Accelerated Screening of Wheat Gluten Strength Using Dual Physicochemical Tests in Diverse Breeding Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wholemeal and Flour Preparation
2.3. Physical Testing
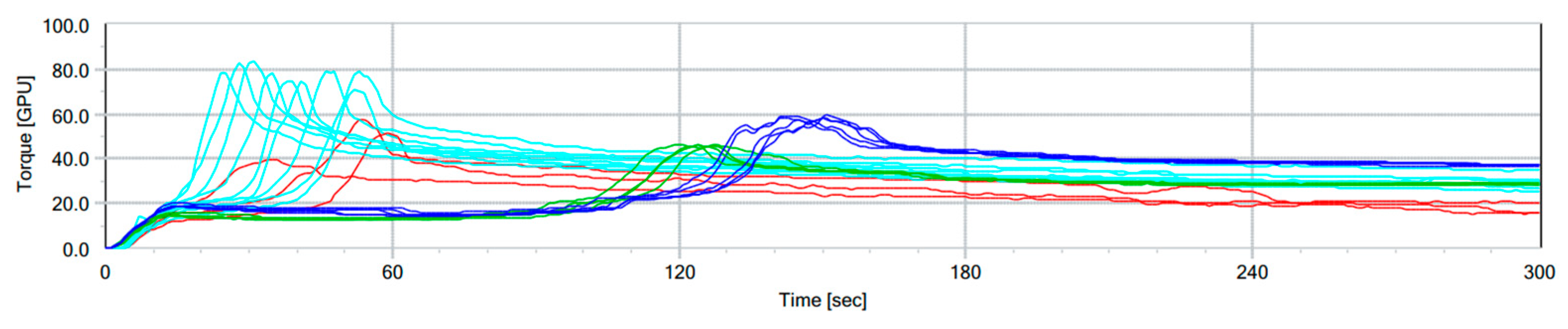
2.4. GlutoPeak Tester (GPT) Protocol
2.5. Protein Fractionation (PF) Protocol
2.6. Statistical Analysis
3. Results
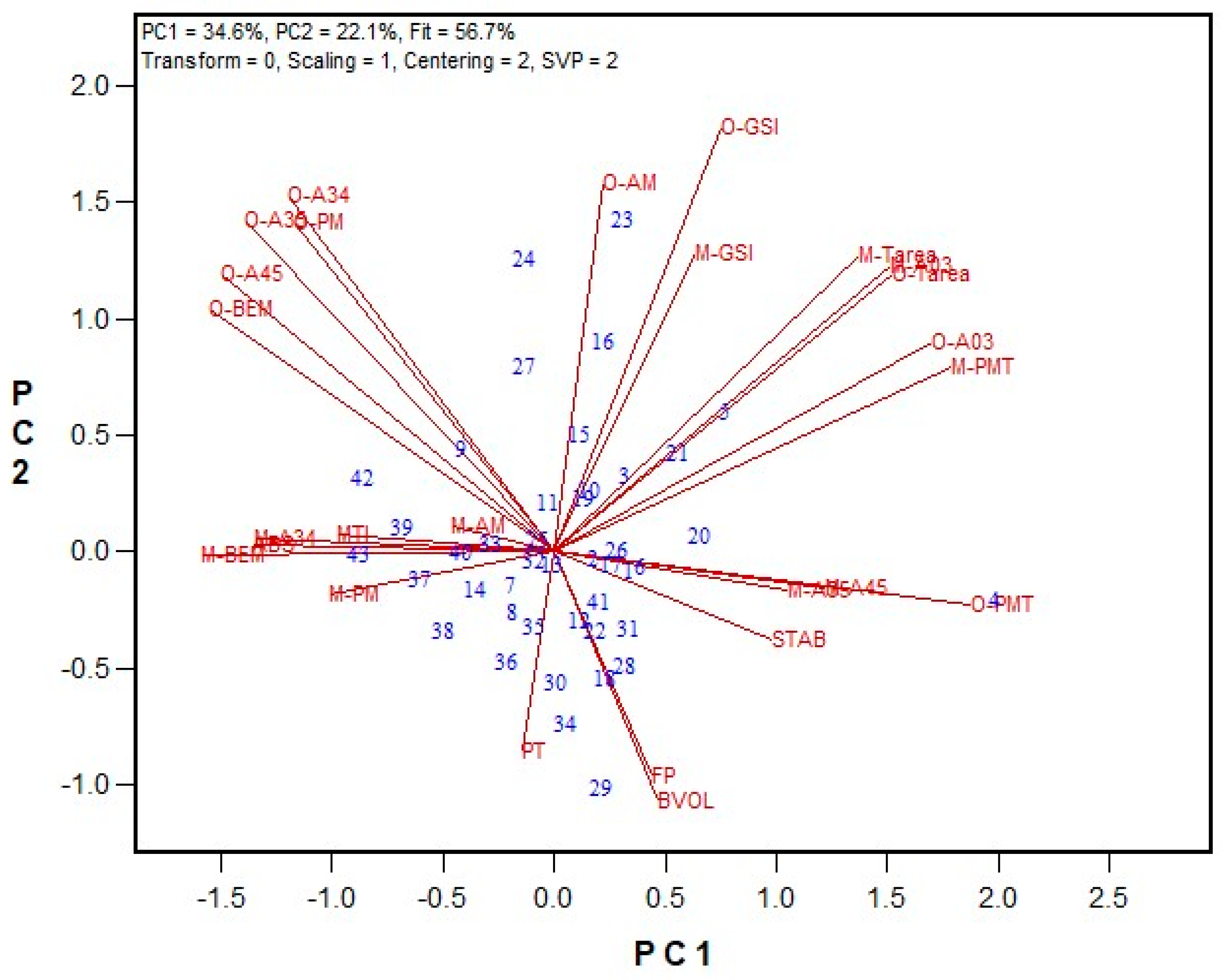
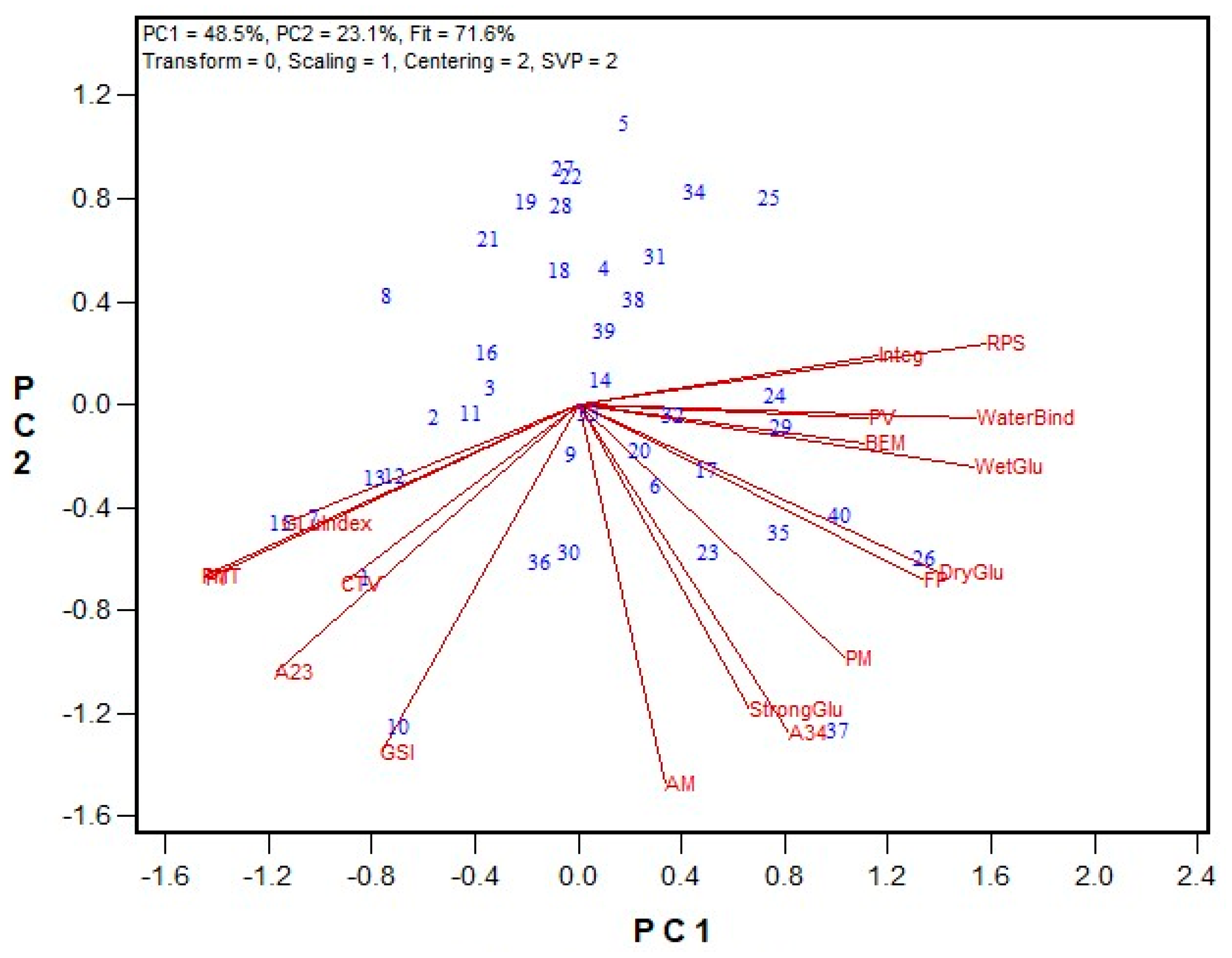
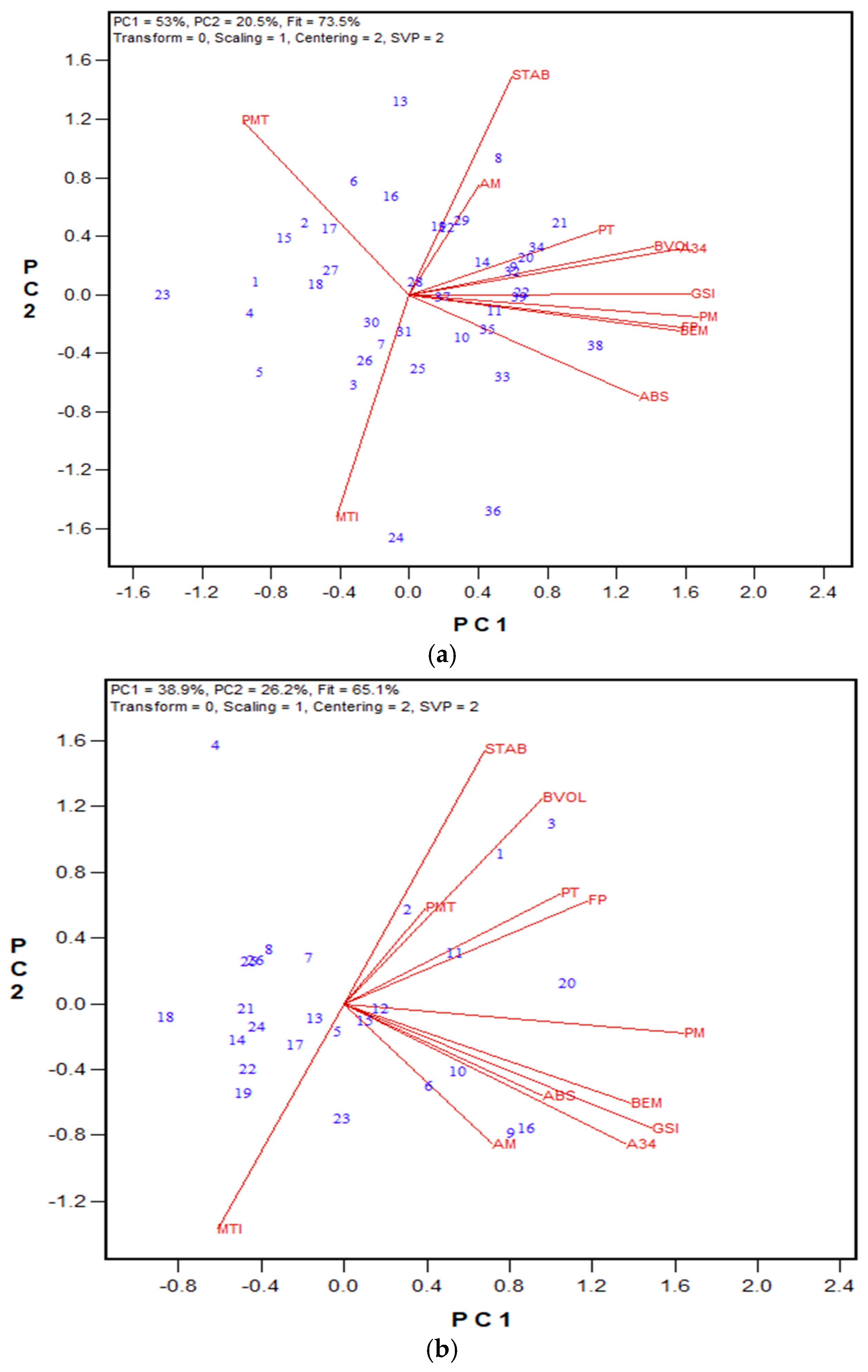
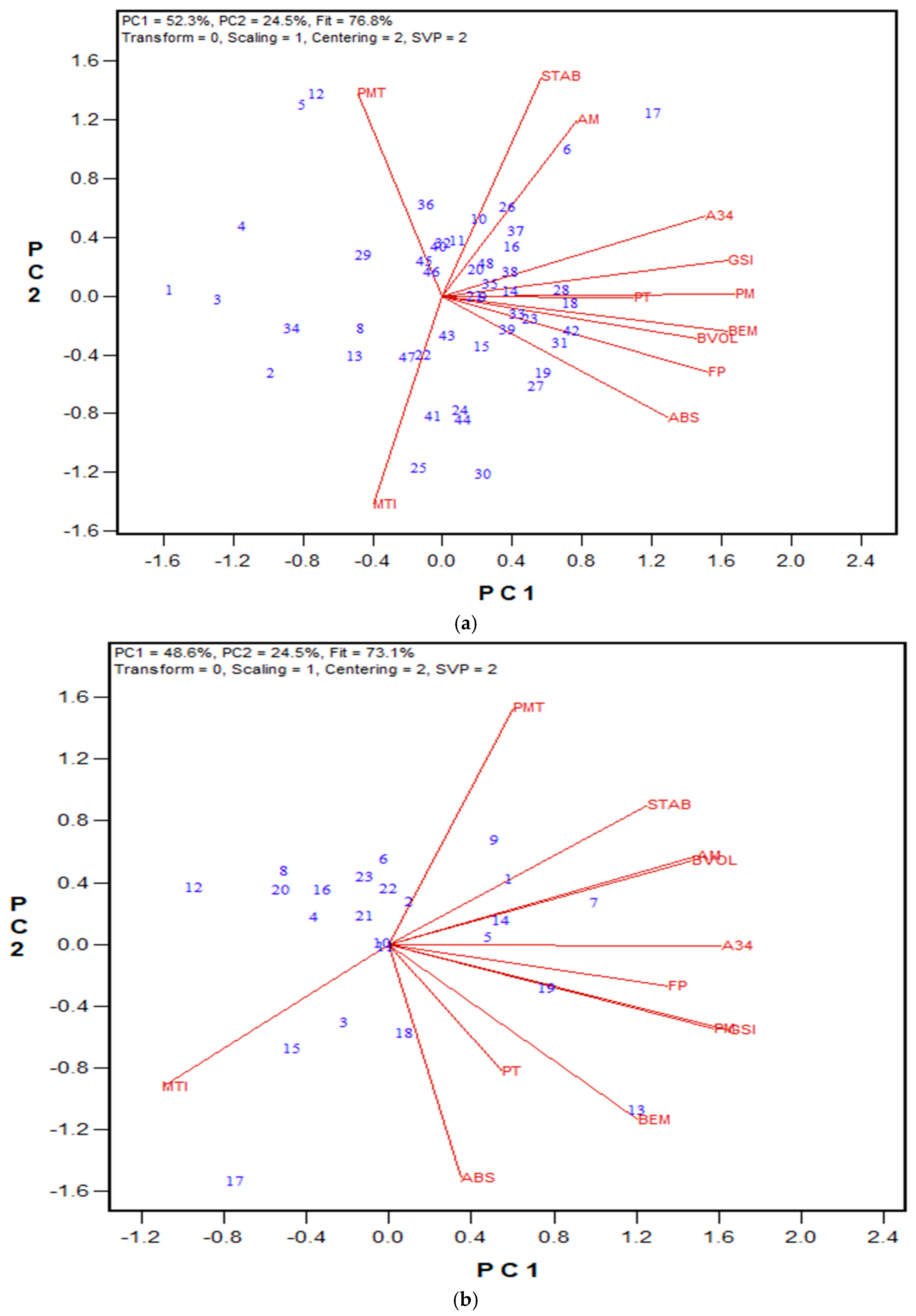
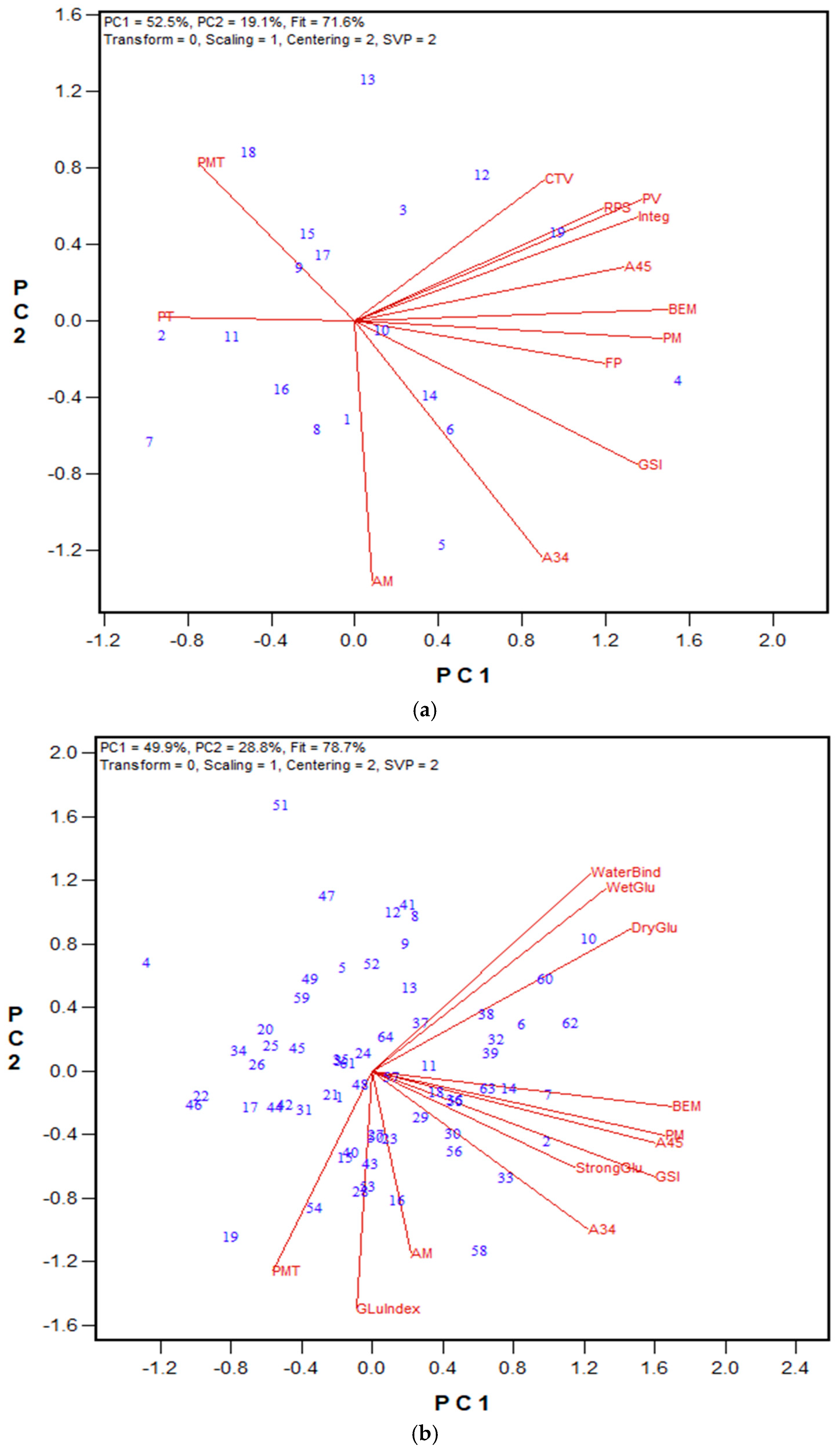
3.1. GPT Protocol Development and Testing
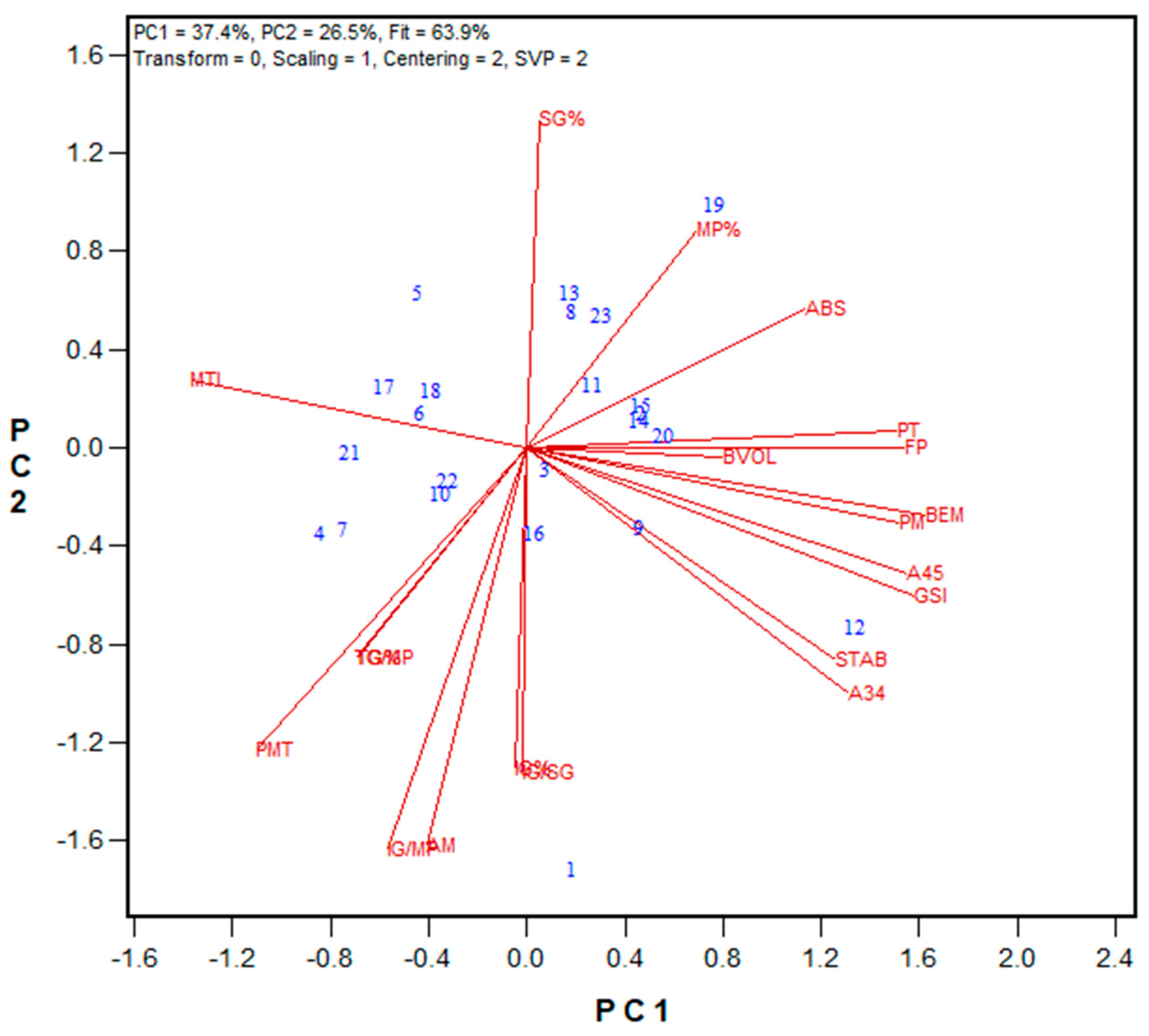
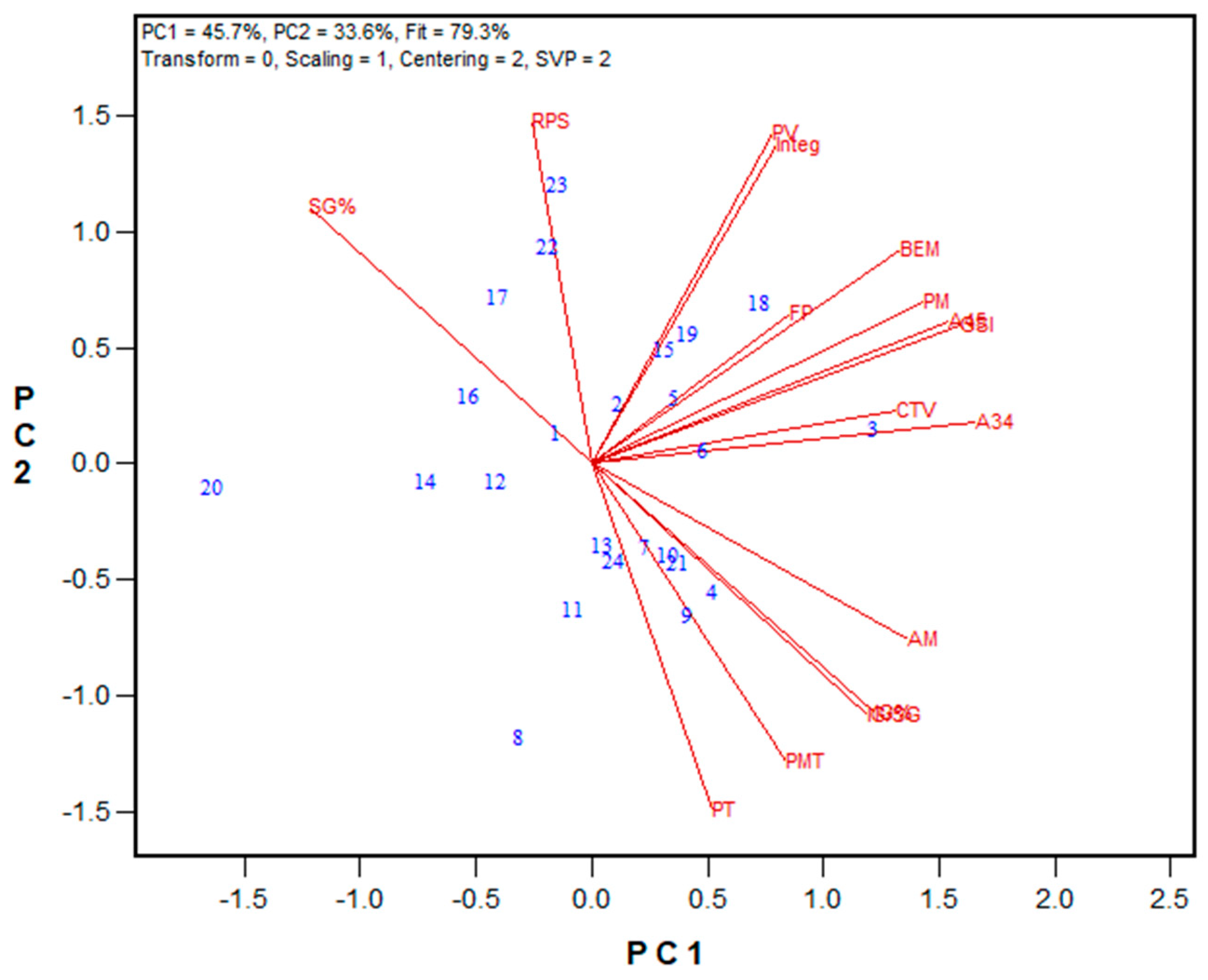
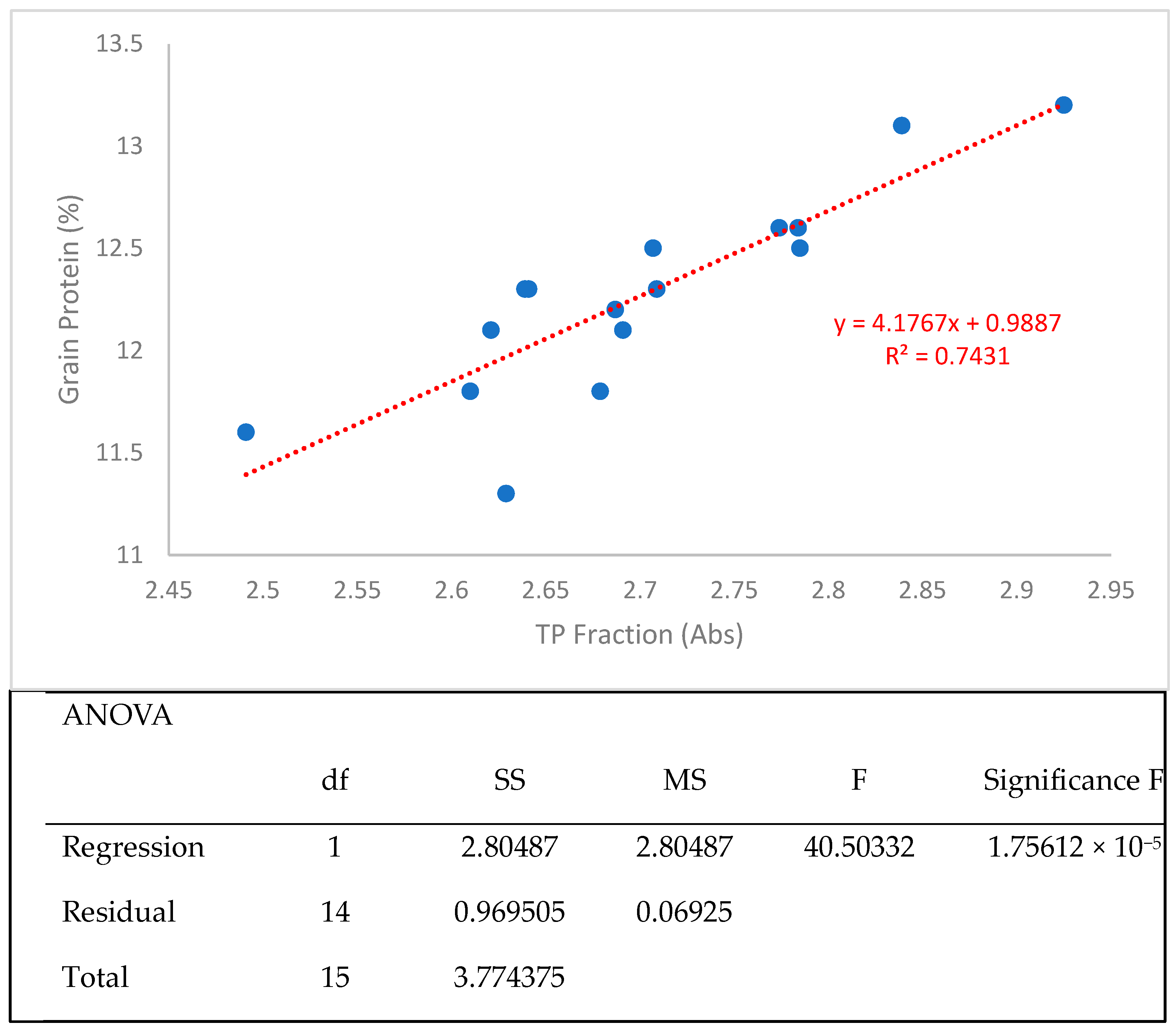
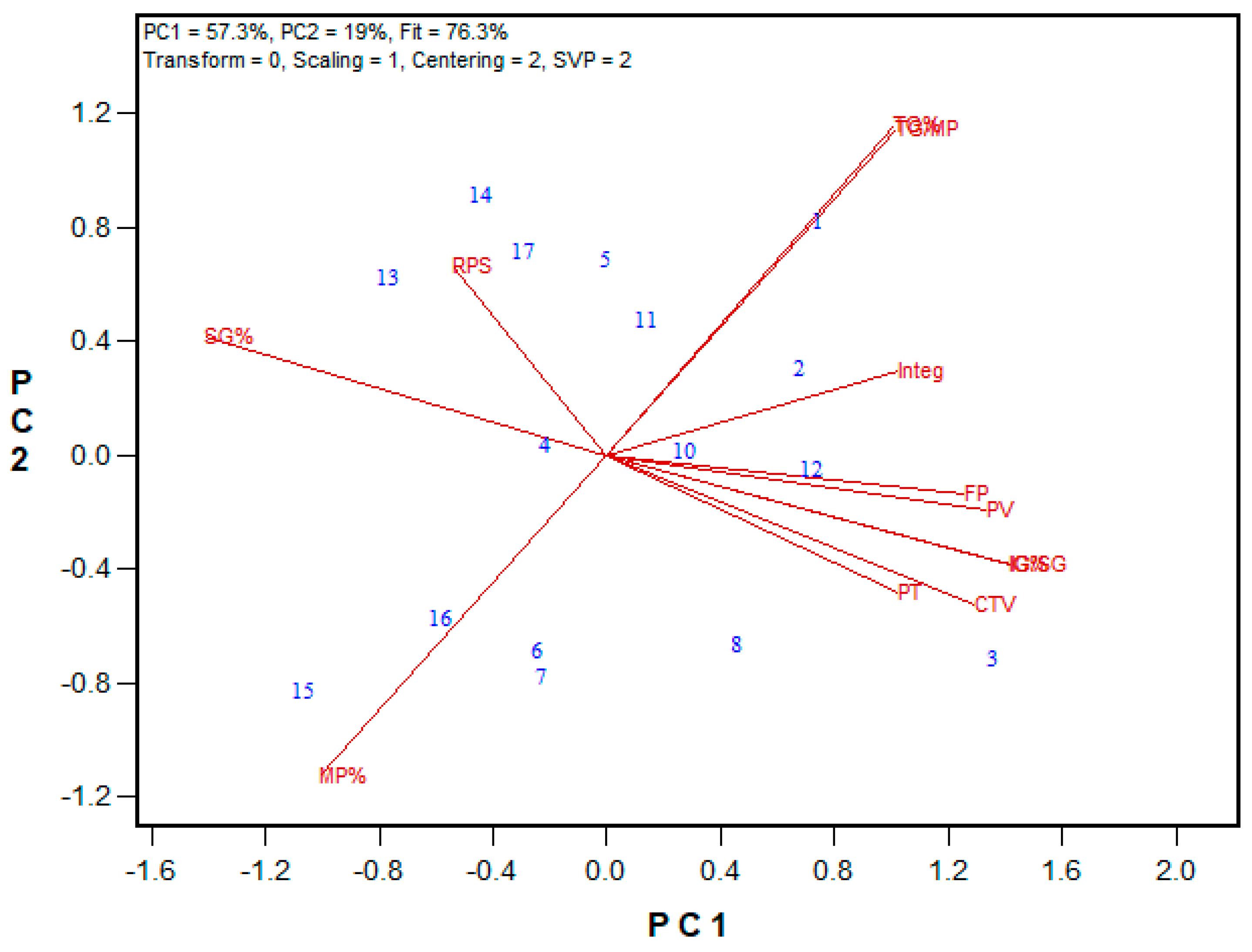
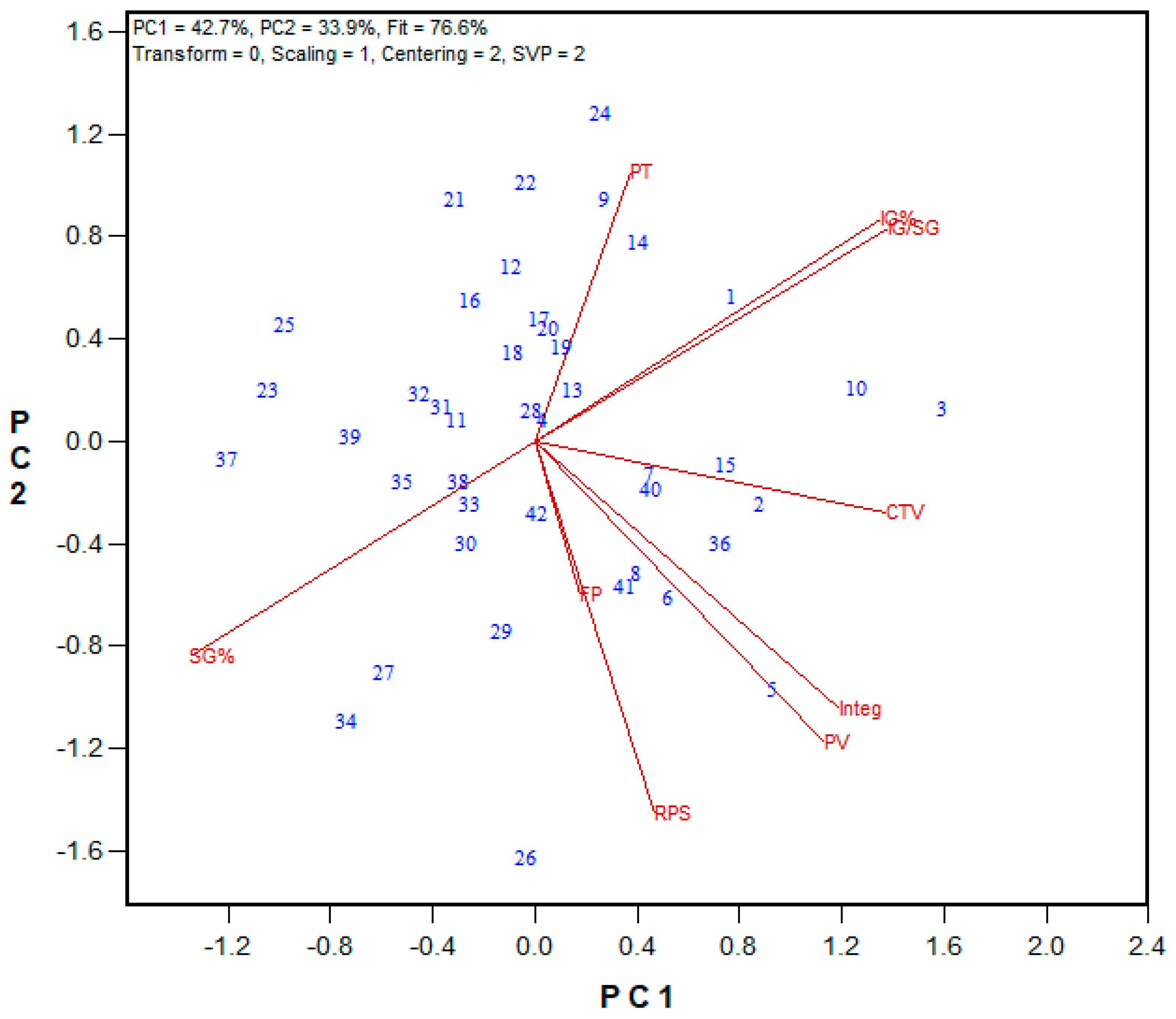
3.2. Protein Fractionation Protocol Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAFC | Agriculture and Agri-Food Canada |
| ARCCC | Atlantic Recommending Committee for Cereal Crop |
| ABS | Farinograph Absorption |
| MTI | Farinograph Mixing Tolerance Index |
| PT | Farinograph Peak Time |
| STAB | Farinograph Stability |
| GPT | GlutoPeak Tester |
| PM-AM | GPT, Differences Between PM and AM Values |
| GSI | GPT, Gluten Strength Index |
| HRS | Hard Red Spring |
| HRW | Hard Red Winter |
| OCCC | Ontario Cereal Crop Committee |
| ORDC | Ottawa Research and Development Centre |
| PF | Protein Fractionation |
| QRCC | Quebec Recommending Committee for Cereals |
References
- Finnie, S.; Atwell, W.A. (Eds.) Wheat Flour, 2nd ed.; AACC International, Inc.: St. Paul, MN, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2016; 164p. [Google Scholar]
- Khan, K.; Shewry, P.R. (Eds.) Wheat: Chemistry and Technology, 4th ed.; AACC International, Inc.: St. Paul, MN, USA, 2009; 480p. [Google Scholar]
- Shewry, P.R.; Lookhart, G.L. (Eds.) Wheat Gluten Protein Analysis; AACC International, Inc.: St. Paul, MN, USA, 2003; 198p. [Google Scholar]
- Gall, S.; Wiertz, J. Technical Note: Brabender GlutoPeak—Rapid Flour Check; Brabender GmbH & Co. KG: Duisburg, Germany, 2023; 4p. [Google Scholar]
