soc. 1996, 125, 819–820. [Google Scholar] [CrossRef]
- Pettersson, L.B.; Brönmark, C. Energetic consequences of an inducible morphological defence in crucian carp. Oecologia 1999, 121, 12–18. [Google Scholar] [CrossRef]
- FLOW-3D®, Version 2023R1; Software for Computational Fluid Dynamics; Flow Science, Inc.: Santa Fe, NM, USA, 2023.
- Quaresma, A.L.; Romão, F.; Branco, P.; Ferreira, M.T.; Pinheiro, A.N. Multi Slot Versus Single Slot Pool-Type Fishways: A Modelling Approach to Compare Hydrodynamics. Ecol. Eng. 2018, 122, 197–206. [Google Scholar] [CrossRef]
- Northcutt, R.G. Evolution of Gnathostome Lateral Line Ontogenies. Brain Behav. Evol. 1997, 50, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.T.; Katopodis, C.; Santos, J.M.; Ferreira, M.T.; Pinheiro, A.N. Cyprinid Swimming Behaviour in Response to Turbulent Flow. Ecol. Eng. 2012, 44, 314–328. [Google Scholar] [CrossRef]
- Yan, X.; Jin, J.; Zheng, T.; Sun, S.; Dai, H.; Dai, L.; Shi, K. The Effect of Rectifier Baffles on the Flow Regime of 180° Turning Pools in Vertical Slot Fishways. Sustainability 2023, 15, 10498. [Google Scholar] [CrossRef]
- Zheng, J.; Li, R.J.; Yu, Y.H. Sediment Carrying Capacity and Sediment Flux of Nearshore Water-Sediment Interface. Acta Oceanol. Sin. 2014, 36, 136. [Google Scholar] [CrossRef]
- Camnasio, E.; Erpicum, S.; Orsi, E.; Pirotton, M.; Schleiss, A.J.; Dewals, B. Coupling Between Flow and Sediment Deposition in Rectangular Shallow Reservoirs. J. Hydraul. Res. 2013, 51, 535–547. [Google Scholar] [CrossRef]
- Tao, J.; Tan, X.; Yang, Z.; Wang, X.; Cai, Y.; Qiao, Y.; Chang, J. Fish Migration Through a Fish Passage Associated with Water Velocities at the Changzhou Fishway (Pearl River, China). J. Appl. Ichthyol. 2014, 31, 72–76. [Google Scholar] [CrossRef]
- Liao, J.C. A Review of Fish Swimming Mechanics and Behaviour in Altered Flows. Philos. Trans. R. Soc. B 2007, 362, 1973–1993. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.T.; Bærum, K.M.; Hedger, R.D.; Baktoft, H.; Fjeldstad, H.-P.; Gjelland, K.Ø.; Økland, F.; Forseth, T. The Effects of Hydrodynamics on the Three-Dimensional Downstream Migratory Movement of Atlantic Salmon. Sci. Total Environ. 2020, 705, 135773. [Google Scholar] [CrossRef]
- Hoover, A.P.; Tytell, E. Decoding the Relationships between Body Shape, Tail Beat Frequency, and Stability for Swimming Fish. Fluids 2020, 5, 215. [Google Scholar] [CrossRef]
- Shi, X.T.; Deng, X.Y.; Chen, X.L.; Jiang, Y.Q.; Wang, Y.; Hu, X.; Tan, J.J. Simulation of the Upstream Behavior of Schizothorax wangchiachii in Vertical Slot Fishway. Trans. Chin. Soc. Agric. Eng. 2025, 41, 175–184. [Google Scholar] [CrossRef]
- Li, M.; An, R.; Chen, M.; Li, J. Evaluation of Volitional Swimming Behavior of Schizothorax prenanti Using an Open-Channel Flume with Spatially Heterogeneous Turbulent Flow. Animals 2022, 12, 752. [Google Scholar] [CrossRef]
- Tritico, H.M.; Cotel, A.J. The Effects of Turbulent Eddies on the Stability and Critical Swimming Speed of Creek Chub (Semotilus atromaculatus). J. Exp. Biol. 2010, 213, 2284–2293. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Z.Y.; Shi, X.T.; Jiang, Y.Q.; Chen, L.; Jin, Z.J. The Reentry Behavior and Its Relationship with Hydraulic Conditions During Upstream Migration of Schizothorax o’connori. Chin. J. Ecol. 2019, 38, 3382–3393. Available online: https://www.cje.net.cn/CN/Y2019/V38/I11/3382 (accessed on 7 October 2025).
