Functional Methods for Studying Sperm–Zona Pellucida Interaction in Mammals
Abstract
1. Introduction
2. Functional Sperm–ZP Binding Assays
2.1. Antibody Blocking Binding Assay
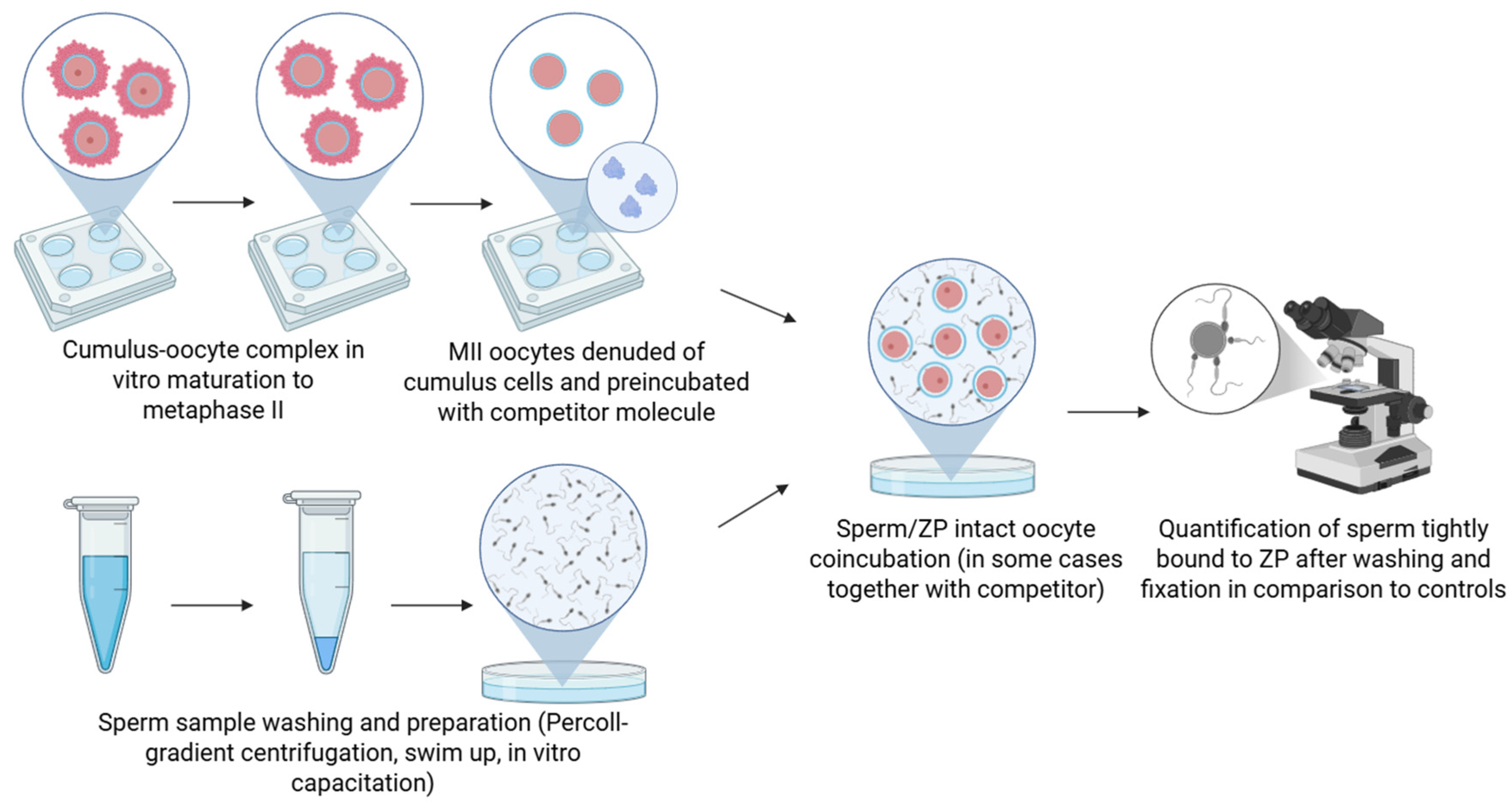
2.2. Competitive Binding Assay
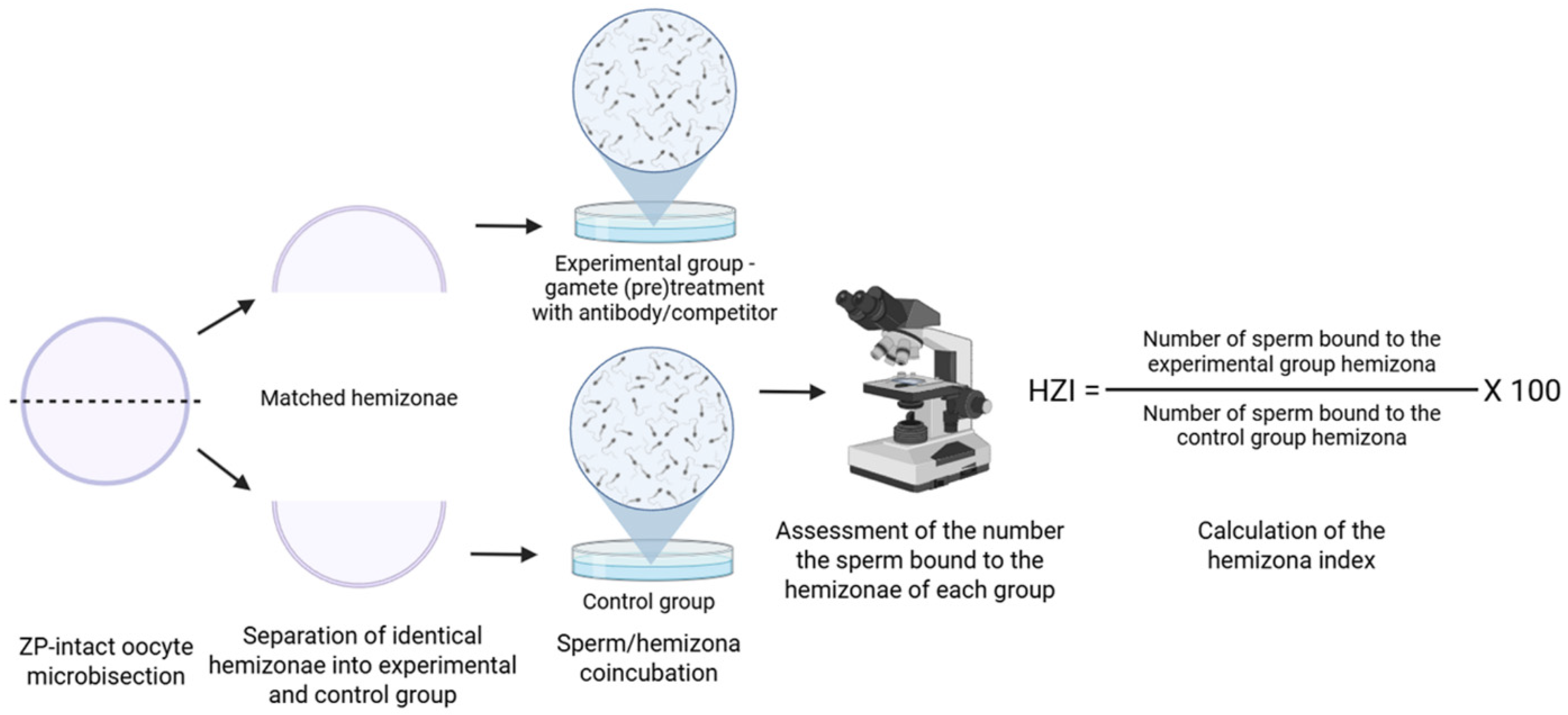
2.3. Hemizona Binding Assay
3. Visualization Methods
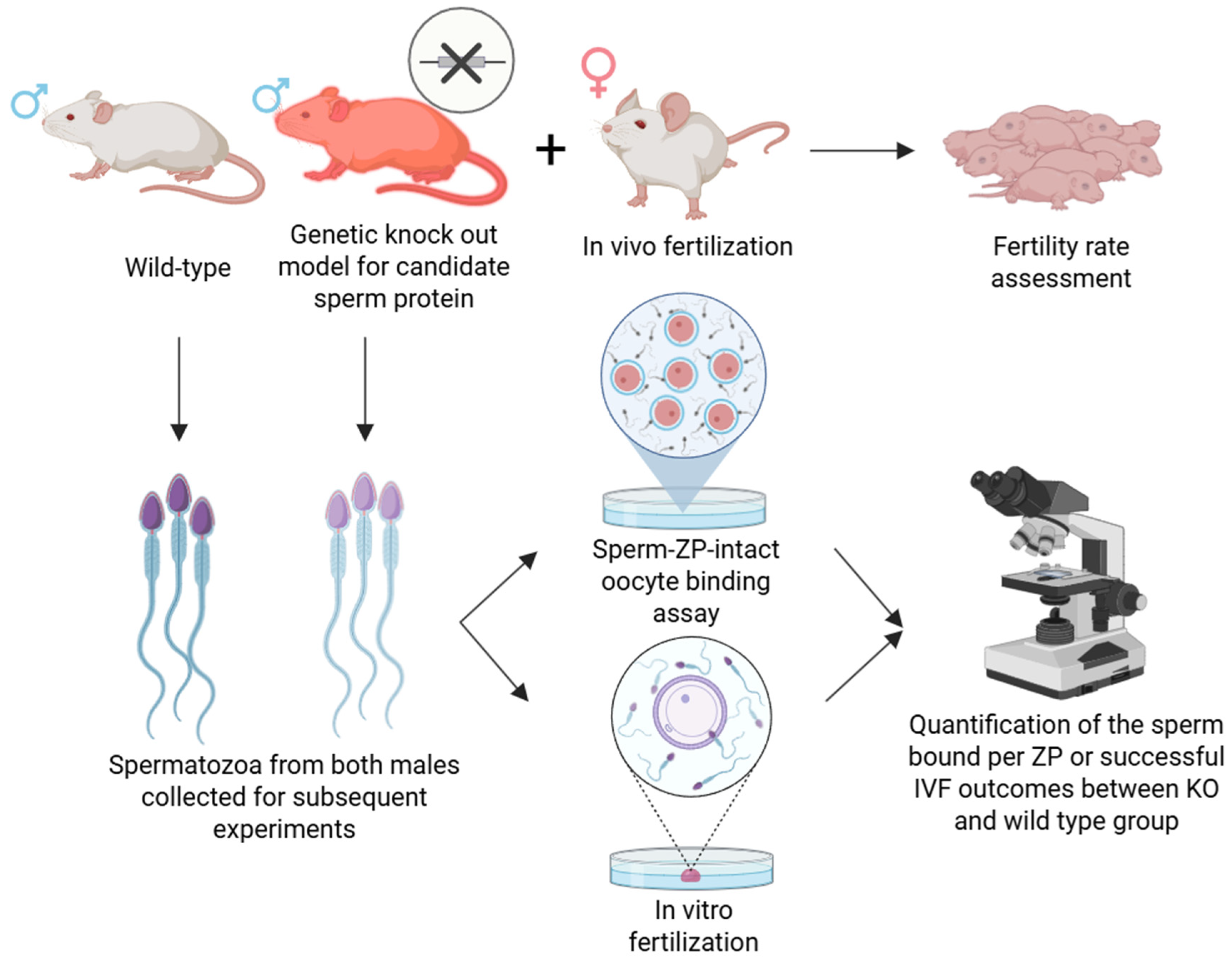
4. In Vivo Methods
4.1. Genetic Knockout Models
4.2. Other Genetic Methods
5. Discussion
6. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Töpfer-Petersen, E.; Romcro, A.; Varcla, P.F.; Ekhlasi-Hundriescr, M.; Dostàlovà, Z.; Sanz, L.; Calvete, J.J. Spermadhesins: A new protein family. Facts, hypotheses and perspectives. Andrologia 1998, 30, 217–224. [Google Scholar] [CrossRef]
- Ikawa, M.; Inoue, N.; Benham, A.M.; Okabe, M. Fertilization: A sperm’s journey to and interaction with the oocyte. J. Clin. Investig. 2010, 120, 984–994. [Google Scholar] [CrossRef]
- Redgrove, K.A.; Aitken, R.J.; Nixon, B. More Than a Simple Lock and Key Mechanism: Unraveling the Intricacies of Sperm-Zona Pellucida Binding. Binding Protein; IntechOpen: Rijeka, Croatia, 2012; pp. 73–122. [Google Scholar]
- Thaler, C.D.; Cardullo, R.A. The initial molecular interaction between mouse sperm and the zona pellucida is a complex binding event. J. Biol. Chem. 1996, 271, 23289–23297. [Google Scholar] [CrossRef]
- Reid, A.T.; Redgrove, K.; Aitken, R.J.; Nixon, B. Cellular mechanisms regulating sperm-zona pellucida interaction. Asian. J. Androl. 2011, 13, 88–96. [Google Scholar] [CrossRef]
- Tanphaichitr, N.; Kongmanas, K.; Kruevaisayawan, H.; Saewu, A.; Sugeng, C.; Fernandes, J.; Souda, P.; Angel, J.B.; Faull, K.F.; Aitken, R.J. Remodeling of the plasma membrane in preparation for sperm–egg recognition: Roles of acrosomal proteins. Asian J. Androl. 2015, 17, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Macek, M.B.; Shur, B.D. Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding. Nature 1992, 357, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Töpfer-Petersen, E.; Henschen, A. Acrosin shows zona and fucose binding, novel properties for a serine proteinase. FEBS Lett. 1987, 226, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Hickox, J.R.; Bi, M.; Hardy, D.M. Heterogeneous Processing and Zona Pellucida Binding Activity of Pig Zonadhesin. J. Biol. Chem. 2001, 276, 41502–41509. [Google Scholar] [CrossRef]
- Tantibhedhyangkul, J.; Weerachatyanukul, W.; Carmona, E.; Xu, H.; Anupriwan, A.; Michaud, D.; Tanphaichitr, N. Role of Sperm Surface Arylsulfatase A in Mouse Sperm-Zona Pellucida Binding. Biol. Reprod. 2002, 67, 212–219. [Google Scholar] [CrossRef]
- Burks, D.J.; Carballada, R.; Moore, H.D.; Saling, P.M. Interaction of a tyrosine kinase from human sperm with the zona pellucida at fertilization. Science 1995, 269, 83–86. [Google Scholar] [CrossRef]
- Kongmanas, K.; Kruevaisayawan, H.; Saewu, A.; Sugeng, C.; Fernandes, J.; Souda, P.; Angel, J.B.; Faull, K.F.; Aitken, R.J.; Whitelegge, J.; et al. Proteomic Characterization of Pig Sperm Anterior Head Plasma Membrane Reveals Roles of Acrosomal Proteins in ZP3 Binding. J. Cell. Physiol. 2015, 230, 449–463. [Google Scholar] [CrossRef]
- van Gestel, R.A.; Brewis, I.A.; Ashton, P.R.; Helms, J.B.; Brouwers, J.F.; Gadella, B.M. Capacitation-dependent concentration of lipid rafts in the apical ridge head area of porcine sperm cells. Mol. Hum. Reprod. 2005, 11, 583–590. [Google Scholar] [CrossRef]