- Melnyk, J.P.; Dreisoerner, J.; Marcone, M.F.; Seetharaman, K. Using the Gluten Peak Tester as a tool to measure physical properties of gluten. J. Cereal Sci. 2012, 56, 561–567. [Google Scholar] [CrossRef]
- Amoriello, T.; Carcea, M. Viscoelastic behavior of wheat dough with salt and a salt substitute studied by means of GlutoPeak®. Cereal Chem. 2020, 97, 216–225. [Google Scholar] [CrossRef]
- Bouachra, S.; Begemann, J.; Aarab, L.; Hüsken, A. Prediction of bread wheat baking quality using an optimized GlutoPeak®-Test method. J. Cereal Sci. 2017, 76, 8–16. [Google Scholar] [CrossRef]
- Chandi, G.K.; Seetharaman, K. Optimization of Gluten Peak Tester: A Statistical Approach. J. Food Qual. 2012, 35, 69–75. [Google Scholar] [CrossRef]
- Daba, S.D.; Simsek, S.; Green, A.J. Predictive ability of four small-scale quality tests for dough rheological properties and baking quality in hard red spring wheat. Cereal Chem. 2021, 98, 660–672. [Google Scholar] [CrossRef]
- Karaduman, Y.; Sayaslan, A.; Akın, A. GlutoPeak parameters of whole wheat flours for gluten quality evaluation in soft wheat breeding programs. J. Cereal Sci. 2020, 95, 103031. [Google Scholar] [CrossRef]
- López-Fernández, M.; Chozas, A.; Benavente, E.; Alonso-Rueda, E.; Isidro y Sánchez, J.; Pascual, L.; Giraldo, P. Genome wide association mapping of end-use gluten properties in bread wheat landraces (Triticum aestivum L.). J. Cereal Sci. 2024, 118, 103956. [Google Scholar] [CrossRef]
- Malegori, C.; Grassi, S.; Ohm, J.-B.; Anderson, J.; Marti, A. GlutoPeak profile analysis for wheat classification: Skipping the refinement process. J. Cereal Sci. 2018, 79, 73–79. [Google Scholar] [CrossRef]
- Mecitoğlu Güçbilmez, Ç.; Şahin, M.; Göçmen Akçacık, A.; Aydoğan, S.; Demir, B.; Hamzaoğlu, S.; Gür, S.; Yakışır, E. Evaluation of GlutoPeak test for prediction of bread wheat flour quality, rheological properties and baking performance. J. Cereal Sci. 2019, 90, 102827. [Google Scholar] [CrossRef]
