High-Dimensional Immune Profiling of Human Retinal Detachment Samples Using Spectral Flow Cytometry: A Protocol for Intraocular Immunotyping
Abstract
1. Introduction
2. Experimental Design
2.1. Materials
- Phosphate-Buffered Saline (PBS) (Cytiva, Marlborough, MA, USA, Cat. no: SH30256.01); stored at room temperature (RT).
- Bovine Serum Albumin (BSA) (Gibco, Waltham, MA, USA, Cat. no: 30063-572); stored at 4 ºC.
- NaN3 (Sigma-Aldrich, St. Louis, MO, USA, Cat. no: S2002-25G); stored in a dry place at RT.
- Wash Buffer (manually prepared; see reagent setup for details).
- LIVE/DEAD Fixable Dead Cell Stain (Invitrogen, Waltham, MA, USA, Cat. No: L34962); stored at 4 °C in the dark.
- Brilliant Stain Buffer Plus (BD Biosciences, Franklin Lakes, NJ, USA, Cat. no: 566385); stored at 4 °C protected from light.
- True-Stain Monocyte Blocker (BioLegend, San Diego, CA, USA; Cat. no: 426103); stored at 4 °C in the dark.
- 10% Neutral Buffered Formalin (Fisher Scientific, Waltham, MA, USA, Cat. no: 316-155); stored at RT in a tightly closed container.
- Paraformaldehyde (PFA) 1% (manually prepared; see reagent setup for details)
- Fluorescently labeled antibodies (see Table 1)
2.2. Equipment
- Cytek Aurora™ cell sorter (CS System) 3-laser (Cytek, Fremont, CA, USA)
- 5-laser Cytek® Aurora (Cytek, Fremont, CA, USA)
2.3. Software
- SpectroFlo® acquisition software v.3.2.1 (Cytek, Fremont, CA, USA)
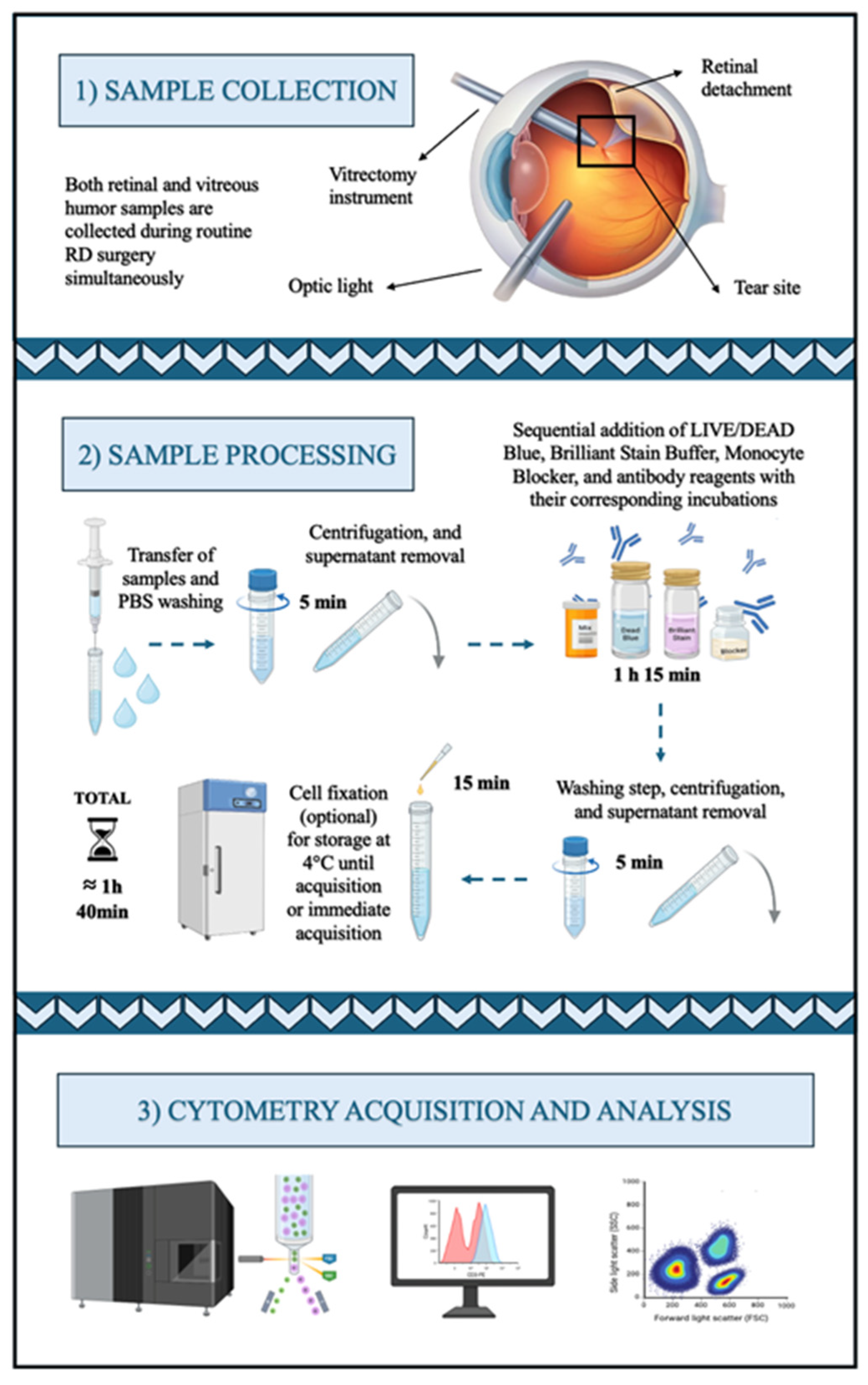
3. Procedure
 All centrifugation steps should be performed at 4 °C to minimize cell loss, which is especially critical in this protocol, as the number of cells recovered from intraocular samples is often low.
All centrifugation steps should be performed at 4 °C to minimize cell loss, which is especially critical in this protocol, as the number of cells recovered from intraocular samples is often low.3.1. Retina and Vitreous Humor Samples Processing for Spectral Cell Sorting