- van Gestel, R.A.; Brewis, I.A.; Ashton, P.R.; Brouwers, J.F.; Gadella, B.M. Multiple proteins present in purified porcine sperm apical plasma membranes interact with the zona pellucida of the oocyte. Mol. Hum. Reprod. 2007, 13, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Aitken, R.J. The biological significance of detergent-resistant membranes in spermatozoa. J. Reprod. Immunol. 2009, 83, 8–13. [Google Scholar] [CrossRef]
- Tumova, L.; Zigo, M.; Sutovsky, P.; Sedmikova, M.; Postlerova, P. Ligands and receptors involved in the sperm-zona pellucida interactions in Mammals. Cells 2021, 10, 133. [Google Scholar] [CrossRef]
- Fujihara, Y.; Herberg, S.; Blaha, A.; Panser, K.; Kobayashi, K.; Larasati, T.; Novatchkova, M.; Theussl, H.-C.; Olszanska, O.; Ikawa, M.; et al. The conserved fertility factor SPACA4/Bouncer has divergent modes of action in vertebrate fertilization. Proc. Natl. Acad. Sci. USA 2021, 118, e2108777118. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Cho, C.; Branciforte, D.R.; Myles, D.G.; Primakoff, P. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin β. Develop. Biol. 2001, 233, 204–213. [Google Scholar] [CrossRef]
- Sanz, L.; Calvete, J.J.; Mann, K.; Schäfer, W.; Schmid, E.R.; Töpfer-Petersen, E. The complete primary structure of the boar spermadhesin AQN-1, a carbohydrate-binding protein involved in fertilization. Eur. J. Biochem. 1992, 205, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Veselský, L.; Pěknicová, J.; Čechová, D.; Kraus, M.; Geussová, G.; Jonáková, V. Characterization of boar spermadhesins by monoclonal and polyclonal antibodies and their role in binding to oocytes. Am. J. Reprod. Immunol. 1999, 42, 187–197. [Google Scholar] [CrossRef]
- Margaryan, H.; Dorosh, A.; Capkova, J.; Manaskova-Postlerova, P.; Philimonenko, A.; Hozak, P.; Peknicova, J. Characterization and possible function of glyceraldehyde-3-phosphate dehydrogenase-spermatogenic protein GAPDHS in mammalian sperm. Reprod. Biol. Endocrinol. 2015, 13, 15. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Yang, X.; Kan, F.W.K. Recombinant hamster oviductin is biologically active and exerts positive effects on sperm functions and sperm-oocyte binding. PLoS ONE 2015, 10, e0123003. [Google Scholar] [CrossRef]
- Tanphaichitr, N.; Smith, J.; Mongkolsirikieart, S.; Gradil, C.; Lingwood, C.A. Role of a Gamete-Specific Sulfoglycolipid Immobilizing Protein on Mouse Sperm-Egg Binding. Dev. Biol. 1993, 156, 164–175. [Google Scholar] [CrossRef]
- White, D.; Weerachatyanukul, W.; Gadella, B.; Kamolvarin, N.; Attar, M.; Tanphaichitr, N. Role of Sperm Sulfogalactosylglycerolipid in Mouse Sperm-Zona Pellucida Binding1. Biol. Reprod. 2000, 63, 147–155. [Google Scholar] [CrossRef]
- Rattanachaiyanont, M.; Weerachatyanukul, W.; Léveillé, M.-C.; Taylor, T.; Amours, D.D.; Rivers, D.; Leader, A.; Tanphaichitr, N. Anti-SLIP1-reactive proteins exist on human spermatozoa and are involved in zona pellucida binding. Mol. Hum. Reprod. 2001, 7, 33–40. [Google Scholar] [CrossRef]
- Carmona, E.; Weerachatyanukul, W.; Soboloff, T.; Fluharty, A.L.; White, D.; Promdee, L.; Ekker, M.; Berger, T.; Buhr, M.; Tanphaichitr, N. Arylsulfatase A is present on the pig sperm surface and is involved in sperm-zona pellucida binding. Dev. Biol. 2002, 247, 182–196. [Google Scholar] [CrossRef]