- Shen, C.Y.; Yang, R.G.; Shi, X.T.; Wang, M.M.; He, S.H. Vortex Identification Based on the Liutex Method and Its Effect on Fish Passage Upstream. J. Hydrodyn. 2024, 36, 130–141. [Google Scholar] [CrossRef]
- Tan, J.; Gao, Z.; Dai, H.; Yang, Z.; Shi, X. Effects of turbulence and velocity on the movement behaviour of bighead carp (Hypophthalmichthys nobilis) in an experimental vertical slot fishway. Ecol. Eng. 2019, 127, 363–374. [Google Scholar] [CrossRef]
- Wu, S.; Rajaratnam, N.; Katopodis, C. Structure of Flow in Vertical Slot Fishway. J. Hydraul. Eng. 1999, 125, 351–360. [Google Scholar] [CrossRef]
- Guiny, E.; Ervine, D.A.; Armstrong, J. Hydraulic and Biological Aspects of Fish Passes for Atlantic Salmon. J. Hydraul. Eng. 2005, 131, 542–550. [Google Scholar] [CrossRef]
- Fuentes-Pérez, J.F.; Eckert, M.; Tuhtan, J.A.; Ferreira, M.T.; Kruusmaa, M.; Branco, P. Spatial preferences of Iberian barbel in a vertical slot fishway under variable hydrodynamic scenarios. Ecol. Eng. 2018, 125, 131–142. [Google Scholar] [CrossRef]