- Michel, S.; Gallee, M.; Löschenberger, F.; Buerstmayr, H.; Kummer, C. Improving the baking quality of bread wheat using rapid tests and genomics: The prediction of dough rheological parameters by gluten peak indices and genomic selection models. J. Cereal Sci. 2017, 77, 24–34. [Google Scholar] [CrossRef]
- Schopf, M.; Scherf, K.A. Predicting vital wheat gluten quality using the gluten aggregation test and the microscale extension test. Curr. Res. Food Sci. 2020, 3, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Sissons, M. GlutoPeak: A Breeding Tool for Screening Dough Properties of Durum Wheat Semolina. Cereal Chem. 2016, 93, 550–556. [Google Scholar] [CrossRef]
- Turksoy, S.; Onar, D. A Rapid Detection of Whole Wheat Gluten Quality by a Novel Chemometric Technique-GlutoPeak. Foods 2022, 11, 1927. [Google Scholar] [CrossRef]
- Wang, J.; Hou, G.G.; Liu, T.; Wang, N.; Bock, J. GlutoPeak method improvement for gluten aggregation measurement of whole wheat flour. LWT 2018, 90, 8–14. [Google Scholar] [CrossRef]
- Brandner, S.; Becker, T.; Jekle, M. Classification of starch-gluten networks into a viscoelastic liquid or solid, based on rheological aspects—A review. Int. J. Biol. Macromol. 2019, 136, 1018–1025. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Wang, Z.; Duan, Y.; Guo, H.; Liang, Y.; Zhang, X.; Zhang, Y.; Wang, J. Effect of high-molecular-weight glutenin subunits silencing on dough aggregation characteristics. Food Chem. 2024, 441, 138371. [Google Scholar] [CrossRef]
- Wrigley, C.; Békés, F.; Bushuk, W. (Eds.) Gliadin and Glutenin, the Unique Balance of Wheat Quality; AACC International: St. Paul, MN, USA, 2006; p. 466. [Google Scholar]
- DuPont, F.M.; Chan, R.; Lopez, R.; Vensel, W.H. Sequential extraction and quantitative recovery of gliadins, glutenins, and other proteins from small samples of wheat flour. J. Agric. Food Chem. 2005, 53, 1575–1584. [Google Scholar] [CrossRef]
- Veraverbeke, W.S.; Delcour, J.A. Wheat protein composition and properties of wheat glutenin in relation to breadmaking functionality. Crit. Rev. Food Sci. Nutr. 2002, 42, 179–208. [Google Scholar] [CrossRef]