- Zayas-Pérez, H.; Casas, E.; Bonilla, E.; Betancourt, M. Inhibition of sperm-zona pellucida binding by a 55 Kda pig sperm protein in vitro. Arch. Androl. 2005, 51, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Maňásková, P.; Liberda, J.; Tichá, M.; Jonáková, V. Aggregated and monomeric forms of proteins in boar seminal plasma: Characterization and binding properties. Folia Biol. 2000, 46, 143–151. [Google Scholar]
- Maňásková, P.; Pěknicová, J.; Elzeinová, F.; Tichá, M.; Jonáková, V. Origin, localization and binding abilities of boar DQH sperm surface protein tested by specific monoclonal antibodies. J. Reprod. Immunol. 2007, 74, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Peknicova, J.; Capkova, L.J.; Geussova, G.; Ivanova, M.; Mollova, M. Monoclonal antibodies to intra-acrosomal proteins inhibit gamete binding in vitro. Theriogenology 2001, 56, 211–223. [Google Scholar] [CrossRef]
- Busso, D.; Cohen, D.J.; Maldera, J.A.; Dematteis, A.; Cuasnicu, P.S. A novel function for CRISP1 in rodent fertilization: Involvement in sperm-zona pellucida interaction. Biol. Reprod. 2007, 77, 848–854. [Google Scholar] [CrossRef]
- Ensslin, M.A.; Shur, B.D. Identification of Mouse Sperm SED1, a Bimotif EGF Repeat and Discoidin-Domain Protein Involved in Sperm-Egg Binding. Cell 2003, 114, 405–417. [Google Scholar] [CrossRef]
- Shur, B.D.; Hall, G.N. A Role for Mouse Sperm Surface Galactosyltransferase in Sperm Binding to the Egg Zona Pellucida. J. Cell Biol. 1982, 95, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Tengowski, M.W.; Wassler, M.J.; Shur, B.D.; Schatten, G. Subcellular localization of β1,4-Galactosyltransferase on bull sperm and its function during sperm-egg interactions. Mol. Reprod. Dev. 2001, 58, 236–244. [Google Scholar] [CrossRef]
- Dubova-Mihailova, M.; Mollova, M.; Ivanova, M.; Kehayov, I.; Kyurkchiev, S.; Kyurkchiev, S. Identification and characterization of human acrosomal antigen defined by a monoclonal antibody with blocking effect on in vitro fertilization. J. Reprod. Immunol. 1991, 19, 251–268. [Google Scholar] [CrossRef]
- Morin, G.; Sullivan, R.; Laflamme, I.; Robert, C.; Leclerc, P. SPAM1 isoforms from two tissue origins are differentially localized within ejaculated bull sperm membranes and have different roles during fertilization. Biol. Reprod. 2010, 82, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Primakoff, P.; Hyatt, H.; Myles, D.G. A Role for the in Guinea Pig Migrating Sperm Surface Antigen PH-20 Sperm Binding to the Egg Zona Pellucida. J. Cell Biol. 1985, 101, 2239–2244. [Google Scholar] [CrossRef]
- Coonrod, S.A.; Herr, J.C.; Westhusin, M.E. Inhibition of bovine fertilization in vitro by antibodies to SP-10. J. Reprod. Fertil. 1996, 107, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Redgrove, K.A.; Anderson, A.L.; Dun, M.D.; McLaughlin, E.A.; O’Bryan, M.K.; Aitken, R.J.; Nixon, B. Involvement of multimeric protein complexes in mediating the capacitation-dependent binding of human spermatozoa to homologous zonae pellucidae. Dev. Biol. 2011, 356, 460–474. [Google Scholar] [CrossRef]
- Cohen, N.; Wassarman, P.M. Association of egg zona pellucida glycoprotein mZP3 with sperm protein sp56 during fertilization in mice. Int. J. Dev. Biol. 2001, 45, 569–576. [Google Scholar]
- Moreno, R.D.; Sqjulveda, M.S.; de Ioannes, A.; Barros, C. The polysulphate binding domain of human proacrosin/acrosin is involved in both the enzyme activation and spermatozoa-zona pellucida interaction. Zygote 1998, 6, 75–83. [Google Scholar] [CrossRef]