- Transfer each sample individually into separate cytometry tubes.
- Add 1 mL PBS and, if necessary, perform mechanical dissociation using a vortex or pipetting up and down.
- Centrifuge and carefully discard the supernatant, leaving approximately 100 μL of residual volume.
CRITICAL STEP: The vitreous sample is viscous; therefore, the first washing step should be performed carefully to avoid cell loss.
- Add 5 µL LIVE/DEAD™ Blue (1:40 dilution) and vortex. Incubate for 15 min at room temperature (RT) in the dark.
- Wash with 1 mL of Wash Buffer and centrifuge.
- Discard the supernatant as step 3.
CRITICAL STEP: This washing step should remove most of the vitreous viscosity. Additional washes before the antibody incubation steps are generally not recommended to avoid cell loss.
- Add 10 µL Brilliant Stain Buffer Plus and vortex.
- Add 5 µL True-Stain Monocyte Blocker and vortex.
- Add 2.5 µL anti-CD45 PerCP antibody and vortex. Incubate for 20 min at RT in the dark.
- Add 1 mL of Wash Buffer and centrifuge.
- Discard the supernatant, leaving approximately 300 µL of cell suspension.OPTIONAL STEP: If cell functionality is not necessary for subsequent experiments, samples can either be acquired fresh or fixed for later acquisition. However, fixation is recommended to ensure consistency across all samples. To fix, add 300 µL 1% PFA and vortex. Incubate for 10 min RT in the dark. Add 1 mL Wash Buffer, centrifuge, and discard the supernatant, leaving approximately 300 µL of cell suspension.
CRITICAL STEP: Use a 100 µm filter if the samples cannot be totally dissociated to prevent clogs in the flow cytometer and ensure accurate acquisition.
- Store at 4 °C until acquisition, preferably within 48 h.
3.2. Retina and Vitreous Humor Samples Processing for Spectral Cytometry Acquisition
- Transfer each sample individually into separate cytometry tubes.