- Marti, A.; Ulrici, A.; Foca, G.; Quaglia, L.; Pagani, M.A. Characterization of common wheat flours (Triticum aestivum L.) through multivariate analysis of conventional rheological parameters and gluten peak test indices. LWT Food Sci. Technol. 2015, 64, 95–103. [Google Scholar] [CrossRef]
- Wang, K.; Dupuis, B.; Fu, B.X. Gluten Aggregation Behavior in High-Shear-Based GlutoPeak Test: Impact of Flour Water Absorption and Strength. Cereal Chem. 2017, 94, 909–915. [Google Scholar] [CrossRef]
- Fu, B.X.; Suchy, J.; Wang, K.; Dupuis, B.; Hatcher, D.; Cuthbert, R.D. Rapid micro-scale assay for functional protein fractions in wheat flour or whole meal. In Proceedings of the LACC/IGW 13th International Gluten Workshop, Mexico City, Mexico, 14–17 March 2018. [Google Scholar]
- Yan, W. GGEbiplot—A Windows Application for Graphical Analysis of Multienvironment Trial Data and Other Types of Two-Way Data. Agron. J. 2001, 93, 1111–1118. [Google Scholar] [CrossRef]
- Goldstein, A.; Ashrafi, L.; Seetharaman, K. Effects of cellulosic fibre on physical and rheological properties of starch, gluten and wheat flour. Int. J. Food Sci. Technol. 2010, 45, 1641–1646. [Google Scholar] [CrossRef]
- Santos, L.S.; de Lima, A.B.S.; Pirozi, M.R. Screening method for baking quality assessment in wheat flour using multivariate techniques associated with protein profile of wheat cultivars. Cereal Res. Commun. 2024, 53, 959–968. [Google Scholar] [CrossRef]
- Durmus, Y.; Anil, M.; Simsek, S. Discrimination of Glutopeak Test and Mixograph Parameters for Evaluation of Wheat Flour Supplemented with Hazelnut Skin, Cross-Linked Starch, and Oxidized Starch. Foods 2023, 12, 328. [Google Scholar] [CrossRef]
- Fu, B.X.; Wang, K.; Dupuis, B. Predicting water absorption of wheat flour using high shear-based GlutoPeak test. J. Cereal Sci. 2017, 76, 116–121. [Google Scholar] [CrossRef]
- Fu, B.X.; Wang, K.; Dupuis, B.; Cuthbert, R.D. High Throughput Testing of Key Wheat Quality Traits in Hard Red Spring Wheat Breeding Programs. In Wheat Quality for Improving Processing and Human Health; Igrejas, G., Ikeda, T.M., Guzmán, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 309–321. [Google Scholar]