- Ranganathan, S.; Ganguly, A.K.; Datta, K. Evidence for presence of hyaluronan binding protein on spermatozoa and its possible involvement in sperm function. Mol. Reprod. Dev. 1994, 38, 69–76. [Google Scholar] [CrossRef]
- Kato, Y.; Kumar, S.; Lessard, C.; Bailey, J.L. ACRBP (Sp32) is involved in priming sperm for the acrosome reaction and the binding of sperm to the zona pellucida in a porcine model. PLoS ONE 2021, 16, e0251973. [Google Scholar] [CrossRef] [PubMed]
- Boue, F.; Berube, B.; De Lamirande, E.; Gagnon, C.; Sullivan, R. Human Sperm-Zona Pellucida Interaction Is Inhibited by an Antiserum against a Hamster Sperm Protein. Biol. Reprod. 1994, 51, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, B.; Sullivan, R. Inhibition of In Vivo Fertilization by Active Immunization of Male Hamsters against a 26-kDa Sperm Glycoprotein. Biol. Reprod. 1994, 51, 1255–1263. [Google Scholar] [CrossRef]
- Bégin, S.; Bérubé, B.; Boué, F.; Sullivan, R. Comparative Immunoreactivity of Mouse and Hamster Sperm Proteins Recognized by an Anti-P26h Hamster Sperm Protein. Mol. Reprod. Dev. 1995, 41, 249–256. [Google Scholar] [CrossRef]
- Phopin, K.; Nimlamool, W.; Lowe-Krentz, L.J.; Douglass, E.W.; Taroni, J.N.; Bean, B.S. Roles of mouse sperm-associated alpha-L-fucosidases in fertilization. Mol. Reprod. Dev. 2013, 80, 273–285. [Google Scholar] [CrossRef]
- Moase, C.E.; Kamolvarin, N.; Kan, F.W.K.; Tanphaichitr, A.N. Localization and Role of Sulfoglycolipid Immobilizing Protein 1 on the Mouse Sperm Head. Mol. Reprod. Dev. 1997, 48, 518–528. [Google Scholar] [CrossRef]
- Aitken, R.J.; Buckingham, D.W.; Fang, H.G. Analysis of the Responses of Human Spermatozoa to A23187 Employing a Novel Technique for Assessing the Acrosome Reaction. J. Androl. 1993, 14, 132–141. [Google Scholar] [CrossRef]
- Tanphaichitr, N.; Moase, C.; Taylor, T.; Surewicz, K.; Hansen, C.; Namking, M.; Bérubé, B.; Kamolvarin, N.; Lingwood, C.A.; Sullivan, R.; et al. Isolation of antiSLIP1-reactive boar sperm P68/62 and its binding to mammalian zona pellucida. Mol. Reprod. Dev. 1998, 49, 203–216. [Google Scholar] [CrossRef]
- Veselský, L.; Jonáková, V.; Sanz, M.L.; Töpfer-Petersen, E.; Čechová, D. Binding of a 15 kDa glycoprotein from spermatozoa of boars to surface of zona pellucida and cumulus oophorus cells. J. Reprod. Fertil. 1992, 96, 593–602. [Google Scholar] [CrossRef]
- Sanz, L.; Calvete, J.J.; Mann, K.; Schäfer, W.; Amselgruber, W.; Sinowatz, F.; Ehrhard, M.; Töpfer-Petersen, E. The complete primary structure of the spermadhesin AWN, a zona pellucida-binding protein isolated from boar spermatozoa. FEBS Lett. 1992, 300, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Datta, K. Sperm surface hyaluronan binding protein (HABP1) interacts with zona pellucida of water buffalo (Bubalus bubalis) through its clustered mannose residues. Mol. Reprod. Dev. 2003, 64, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A.; Ram Tuisiani, D.P.; Orgebin-Crist, M. Inhibition of the Mouse Sperm Surface a-D-Mannosidase Inhibits Sperm-Egg Binding in Vitro. Biol. Reprod. 1991, 44, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Buffone, M.G.; Zhuang, T.; Ord, T.S.; Hui, L.; Moss, S.B.; Gerton, G.L. Recombinant mouse sperm ZP3-binding protein (ZP3R/sp56) forms a high order oligomer that binds eggs and inhibits mouse fertilization in vitro. J. Biol. Chem. 2008, 283, 12438–12445. [Google Scholar] [CrossRef]