- Add 1 mL PBS and, if necessary, perform mechanical dissociation using a vortex or by pipetting up and down.
- Centrifuge and carefully discard the supernatant, leaving approximately 100 μL of residual volume.
CRITICAL STEP: The vitreous sample is viscous; therefore, the first washing step should be performed carefully to avoid cell loss.
- Add 5 µL LIVE/DEAD™ Blue (1:40 dilution) and vortex. Incubate for 15 min at RT in the dark.
- Wash with 1 mL of Wash Buffer and centrifuge at 500× g for 5 min at 4 °C.
- Discard the supernatant as step 3.
CRITICAL STEP: This washing step should remove most of the vitreous viscosity. Additional washes before the antibody incubation steps are generally not recommended to avoid cell loss.
- Add 10 µL Brilliant Stain Buffer Plus and vortex.
- Add 5 µL True-Stain Monocyte Blocker and vortex.
- Add 2.5 µL of anti-IgG BV605 antibody and vortex. Incubate for 10 min at RT in the dark.
CRITICAL STEP: Do not wash after this step.
- Add 1.25 µL anti-TCRγδ PerCP/eFluor 710 antibody and vortex. Incubate for 10 min further at RT in the dark.
CRITICAL STEP: Do not wash after this step.
- Add 1.25 µL anti-CXCR5 BV750 and 2.5 µL anti-CCR5 BV563 antibodies and vortex. Incubate for 10 min further at RT in the dark.
CRITICAL STEP: Do not wash after this step.
- Add 100 µL of the modified OMIP-069 antibody mix and vortex. Incubate for 30 min further at RT in the dark.
- Add 1 mL of Wash Buffer and centrifuge.
- Discard the supernatant, leaving approximately 300 µL of cell suspension.OPTIONAL STEP: If cell functionality is not necessary for subsequent experiments, samples can either be acquired fresh or fixed for later acquisition. However, fixation is recommended to ensure consistency across all samples. To fix, add 300 µL 1% PFA