- Zheng, T.; Tu, C.; Huang, W.; Ren, W.; Li, G.; Liu, H. Testing Three Vertical Slot Fishway Configurations for a Chinese Endemic Fish. J. Hydraul. Eng. 2023, 149, 04023025. [Google Scholar] [CrossRef]
- Ouyang, L.; Li, D.; Cui, S.; Wu, X.; Liu, Y.; Han, X.; Zhou, S.; Xu, G.; Tu, X.; Chen, K.; et al. Fish Swimming Behavior and Strategies Under Different Hydrodynamic Conditions in Fishways with Various Vertical Slot Configurations. Fishes 2025, 10, 415. [Google Scholar] [CrossRef]
- Koed, A.; Thorstad, E.B. Long-term effect of radio-tagging on the swimming performance of pikeperch. J. Fish Biol. 2001, 58, 1753–1756. [Google Scholar] [CrossRef]
- Cao, C.; Fan, Z.; Wang, Y.; Liu, X.; Shi, H.; Yang, Y. Effects of continuous exposure to total dissolved gas supersaturation on swimming ability and recovery in grass carp (Ctenopharyngodon idella). Ecol. Freshw. Fish 2021, 31, 578–589. [Google Scholar] [CrossRef]
- Huang, Y.; Pang, R.; Li, X.; Li, W.; Yang, Z.; Wang, D.; Dong, K. Ecology Health Evaluation System Based on Fish Movement Behavior Response. Water 2023, 15, 4066. [Google Scholar] [CrossRef]

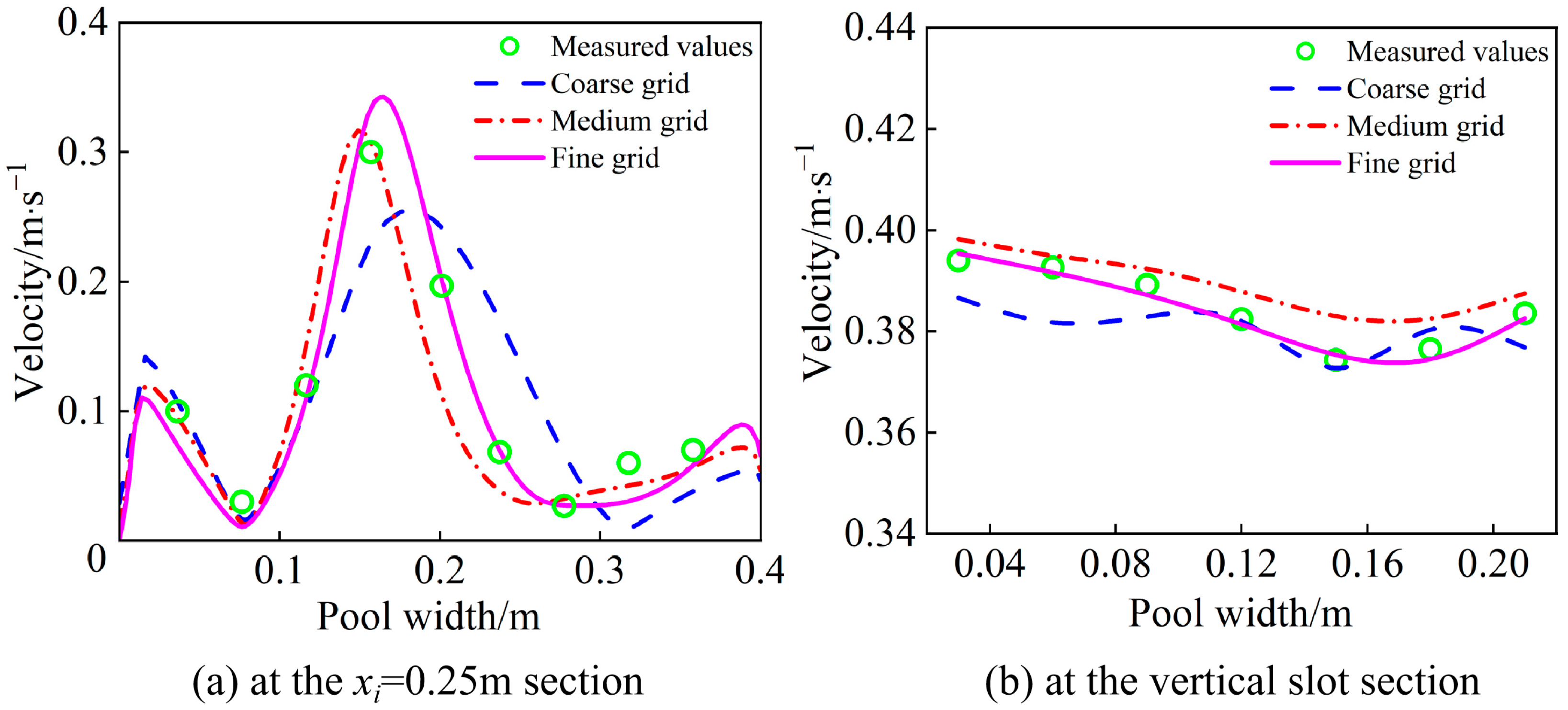
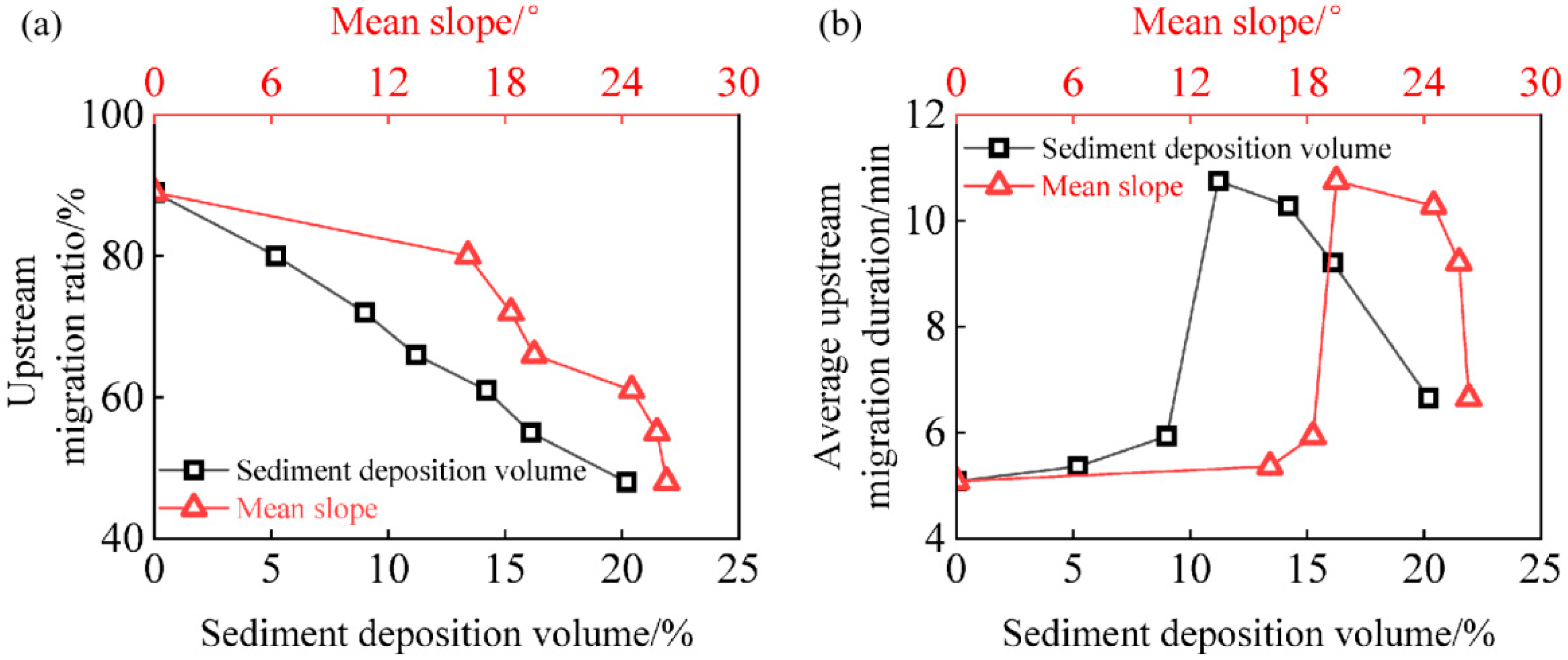
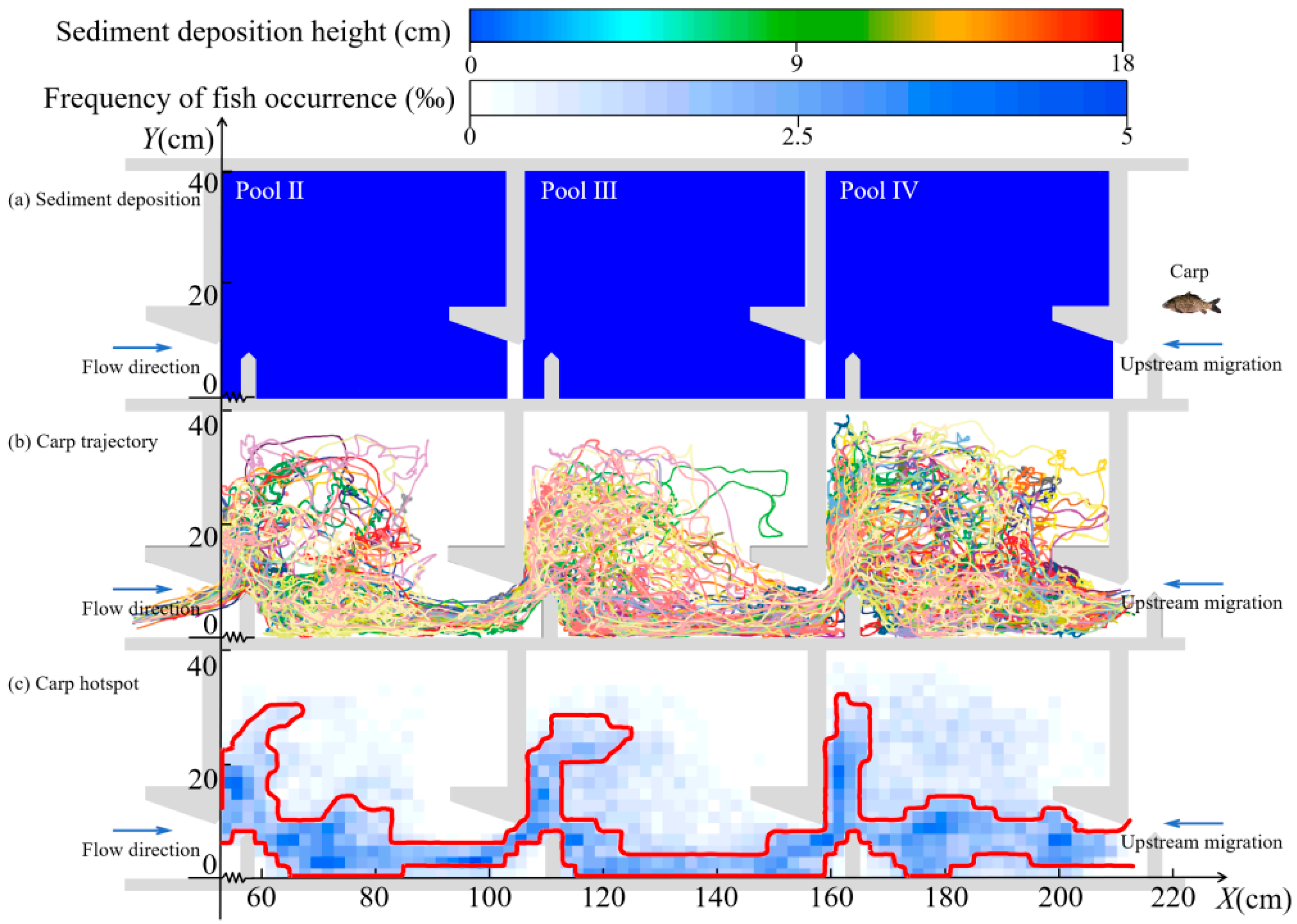
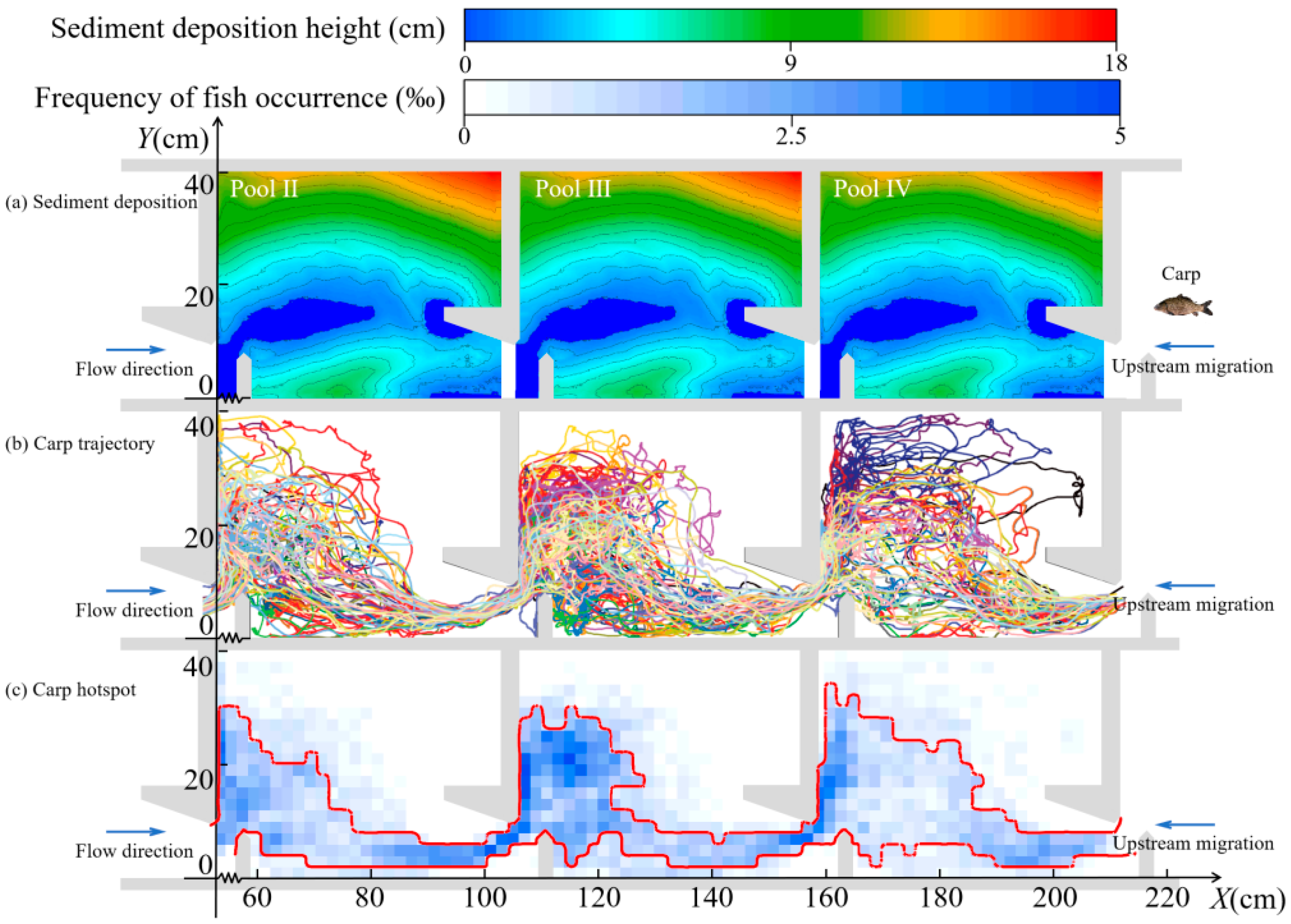
| Conditions | Sediment Deposition Height (cm) | Sediment Deposition Volume (%) | Mean Slopes (°) |
|---|---|---|---|
| 1 | 0 | 0.0 | 0.0 |
| 2 | 3 | 5.2 | 16.1 |
| 3 | 5 | 9.0 | 18.3 |
| 4 | 7 | 11.2 | 19.5 |
| 5 | 10 | 14.2 | 24.5 |
| 6 | 13 | 16.1 | 25.8 |
| 7 | 15 | 20.2 | 26.3 |
| Grid | Coarse Grid | Medium Grid | Fine Grid |
|---|---|---|---|
| Grid size/cm | 1.00 | 0.60 | 0.47 |
| Total number of grids | 1,103,520 | 5,029,846 | 10,646,064 |
| Average relative error | 9% | 5% | 2% |
| Deposition Conditions | Average Water Flow Resistance (N) | Cumulative Energy Consumption (J) |
|---|---|---|
| No sediment deposition | 1.0 × 10−3 | 2.4 × 10−3 |
| Maximum sediment deposition | 8.0 × 10−4 | 3.1 × 10−3 |