- Velásquez, J.G.; Canovas, S.; Barajas, P.; Marcos, J.; Jiménez-Movilla, M.; Gallego, R.G.; Ballesta, J.; Avilés, M.; Coy, P. Role of sialic acid in bovine sperm-zona pellucida binding. Mol. Reprod. Dev. 2007, 74, 617–628. [Google Scholar] [CrossRef]
- Kashyap, P.; Solanki, S.; Datta, T.K.; Kumar, R. Buffalo sperm membrane glycan-binding proteins reveal precise and preferential binding signatures with specific glycans targets on oviduct epithelium and zona pellucida-an implication in fertilization. Theriogenology 2023, 207, 96–109. [Google Scholar] [CrossRef]
- Fernandez-Fuertes, B.; Blanco-Fernandez, A.; Reid, C.J.; Meade, K.G.; Fair, S.; Lonergan, P. Removal of sialic acid from bull sperm decreases motility and mucus penetration ability but increases zona pellucida binding and polyspermic penetration in vitro. Reproduction 2018, 155, 481–492. [Google Scholar] [CrossRef]
- Hamze, J.G.; Canha-Gouveia, A.; Algarra, B.; Gómez-Torres, M.J.; Olivares, M.C.; Romar, R.; Jiménez-Movilla, M. Mammalian spermatozoa and cumulus cells bind to a 3D model generated by recombinant zona pellucida protein-coated beads. Sci. Rep. 2019, 9, 17989. [Google Scholar] [CrossRef]
- Chen, L.; Song, J.; Zhang, J.; Luo, Z.; Chen, X.; Zhou, C.; Shen, X. Spermatogenic cell-specific SPACA4 is essential for efficient sperm-zona pellucida binding in vitro. Front. Cell Dev. Biol. 2023, 11, 1204017. [Google Scholar] [CrossRef]
- Burkman, L.J.; Coddington, C.C.; Daniel Franken, I.R.; Kruger, T.F.; Rosenwaks, Z.; Hodgen, G.D. The hemizona assay (HZA): Development of a diagnostic test for the binding of human spermatozoa to the human hemizona pellucida to predict fertilization potential. Fertil. Steril. 1988, 49, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Jean, M.; Dacheux, J.-L.; Dacheux, F.; Sagot, P.; Lopes, P.; Barriere, P. Increased zona-binding ability after incubation of spermatozoa with proteins extracted from spermatozoa of fertile semen. J. Reprod. Fertil. 1995, 105, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Q.; Yeung, W.S.B.; Ho, P.C. The factors affecting sperm binding to the zona pellucida in the hemizona binding assay. Hum. Reprod. 1996, 11, 1516–1519. [Google Scholar] [CrossRef]
- Oehninger, S.; Morshedi, M.; Franken, D. The Hemizona Assay for Assessment of Sperm Function. Methods Mol. Biol. 2013, 927, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Franken, D.R.; Windt, M.L.; Kruger, T.F.; Oehninger, S.; Hodgen, G.D. Comparison of Sperm Binding Potential of Uninseminated, Inseminated-Unfertilized, and Fertilized-Noncleaved Human Oocytes Under Hemizona Assay Conditions. Mol. Reprod. Dev. 1991, 30, 56–61. [Google Scholar] [CrossRef]
- Kruger, T.F.; Oehninger, S.; Franken, D.R.; Hodgen, G.D. Hemizona Assay: Use of Fresh Versus Salt-Stored Human Oocytes to Evaluate Sperm Binding Potential to the Zona Pellucida. J. Vitr. Fertil. Embryo Transf. 1991, 8, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Yogev, L.; Homonnai, Z.T.; Gamzu, R.; Amit, A.; Lessing, J.B.; Paz, G.; Yavetz, H. The use of hemizona assay in the evaluation of the optimal sperm preparation technique. Hum. Reprod. 1995, 10, 851–854. [Google Scholar] [CrossRef]
- Kadam, A.L.; Fateha, M.; Nazis, R.K. Fertilization antigen (FA-1) completely blocks human sperm binding to human zona pellucida: FA-1 antigen may be a sperm receptor for zona pellucida in humans. J. Reprod. Immunol. 1995, 29, 19–30. [Google Scholar] [CrossRef]