and vortex. Incubate for 10 min RT in the dark. Add 1 mL Wash Buffer, centrifuge, and discard the supernatant, leaving approximately 300 µL of cell suspension.
CRITICAL STEP: Use a 100 µm filter if the samples cannot be totally dissociated to prevent clogs in the flow cytometer and ensure accurate acquisition.
- Store at 4 °C until acquisition, preferably within 48 h.
4. Expected Results
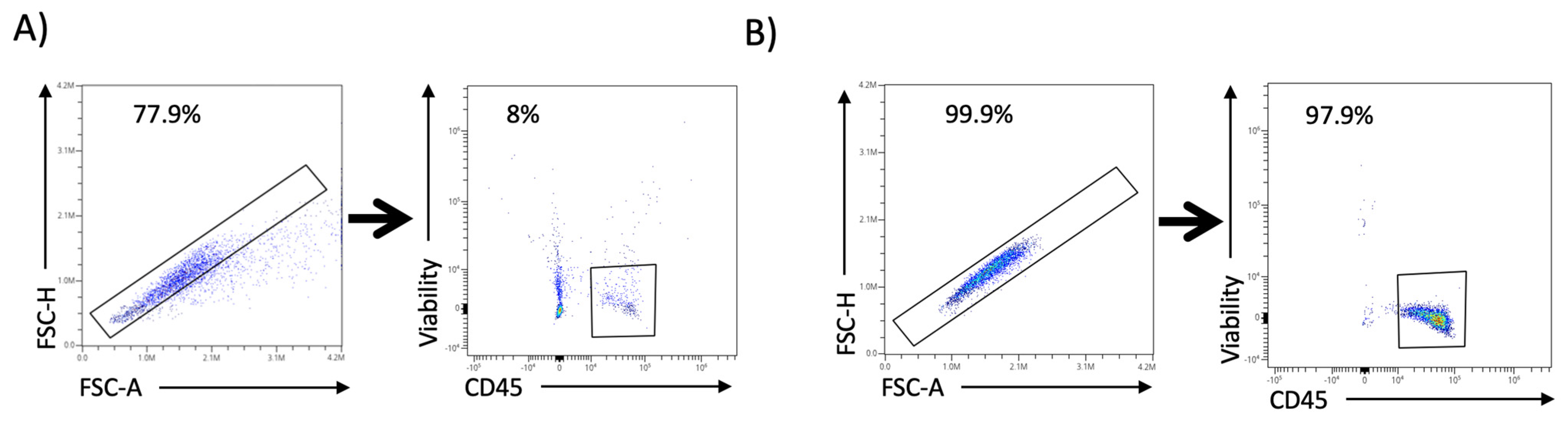
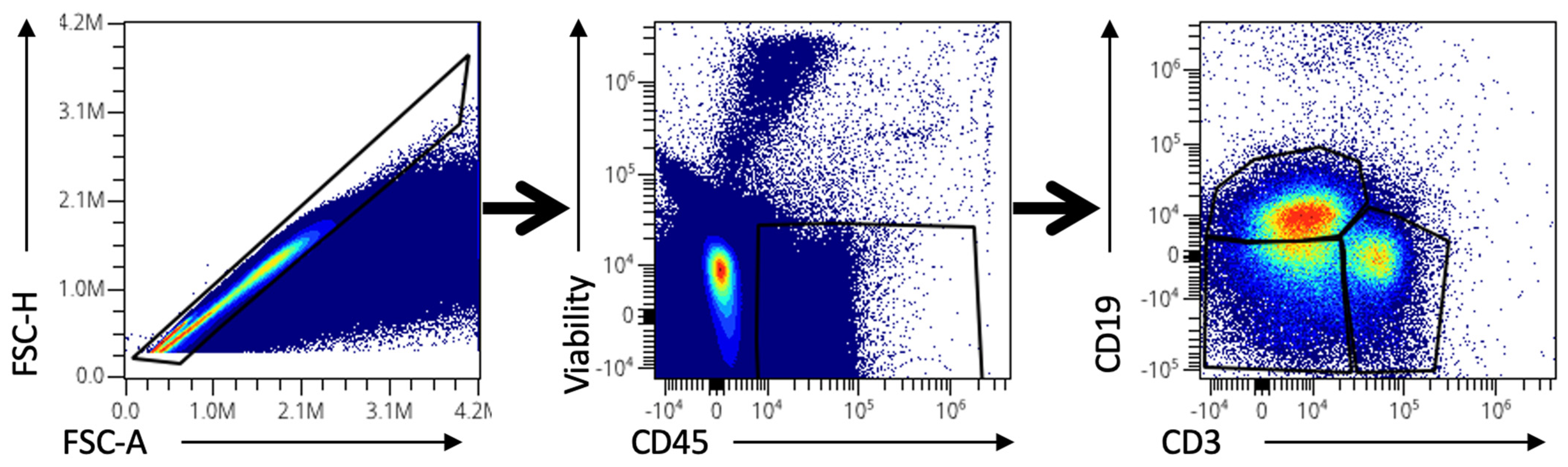
4.1. Spectral Cell-Sorter Enrichment
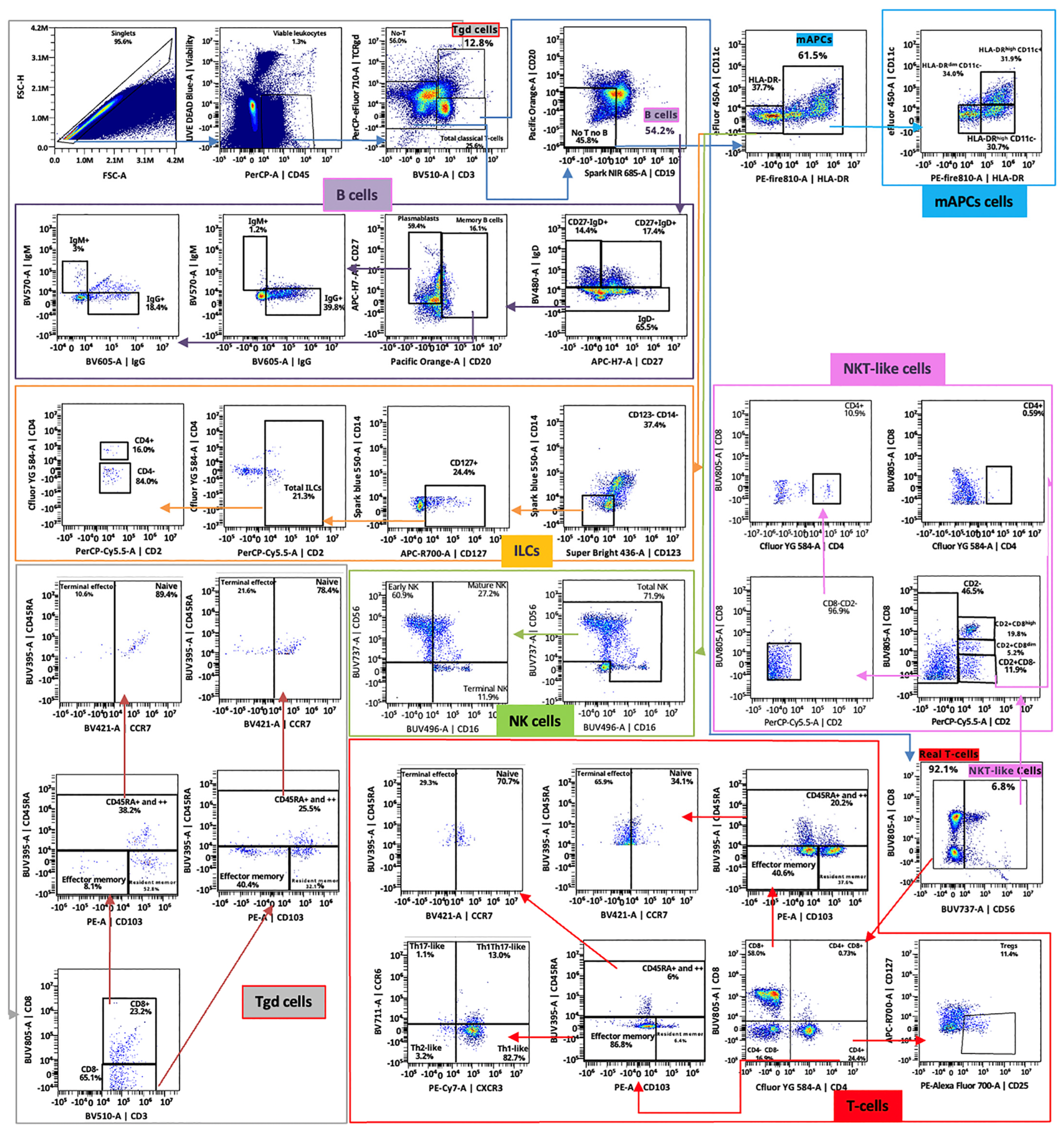
4.2. Spectral Cytometry Analysis
5. Reagents Setup
- Wash Buffer: prepared fresh every week with the following composition: 500 mL PBS, 1 g BSA, and 0.445 g NaN3. Stored at 4 °C and used within one week.
- PFA 1%: prepared by mixing 90% PBS with 10% neutral buffered formalin; stored at 4 °C and used within one month of preparation.