| Deposition Conditions | Variables | Nominal by Nominal | ||
|---|---|---|---|---|
| Phi Coefficient | Cramer’s V | Contingency Coefficient | ||
| No sediment deposition | Velocity | 6.532 | 0.996 | 0.988 |
| TKE | 6.187 | 0.994 | 0.987 | |
| Maximum sediment deposition | Velocity | 6.648 | 0.991 | 0.989 |
| TKE | 6.378 | 0.951 | 0.988 | |
| Model | R2 | Adjusted R2 | Std. Error of the Estimate | Durbin-Watson |
|---|---|---|---|---|
| Model A | 0.996 | 0.995 | 0.981223 | 1.225 |
| Model B | 0.983 | 0.974 | 0.01281 | 2.651 |
| Model | B | Std. Error | Beta | t | Tolerance | VIF | |
|---|---|---|---|---|---|---|---|
| Model A | (Constant) | 88.977 | 1.967 | / | 45.237 | / | / |
| Mean height | −148.630 | 21.732 | −0.949 | −6.839 | 0.170 | 5.874 | |
| Mean slope | −0.077 | 0.217 | −0.049 | −0.353 | 0.170 | 5.874 | |
| Model B | (Constant) | 0.147 | 0.013 | / | 11.618 | / | / |
| Mean height | 0.936 | 0.139 | 1.074 | 6.718 | 0.170 | 5.874 | |