- Wang, K.; Sangha, J.; Cuthbert, R.; Fu, B.X. Effectiveness and biochemical basis of wholemeal GlutoPeak test in predicting water absorption and gluten strength of Canadian hard red spring wheat. Cereal Chem. 2021, 98, 878–890. [Google Scholar] [CrossRef]
- Zawieja, B.; Makowska, A.; Gutsche, M. Prediction of selected rheological characteristics of wheat based on Glutopeak test parameters. J. Cereal Sci. 2020, 91, 102898. [Google Scholar] [CrossRef]
- Hoeller, N.; Scherf, K.A. Influence of salts on the protein composition and functionality of gluten. J. Cereal Sci. 2024, 118, 103978. [Google Scholar] [CrossRef]
- Melnyk, J.P.; Dreisoerner, J.; Bonomi, F.; Marcone, M.F.; Seetharaman, K. Effect of the Hofmeister series on gluten aggregation measured using a high shear-based technique. Food Res. Int. 2011, 44, 893–896. [Google Scholar] [CrossRef]
- Park, S.H.; Bean, S.R.; Chung, O.K.; Seib, P.A. Levels of Protein and Protein Composition in Hard Winter Wheat Flours and the Relationship to Breadmaking. Cereal Chem. 2006, 83, 418–423. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Quantitative composition. Cereal Chem. 2023, 100, 36–55. [Google Scholar] [CrossRef]
- Marti, A.; Augst, E.; Cox, S.; Koehler, P. Correlations between gluten aggregation properties defined by the GlutoPeak test and content of quality-related protein fractions of winter wheat flour. J. Cereal Sci. 2015, 66, 89–95. [Google Scholar] [CrossRef]



| Year | Wheat Class | Region | Check Variety Name/s |
|---|---|---|---|
| 2018 | HRW HRW | Ontario Registration Trials Quebec Registration Trials | AC Morley and Gallus Lexington |
| HRS HRS | Ontario Registration Trials Quebec Registration Trials | AC Carberry and Norwell Helios | |
| 2020 | HRS | Advanced Breeding Trials | AAC Scotia, AC Helena, and AC Walton |
| 2021 | HRW HRW | Ontario Registration Trials Quebec Registration Trials | Pro 81 and Gallus Lexington |
| HRS HRS HRS | Ontario Registration Trials Quebec Registration Trials Maritimes Registration Trials | AC Carberry and Ventry Helios and Touran AC Helena | |
| 2022 | HRW HRW | Ontario Registration Trials Quebec Registration Trials | Pro 81 and Gallus Lexington |