- Chiu, P.C.N.; Chung, M.K.; Koistinen, R.; Koistinen, H.; Seppala, M.; Ho, P.C.; Ng, E.H.Y.; Lee, K.F.; Yeung, W.S.B. Glycodelin-A interacts with fucosyltransferase on human sperm plasma membrane to inhibit spermatozoa-zona pellucida binding. J. Cell Sci. 2007, 120, 33–44. [Google Scholar] [CrossRef]
- Naz, R.K.; Dhandapani, L. Identification of human sperm proteins that interact with human zona pellucida3 (ZP3) using yeast two-hybrid system. J. Reprod. Immunol. 2010, 84, 24–31. [Google Scholar] [CrossRef]
- Mei, S.; Chen, P.; Lee, C.-L.; Zhao, W.; Wang, Y.; Ho, P.-C.; Yeung, W.S.; Fang, C.; Chiu, P.C.; Chi Ngong Chiu, P. The role of galectin-3 in spermatozoa-zona pellucida binding and its 1 association with fertilization in vitro. Mol. Hum. Reprod. 2019, 25, 458–470. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Mei, S.; Chen, P.; Leung, T.Y.; Lee, C.L.; Yeung, W.S.B.; Ou, J.P.; Liang, X.; Chiu, P.C.N. Identification of Sialyl-Lewis(x)-Interacting Protein on Human Spermatozoa. Front. Cell Dev. Biol. 2021, 9, 700396. [Google Scholar] [CrossRef]
- Hamatani, T.; Tanabe, K.; Kamei, K.; Sakai, N.; Yamamoto, Y.; Yoshimura, Y. A Monoclonal Antibody to Human SP-10 Inhibits In Vitro the Binding of Human Sperm to Hamster Oolemma but Not to Human Zona Pellucida. Biol. Reprod. 2000, 62, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Zhu, X.; Kadam, A.L. Identification of Human Sperm Peptide Sequence Involved in Egg Binding for Immunocontraception. Biol. Reprod. 2000, 62, 318–324. [Google Scholar] [CrossRef]
- Maldera, J.A.; Muñoz, M.W.; Chirinos, M.; Busso, D.; Raffo, F.G.E.; Battistone, M.A.; Blaquier, J.A.; Larrea, F.; Cuasnicu, P.S. Human fertilization: Epididymal hCRISP1 mediates sperm-zona pellucida binding through its interaction with ZP3. Mol. Hum. Reprod. 2014, 20, 341–349. [Google Scholar] [CrossRef]
- Copland, S.D.; Murphy, A.A.; Shur, B.D. The mouse gamete adhesin, SED1, is expressed on the surface of acrosome-intact human sperm. Fertil. Steril. 2009, 92, 2014–2019. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Lakamp, A.; Gentzel, M.; Ekhlasi-Hundrieser, M.; Topfer-Petersen, E. Fate of lactadherin P47 during post-testicular maturation and capacitation of boar spermatozoa. Reproduction 2003, 125, 377–387. [Google Scholar] [CrossRef][Green Version]
- Dun, M.D.; Smith, N.D.; Baker, M.A.; Lin, M.; John Aitken, R.; Nixon, B. The chaperonin containing TCP1 complex (CCT/TRiC) is involved in mediating sperm-oocyte interaction. J. Biol. Chem. 2011, 286, 36875–36887. [Google Scholar] [CrossRef]
- Cheng, F.P.; Fazeli, A.; Voorhout, W.F.; Marks, A.; Bevers, M.M.; Colenbrander, B. Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa. J. Androl. 1996, 17, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Esterhuizen, A.D.; Franken, D.R.; Lourens, J.G.H.; Van Rooyen, L.H. Clinical importance of zona pellucida-induced acrosome reaction and its predictive value for IVF. Hum. Reprod. 2001, 16, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Carreras, A.; Moos, J.; Tesarik, J. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J. Reprod. Fertil. 1992, 95, 755–763. [Google Scholar] [CrossRef]
- Baba, T.; Azuma, S.; Kashiwabara, S.I.; Toyoda, Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J. Biol. Chem. 1994, 269, 31845–31849. [Google Scholar] [CrossRef]
- Lu, Q.; Shur, B.D. Sperm from beta 1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development 1997, 124, 4121–4131. [Google Scholar] [CrossRef]
- Cho, C.; Bunch, D.O.; Faure, J.-E. Fertilization defects in sperm from mice lacking fertilin beta. Science 1998, 281, 1857–1859. [Google Scholar] [CrossRef]