- Modified OMIP-069 antibody mix: prepared based on the panel described by Park LM et al. [13], with slight modifications. Our version includes 38 antibodies instead of the original 39. CD337, CD24, CD39, and CD159c-specific antibodies are omitted, and VISTA, CD103, and CTLA4 ones are added. For the spectral cytometry protocol (see Section 3.2), the antibody mix used in step 15 contains all antibodies that were not pre-incubated individually (anti-IgG BV605, anti-TCRγδ PerCP/eFluor 710, anti-CXCR5 BV750, and anti-CCR5 BV563). Thus, a total of 34 antibodies are combined as indicated in Table 1.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine Serum Albumin |
| OMIP | Optimized Multicolor Immunofluorescence Panel |
| PBS | Phosphate-Buffered Saline |
| PFA | Paraformaldehyde |
| PR | Photoreceptor |
| RD | Retinal Detachment |
| RT | Room Temperature |
References
- Katamay, R.; Nussenblatt, R.B. Chapter 27-Blood–Retinal Barrier, Immune Privilege, and Autoimmunity. In Retina, 5th ed.; Ryan, S.J., Sadda, S.R., Hinton, D.R., Schachat, A.P., Wilkinson, C.P., Wiedemann, P., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 579–589. [Google Scholar] [CrossRef]
- Redruello-Guerrero, P.; Gómez-Tomás, M.; Rechi-Sierra, T.; Molinero-Sicilia, L.; Galindo-Cabello, N.; Usategui-Martín, R.; Pastor-Idoate, S. Inflammatory Mechanisms in the Management and Treatment of Retinal Detachment. Metabolites 2025, 15, 442. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Choi, K.S.; Sun, H.J.; Lee, S.J. Factors associated with visual outcome after macula-off rhegmatogenous retinal detachment surgery. Retina 2018, 38, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, S.; Castrejón, M.; Fernández, I.; Rojas, J.; Coco, R.M.; Sanabria, M.R.; Rodríguez de la Rua, E.; Pastor, J.C. Loss of Visual Acuity after Successful Surgery for Macula-On Rhegmatogenous Retinal Detachment in a Prospective Multicentre Study. J. Ophthalmol. 2015, 2015, 821864. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.M.; Khan, N.; Weichel, E.D.; Berinstein, D.M. Anatomic and Visual Outcomes in Early versus Late Macula-On Primary Retinal Detachment Repair. Retina 2011, 31, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Hassman, L.; Kodati, S.; Chu, C.J. Intraocular Immune Response in Human Uveitis: Time to Look Beyond Animal Models. Am. J. Ophthalmol. 2024, 266, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Tabor, S.J.; Yuda, K.; Deck, J.; Gnanaguru, G.; Connor, K.M. Retinal Injury Activates Complement Expression in Müller Cells Leading to Neuroinflammation and Photoreceptor Cell Death. Cells 2023, 12, 1754. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Shahror, R.A.; Morris, C.A.; Caldwell, R.B.; Fouda, A.Y. Multi-Color Flow Cytometry Protocol to Characterize Myeloid Cells in Mouse Retina Research. Bio. Protoc. 2023, 13, e4745. [Google Scholar] [CrossRef] [PubMed]
- Maidana, D.E.; Gonzalez-Buendia, L.; Pastor-Puente, S.; Naqvi, A.; Paschalis, E.; Kazlauskas, A.; Miller, J.W.; Vavvas, D.G. Peripheral Monocytes and Neutrophils Promote Photoreceptor Cell Death in an Experimental Retinal Detachment Model. Cell Death Dis. 2023, 14, 834. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, Z.; Pan, L.; Zhuang, D.; Cho, K.-S.; Robert, K.; Chen, X.; Shu, L.; Tang, G.; Wu, J.; et al. Therapeutic Targeting of Retinal Immune Microenvironment with CSF-1 Receptor Antibody Promotes Visual Function Recovery After Ischemic Optic Neuropathy. Front. Immunol. 2020, 11, 585918. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, P.; Qi, Y.; Zhao, Y.; Wei, X. Progress and Challenges of in Vivo Flow Cytometry and Its Applications in Circulating Cells of Eyes. Cytometry Pt A 2024, 105, 437–445. [Google Scholar] [CrossRef]