| HRW | Advanced Breeding Trials | Adrianus, Pro 81, and Gallus | |
| HRS HRS HRS | Ontario Registration Trials Quebec Registration Trials Maritimes Registration Trials | AC Carberry and Ventry Helios and Touran AC Helena and AC Walton | |
| HRS HRS | Advanced Breeding Trials Preliminary Breeding Trials | AAC Scotia, AC Helena, and Ventry AAC Scotia and Ventry | |
| 2023 | HRW | Advanced Breeding Trials | PRO 81 and Adrianus |
| HRS | Advanced Breeding Trials | AAC Scotia, AC Walton, and Ventry | |
| 2024 | HRW HRW | Ontario Registration Trials Quebec Registration Trials | PRO 81 and Adrianus Lexington and Mirador |
| HRW | Advanced Breeding Trials | PRO 81 and Adrianus | |
| HRS | Advanced Breeding Trials | AAC Scotia, Raven, and Ventry |
| Method Number | Reference | Flour (g):Solvent (g) | Salt/Solution Type | Temperature (°C) | Speed (RPM) | Run Time (s) |
|---|---|---|---|---|---|---|
| 1 | Brabender method | 8: 9 | 1.6% (w/v) NaCl | 36 | 2750 | 300 |
| 2 | Brabender method, adjusted for P% and M% | 8: 9 | 1.6% (w/v) NaCl | 36 | 2750 | 300 |
| 3 | [7] | 10.2: 11.2 | 1.0% (w/v) NaCl | 36 | 2750 | 300 |
| 4 | [24] | 9: 10 | 2.0% (w/v) NaCl | 35 | 3000 | 600 |
| 5 | [14] | 9: 10 | Distilled H2O | 35 | 2200 | 600 |
| 6 | [25] | 8: 10 | Distilled H2O | 34 | 2700 | 350 |
| Sample @ | GPT Parameters | Method#1 * | Method#2 | Method#3 | Method#4 | Method#5 | Method#6 |
|---|---|---|---|---|---|---|---|
| 1 | PMT | 119.7 ± 3.0 | 56.7 ± 2.2 | 100.0 ± 0.7 | 96.0 ± 0.0 | 176.0 ± 3.0 | 318.0 ± 9.0 |
| 2 | 103.0 ± 5.0 | 58.5 ± 1.5 | 82.3 ± 1.2 | 86.0 ± 1.0 | 99.0 ± 4.5 | 198.2 ± 12.0 | |
| 3 | 126.3 ± 4.1 | 83.7 ± 4.4 | 100.3 ± 2.4 | 100.3 ± 0.9 | 169.3 ± 2.4 | 251.3 ± 1.2 | |
| 4 | 295.0 ± 3.5 | 16.5 ± 0.5 | 173.7 ± 3.4 | 377.3 ± 26.7 | 145.5 ± 4.5 | 257.0 ± 11.2 | |
| 1 | BEM | 53.0 ± 0.6 | 65.0 ± 0.6 | 71.3 ± 0.9 | 68.0 ± 0.0 | 51.7 ± 0.7 | 36.5 ± 0.5 |
| 2 | 55.0 ± 1.0 | 68.0 ± 2.0 | 78.0 ± 1.0 | 74.0 ± 0.6 | 60.2 ± 1.4 | 47.3 ± 0.9 | |
| 3 | 51.3 ± 0.3 | 60.0 ± 0.9 | 72.0 ± 1.5 | 69.0 ± 0.6 | 52.3 ± 0.9 | 42.3 ± 0.3 | |
| 4 | 19.7 ± 6.8 | 97.5 ± 0.5 | 38.0 ± 0.0 | 33.3 ± 0.3 | 28.5 ± 0.5 | 21.0 ± 1.0 | |