- Shetty, J.; Wolkowicz, M.J.; Digilio, L.C.; Klotz, K.L.; Jayes, F.L.; Diekman, A.B.; Westbrook, V.A.; Farris, E.M.; Hao, Z.; Coonrod, S.A.; et al. SAMP14, a novel, acrosomal membrane-associated, glycosylphosphatidylinositol-anchored member of the Ly-6/urokinase-type plasminogen activator receptor superfamily with a role in sperm-egg interaction. J. Biol. Chem. 2003, 278, 30506–30515. [Google Scholar] [CrossRef] [PubMed]
- Devlin, D.J.; Agrawal Zaneveld, S.; Nozawa, K.; Han, X.; Moye, A.R.; Liang, Q.; Harnish, J.M.; Matzuk, M.M.; Chen, R. Knockout of mouse receptor accessory protein 6 leads to sperm function and morphology defects. Biol. Reprod. 2020, 102, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Kim, E.; Nakanishi, T.; Baba, T. Possible function of the ADAM1a/ADAM2 fertilin complex in the appearance of ADAM3 on the sperm surface. J. Biol. Chem. 2004, 279, 34957–34962. [Google Scholar] [CrossRef] [PubMed]
- Tokuhiro, K.; Ikawa, M.; Benham, A.M.; Okabe, M. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male infertility. Proc. Natl. Acad. Sci. USA 2012, 109, 3850–3855. [Google Scholar] [CrossRef] [PubMed]
- Tardif, S.; Wilson, M.D.; Wagner, R.; Hunt, P.; Gertsenstein, M.; Nagy, A.; Lobe, C.; Koop, B.F.; Hardy, D.M. Zonadhesin Is Essential for Species Specificity of Sperm Adhesion to the Egg Zona Pellucida. J. Biol. Chem. 2010, 285, 24863–24870. [Google Scholar] [CrossRef]
- Adham, I.M.; Nayernia, K.; Engel, W. Spermatozoa lacking acrosin protein show delayed fertilization. Mol. Reprod. Dev. 1997, 46, 370–376. [Google Scholar] [CrossRef]
- Isotani, A.; Matsumura, T.; Ogawa, M.; Tanaka, T.; Yamagata, K.; Ikawa, M.; Okabe, M. A delayed sperm penetration of cumulus layers by disruption of acrosin gene in rats. Biol. Reprod. 2017, 97, 61–68. [Google Scholar] [CrossRef]
- Hirose, M.; Honda, A.; Fulka, H.; Tamura-Nakano, M.; Matoba, S.; Tomishima, T.; Mochida, K.; Hasegawa, A.; Nagashima, K.; Inoue, K.; et al. Acrosin is essential for sperm penetration through the zona pellucida in hamsters. Proc. Natl. Acad. Sci. USA 2020, 117, 2513–2518. [Google Scholar] [CrossRef]
- Bleil, J.D.; Wassarman, P.M. Identification of a ZP3-binding protein on acrosome-intact mouse sperm by photoaffinity crosslinking. Proc. Natl. Acad. Sci. USA 1990, 87, 5563–5567. [Google Scholar] [CrossRef]
- Muro, Y.; Buffone, M.G.; Okabe, M.; Gerton, G.L. Function of the acrosomal matrix: Zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization. Biol. Reprod. 2012, 86, 1–6. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, R.; Guo, R.; Yang, F.; Sha, X.; Li, Y.; Hua, R.; Li, G.; Shen, Q.; Li, K.; et al. CALR3 defects disrupt sperm-zona pellucida binding in humans: New insights into male factor fertilization failure and relevant clinical therapeutic approaches. Hum. Reprod. 2024, 39, 2608–2617. [Google Scholar] [CrossRef]
- Ikawa, M.; Tokuhiro, K.; Yamaguchi, R.; Benham, M.A.; Tamura, T.; Wada, I.; Satouh, Y.; Inoue, N.; Okabe, M. Calsperin is a testis-specific chaperone required for sperm fertility. J. Biol. Chem. 2011, 286, 5639–5646. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Honda, A.; Ogura, A.; Kashiwabara, S.I.; Fukami, K.; Baba, T. Reduced fertility of mouse epididymal sperm lacking Prss21/Tesp5 is rescued by sperm exposure to uterine microenvironment. Genes Cells 2008, 13, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef]