- Stepp, M.A.; Menko, A.S. Immune Responses to Injury and Their Links to Eye Disease. Transl. Res. 2021, 236, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Park, L.M.; Lannigan, J.; Jaimes, M.C. OMIP-069: Forty-Color Full Spectrum Flow Cytometry Panel for Deep Immunophenotyping of Major Cell Subsets in Human Peripheral Blood. Cytometry Pt A 2020, 97, 1044–1051. [Google Scholar] [CrossRef]
- Galindo-Cabello, N.; Sobas-Abad, E.M.; Lapresa, R.; Agulla, J.; Almeida, Á.; López, A.; Pastor, J.C.; Pastor-Idoate, S.; Usategui-Martín, R. The TP53 Arg72Pro Polymorphism Predicts Visual and Neurodegenerative Outcomes in Retinal Detachment. Cell Death Dis. 2025, 16, 415. [Google Scholar] [CrossRef] [PubMed]
| Specificity | Clone | Fluorochrome | Supplier (Cat. No) | Purpose | Dilution | Volume (mL)/Test |
|---|---|---|---|---|---|---|
| CD1c | L161 | Alexa Fluor 647 | BioLegend (331510) | Dendritic cells, NKT-Like cells | 1/20 | 5 |
| NKG2A (CD159a) | REA110 | APC | Miltenyi (Bergisch Gladbach, Germany) (130-113-563) | NK, NKT-Like, and γδ T cell activation/differentiation | 1/50 | 2 |
| CD38 | HIT2 | APC/Fire810 | BioLegend (356644) | Monocyte, dendritic cell, T cell, and B cell activation/differentiation | 1/40 | 2.5 |
| CD27 | M-T271 | APC/H7 | BD Biosciences (560222) | T and B cell differentiation | 1/20 | 5 |
| CD127 | HIL-7R-M21 | APC/R700 | BD Biosciences (565185) | Cytokine receptor; T cell differentiation | 1/20 | 5 |
| CD141 | 1A4 | BB 515 | BD Biosciences (565084) | Dendritic cell differentiation | 1/80 | 1.25 |
| CD45RA | 5H9 | BUV 395 | BD Biosciences (569489) | T cell differentiation | 1/80 | 1.25 |
| CD16 | 3G8 | BUV 496 | BD Biosciences (612944) | Monocyte, NK cell, and dendritic cell differentiation | 1/160 | 0.625 |
| CCR5 (CD195) | 2D7/CCR5 | BUV 563 | BD Biosciences (741401) | Chemokine receptor; Monocyte, dendritic cell, T cell, and B cell differentiation | 1/20 | 5 |
| NKG2D (CD314) | 1D11 | BUV 615 | BD Biosciences (751232) | NK cell differentiation | 1/20 | 5 |
| VISTA | TU66 | BUV 661 | BD Biosciences (750502) | Immune checkpoint | 1/50 | 2 |
| CD56 | NCAM16.2 | BUV 737 | BD Biosciences (612766) | Pan NK cell, γδ T cell activation | 1/80 | 1.25 |
| CD8 | SK1 | BUV 805 | BD Biosciences (612889) | T cell, NK, and NKT-Like cell lineage | 1/160 | 0.625 |
| CCR7 (CD197) | G043H7 | BV 421 | BioLegend (353208) | Chemokine receptor; T cell differentiation | 1/20 | 5 |
| IgD | IA6-2 | BV 480 | BD Biosciences (566138) | B cell differentiation | 1/160 | 0.625 |
| IgM | MHM-88 | BV 570 | BioLegend (314518) | B cell differentiation | 1/32 | 3.125 |
| IgG | IA6-2 | BV 605 | BD Biosciences (566138) | B cell differentiation | 1/40 | 2.5 |
| CD3 | SK7 | BV510 | BioLegend (44828) | Pan T cell, NKT-Like cells | 1/20 | 5 |
| CD28 | CD28.2 | BV 650 | Bio Legend (302946) | T cell and NK cell differentiation | 1/40 | 2.5 |
| CCR6 (CD196) | G034E3 | BV 711 | BioLegend (353436) | Chemokine receptor; T cell and B cell differentiation | 1/80 | 1.25 |