| 1 | AM | 23.3 ± 0.9 | 43.0 ± 3.5 | 27.0 ± 0.6 | 24.5 ± 0.5 | 25.7 ± 2.6 | 16.0 ± 0.0 |
| 2 | 38.0 ± 1.0 | 41.0 ± 3.0 | 28.7 ± 0.3 | 28.0 ± 1.0 | 44.6 ± 3.6 | 18.2 ± 0.6 | |
| 3 | 26.0 ± 2.0 | 30.7 ± 1.8 | 27.0 ± 1.5 | 26.7 ± 0.9 | 27.3 ± 0.9 | 17.3 ± 0.9 | |
| 4 | 9.3 ± 2.9 | N/R | 20.0 ± 0.6 | 17.7 ± 0.3 | 11.5 ± 0.5 | 7.5 ± 0.5 | |
| 1 | PM | 38.0 ± 0.6 | 51.7 ± 1.2 | 54.7 ± 0.7 | 50.0 ± 0.0 | 41.0 ± 0.0 | 29.0 ± 1.0 |
| 2 | 41.0 ± 0.0 | 52.0 ± 2.0 | 60.0 ± 0.6 | 56.0 ± 1.0 | 49.0 ± 3.9 | 35.8 ± 2.6 | |
| 3 | 39.3 ± 0.9 | 46.7 ± 0.7 | 56.0 ± 0.6 | 52.0 ± 1.2 | 42.7 ± 0.9 | 32.3 ± 0.3 | |
| 4 | N/R | 57.5 ± 8.5 | 31.3 ± 0.3 | 28.3 ± 0.3 | 22.5 ± 0.5 | 15.5 ± 0.5 | |
| 1 | A34 | 652 ± 13 | 901 ± 9 | 784 ± 15 | 672 ± 14 | 633 ± 55 | 390 ± 5 |
| 2 | 771 ± 9 | 913 ± 42 | 854 ± 22 | 834 ± 40 | 871 ± 46 | 527 ± 15 | |
| 3 | 665 ± 17 | 786 ± 17 | 783 ± 40 | 803 ± 11 | 699 ± 10 | 478 ± 26 | |
| 4 | 253 ± 84 | 948 ± 40 | 488 ± 8 | 424 ± 2 | 310 ± 3 | 230 ± 19 | |
| 1 | A45 | 695 ± 10 | 861 ± 30 | 952 ± 17 | 903 ± 6 | 704 ± 11 | 501 ± 13 |
| 2 | 715 ± 2 | 913 ± 1 | 1067 ± 10 | 1000 ± 23 | 811 ± 29 | 592 ± 25 | |
| 3 | 695 ± 18 | 799 ± 6 | 988 ± 8 | 922 ± 19 | 724 ± 10 | 538 ± 5 | |
| 4 | 975 ± 136 | 1257 ± 28 | 525 ± 2 | 471 ± 3 | 385 ± 3 | 287 ± 12 | |
| 1 | GSI ^ | 101,932 ± 3806 | 125,276 ± 1384 | 171,556 ± 4257 | 136,952 ± 2312 | 158,709 ± 6441 | 100,836 ± 8424 |
| 2 | 116,364 ± 2748 | 129,877 ± 7183 | 173,438 ± 4564 | 158,637 ± 3506 | 161,820 ± 5132 | 105,139 ± 3541 | |
| 3 | 100,131 ± 1853 | 119,429 ± 1831 | 172,115 ± 14,007 | 150,849 ± 7672 | 153,576 ± 5584 | 100,839 ± 4457 | |
| 4 | 20,999 ± 8468 | 92,499 ± 4423 | 65,220 ± 2480 | 74,608 ± 4464 | 29,745 ± 1633 | 16,401 ± 2981 |
| Sample | GPT Parameter | 1.0% NaCl | 1.6% NaCl |
|---|---|---|---|
| AACCI Hard Wheat | PMT | 115.0 ± 0.7 | 116.3 ± 1.7 |
| BEM | 63.0 ± 0.4 | 62.3 ± 0.6 | |
| A23 | 813 ± 77 | 809 ± 39 | |
| A34 | 753 ± 10 | 801 ± 5 | |
| A45 | 857 ± 8 | 873 ± 3 | |
| GSI | 98,757 ± 5742 | 100,173 ± 1655 | |
| Hard Red Spring Wheat | PMT | 139.8 ± 1.1 | 148.3 ± 2.1 |
| BEM | 59.3 ± 0.5 | 58.5 ± 0.3 | |