| Mean slope | −0.001 | 0.001 | −0.092 | −0.574 | 0.170 | 5.874 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Z.; Chen, J.; Jiang, C.; Liao, Y.; Ding, T.; Chen, Y.; Deng, B.; Meng, W. Sediment Deposition Impacts on Fish Migration in Vertical Slot Fishways. Fishes 2025, 10, 590. https://doi.org/10.3390/fishes10110590
Ning Z, Chen J, Jiang C, Liao Y, Ding T, Chen Y, Deng B, Meng W. Sediment Deposition Impacts on Fish Migration in Vertical Slot Fishways. Fishes. 2025; 10(11):590. https://doi.org/10.3390/fishes10110590
Chicago/Turabian StyleNing, Zihao, Jie Chen, Changbo Jiang, Yihan Liao, Tianshun Ding, Yulin Chen, Bin Deng, and Wenkang Meng. 2025. "Sediment Deposition Impacts on Fish Migration in Vertical Slot Fishways" Fishes 10, no. 11: 590. https://doi.org/10.3390/fishes10110590
APA StyleNing, Z., Chen, J., Jiang, C., Liao, Y., Ding, T., Chen, Y., Deng, B., & Meng, W. (2025). Sediment Deposition Impacts on Fish Migration in Vertical Slot Fishways. Fishes, 10(11), 590. https://doi.org/10.3390/fishes10110590