| CXCR5 | RF8B2 | BV 750 | BD Biosciences (747111) | Chemokine receptor; T cell differentiation | 1/80 | 1.25 |
| CD279 (PD-1) | EH12.2H7 | BV 785 | BioLegend (329930) | T cell inhibitory receptor | 1/20 | 5 |
| CD4 | SK3 | cFluor YG584 | Cytek (R7-20041-100T) | T and NKT-Like cell lineages | 1/200 | 0.5 |
| CD11c | 3.9 | eFluor 450 | eBioscience (Santa Clara, CA, USA) (48-0116-42) | Pan myeloid lineage | 1/20 | 5 |
| CD57 | HNK-1 | FITC | BioLegend (359604) | NK and CD8+ T cell immune senescence | 1/80 | 1.25 |
| CD20 | HI47 | Pacific Orange | Invitrogen (MHCD2030) | Pan-B cells | 1/20 | 5 |
| CD103 | REA205 | PE | BioLegend (350206) | Integrin for tissue-resident cells | 1/100 | 1 |
| CD25 | CD25-3G10 | PE/Alexa 700 | Life Technologies (Carlsbad, CA, USA) (MHCD2524) | IL-2 receptor | 1/40 | 2.5 |
| CD95 (Fas) | DX2 | PE/Cy5 | BioLegend (305610) | Cytotoxicity marker | 1/160 | 0.625 |
| CXCR3 (CD183) | G025H7 | PE/Cy7 | BioLegend (353720) | Chemokine receptor; Dendritic cell, T cell, and B cell differentiation | 1/20 | 5 |
| CTLA4 | P30-15 | PE/Dazzle 594 | BioLegend (369616) | Immune checkpoint | 1/50 | 2 |
| HLA-DR | L243 | PE/Fire 810 | BioLegend (307683) | T cell and monocyte activation, NK cell lineage discrimination, and dendritic cell marker | 1/20 | 5 |
| CD45 | HI30 | PerCP | Invitrogen (MHCD4531) | Pan leukocytes | 1/40 | 2.5 |
| CD2 | TS1/8 | PerCP/Cy5.5 | BioLegend (309226) | NK cell differentiation | 1/20 | 5 |
| TCRyg | B1.1 | PerCP/eFluor710 | Thermofisher (Waltham, MA, USA) (46-9959-42) | Pan γδ T cell | 1/80 | 1.25 |
| CD14 | 63D3 | Spark Blue 550 | BioLegend (367148) | Monocyte differentiation | 1/40 | 2.5 |
| CD19 | HIB19 | Spark NIR 685 | BioLegend (302270) | Pan B cells | 1/80 | 1.25 |
| CD123 | 6H6 | Super Bright 436 | eBioscience (62-1239-42) | Il-3 receptor | 1/40 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinero-Sicilia, L.; del Hierro, A.G.; Galindo-Cabello, N.; Redruello-Guerrero, P.; Pastor-Idoate, S.; Usategui-Martín, R.; Bernardo, D. High-Dimensional Immune Profiling of Human Retinal Detachment Samples Using Spectral Flow Cytometry: A Protocol for Intraocular Immunotyping. Methods Protoc. 2025, 8, 141. https://doi.org/10.3390/mps8060141
Molinero-Sicilia L, del Hierro AG, Galindo-Cabello N, Redruello-Guerrero P, Pastor-Idoate S, Usategui-Martín R, Bernardo D. High-Dimensional Immune Profiling of Human Retinal Detachment Samples Using Spectral Flow Cytometry: A Protocol for Intraocular Immunotyping. Methods and Protocols. 2025; 8(6):141. https://doi.org/10.3390/mps8060141
Chicago/Turabian StyleMolinero-Sicilia, Laura, Alejandro G. del Hierro, Nadia Galindo-Cabello, Pablo Redruello-Guerrero, Salvador Pastor-Idoate, Ricardo Usategui-Martín, and David Bernardo. 2025. "High-Dimensional Immune Profiling of Human Retinal Detachment Samples Using Spectral Flow Cytometry: A Protocol for Intraocular Immunotyping" Methods and Protocols 8, no. 6: 141. https://doi.org/10.3390/mps8060141
APA StyleMolinero-Sicilia, L., del Hierro, A. G., Galindo-Cabello, N., Redruello-Guerrero, P., Pastor-Idoate, S., Usategui-Martín, R., & Bernardo, D. (2025). High-Dimensional Immune Profiling of Human Retinal Detachment Samples Using Spectral Flow Cytometry: A Protocol for Intraocular Immunotyping. Methods and Protocols, 8(6), 141. https://doi.org/10.3390/mps8060141

