| A23 | 1191 ± 63 | 1329 ± 54 | |
| A34 | 796 ± 6 | 801 ± 8 | |
| A45 | 806 ± 2 | 815 ± 10 | |
| GSI | 117,811 ± 4951 | 124,564 ± 2807 | |
| Hard Red Winter Wheat | PMT | 123.8 ± 1.4 | 135.3 ± 0.8 |
| BEM | 46.3 ± 0.3 | 46.3 ± 0.3 | |
| A23 | 938 ± 58 | 991 ± 42 | |
| A34 | 576 ± 9 | 579 ± 13 | |
| A45 | 632 ± 9 | 649 ± 7 | |
| GSI | 69,966 ± 2201 | 72,626 ± 2613 |
| Sample | GPT Parameter | 1% NaCl | 0.5 M CaCl2 |
|---|---|---|---|
| Hard Red Spring Wheat | PMT | 71.5 ± 2.3 | 43.3 ± 0.3 |
| BEM | 63.5 ± 1.6 | 68.7 ± 0.9 | |
| AM | 24.2 ± 0.4 | 24.2 ± 0.5 | |
| PM | 43.3 ± 0.7 | 45.0 ± 0.6 | |
| PM-AM | 19.2 ± 0.5 | 20.8 ± 0.6 | |
| A34 | 630 ± 10 | 686 ± 9 | |
| GSI | 40,094 ± 1684 | 47,105 ± 1111 | |
| Hard Red Winter Wheat | PMT | 68.7 ± 3.6 | 42.8 ± 0.5 |
| BEM | 54.5 ± 1.4 | 57.2 ± 0.9 | |
| AM | 21.3 ± 0.9 | 19.8 ± 0.5 | |
| PM | 35.7 ± 0.6 | 37.0 ± 0.4 | |
| PM-AM | 14.3 ± 0.6 | 17.2 ± 0.7 | |
| A34 | 562 ± 15 | 560 ± 10 | |
| GSI | 30,718 ± 1610 | 31,975 ± 638 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadinezhad, M.; Frégeau-Reid, J.; Giles, M.; Ballentine, J.; Carkner, B. Accelerated Screening of Wheat Gluten Strength Using Dual Physicochemical Tests in Diverse Breeding Lines. Methods Protoc. 2025, 8, 124. https://doi.org/10.3390/mps8050124
Hadinezhad M, Frégeau-Reid J, Giles M, Ballentine J, Carkner B. Accelerated Screening of Wheat Gluten Strength Using Dual Physicochemical Tests in Diverse Breeding Lines. Methods and Protocols. 2025; 8(5):124. https://doi.org/10.3390/mps8050124
Chicago/Turabian StyleHadinezhad, Mehri, Judith Frégeau-Reid, Makayla Giles, Jeremy Ballentine, and Brittany Carkner. 2025. "Accelerated Screening of Wheat Gluten Strength Using Dual Physicochemical Tests in Diverse Breeding Lines" Methods and Protocols 8, no. 5: 124. https://doi.org/10.3390/mps8050124
APA StyleHadinezhad, M., Frégeau-Reid, J., Giles, M., Ballentine, J., & Carkner, B. (2025). Accelerated Screening of Wheat Gluten Strength Using Dual Physicochemical Tests in Diverse Breeding Lines. Methods and Protocols, 8(5), 124. https://doi.org/10.3390/mps8050124