- Miles, E.L.; O’Gorman, C.; Zhao, J.; Samuel, M.; Walters, E.; Yi, Y.J.; Sutovsky, M.; Prather, R.S.; Wells, K.D.; Sutovsky, P. Transgenic pig carrying green fluorescent proteasomes. Proc. Natl. Acad. Sci. USA 2013, 110, 6334–6339. [Google Scholar] [CrossRef]
- Navarro-Serna, S.; Piñeiro-Silva, C.; Fernández-Martín, I.; Dehesa-Etxebeste, M.; López de Munain, A.; Gadea, J. Oocyte electroporation prior to in vitro fertilization is an efficient method to generate single, double, and multiple knockout porcine embryos of interest in biomedicine and animal production. Theriogenology 2024, 218, 111–118. [Google Scholar] [CrossRef]
- Fernandez-Fuertes, B.; Laguna-Barraza, R.; Fernandez-Gonzalez, R.; Gutierrez-Adan, A.; Blanco-Fernandez, A.; O’Doherty, M.A.; Di Fenza, M.; Kelly, K.A.; Kölle, S.; Lonergan, P. Subfertility in bulls carrying a nonsense mutation in transmembrane protein 95 is due to failure to interact with the oocyte vestments. Biol. Reprod. 2017, 97, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hagaman, J.R.; Moyer, J.S.; Bachman, E.S.; Sibony, M.; Magyar, P.L.; Welch, J.E.; Smithies, O.; Krege, J.H.; O’Brien, D.A. Angiotensin-converting enzyme and male fertility. Proc. Natl. Acad. Sci. USA 1998, 95, 2552–2557. [Google Scholar] [CrossRef]
- Baba, D.; Kashiwabara, S.I.; Honda, A.; Yamagata, K.; Wu, Q.; Ikawa, M.; Okabe, M.; Baba, T. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J. Biol. Chem. 2002, 277, 30310–30314. [Google Scholar] [CrossRef]
- Baibakov, B.; Boggs, N.A.; Yauger, B.; Baibakov, G.; Dean, J. Human sperm bind to the N-terminal domain of ZP2 in humanized zonae pellucidae in transgenic mice. J. Cell Biol. 2012, 197, 897–905. [Google Scholar] [CrossRef]
- Gupta, S.K. Human Zona Pellucida Glycoproteins: Binding Characteristics With Human Spermatozoa and Induction of Acrosome Reaction. Front. Cell Dev. Biol. 2021, 9, 619868. [Google Scholar] [CrossRef]
- Fields, S.; Sternglanz, R. The two-hybrid system: An assay for protein-protein interactions. Trends Genet. 1994, 10, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Franken, D.R.; Kruger, T.F.; Oehninger, S.C.; Kaskar, K.; Hodgen, G.D. Sperm binding capacity of human zona pellucida derived from oocytes obtained from different sources. Andrologia 1994, 26, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Saito, T.; Shimada, Y.; Nishimura, H. Fertilization mechanisms in hermaphroditic ascidians and nematodes: Common mechanisms with mammals and plants. Curr. Top. Dev. Biol. 2025, 162, 55–114. [Google Scholar] [CrossRef]
- Romar, R.; Cánovas, S.; Matás, C.; Gadea, J.; Coy, P. Pig in vitro fertilization: Where are we and where do we go? Theriogenology 2019, 137, 113–121. [Google Scholar] [CrossRef] [PubMed]
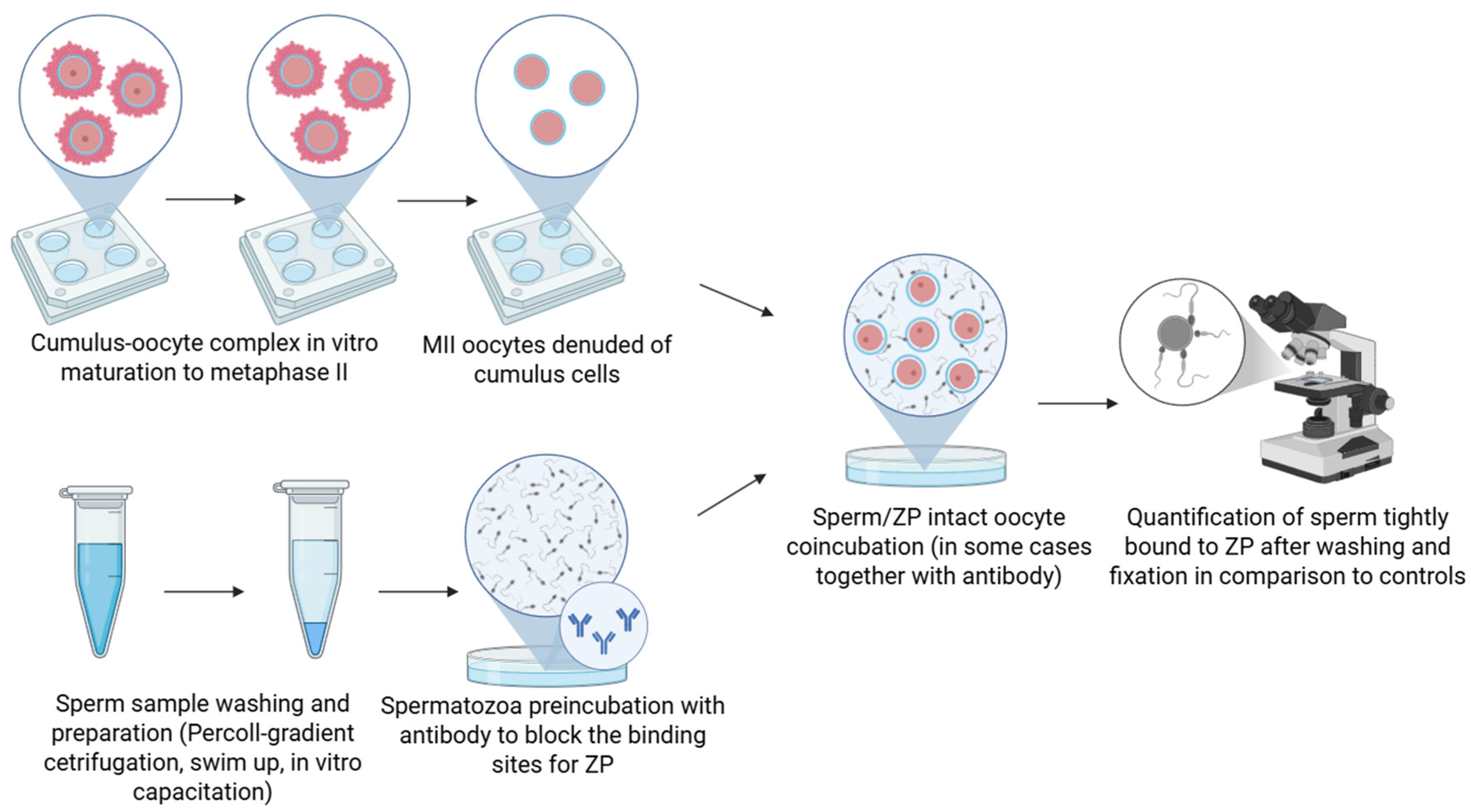
Antibody Block Binding Assay | |||||||
|---|---|---|---|---|---|---|---|
| Species | Sperm Source/Preparation | Oocyte Source | Antibody Target (Type of Antibody) | Antibody Co-Incubation Timing | Methodological Advances/Differences | Key Findings | Study |
| Porcine | Percoll gradient centrifuged capacitated boar sperm | Porcine ZP-intact oocytes | AWN spermadhesin (in-house polyclonal affinity-purified Ab and monoclonal Ab Bo.5) | Sperm/oocyte coincubation (30 min, stopped with NaN3) | Compared mono- vs. polyclonal Ab; highlighted epitope coverage and blocking efficiency differences | All anti-AWN antibodies inhibited sperm–ZP binding; polyclonal Ab blocked ZP binding more effectively (87%) than monoclonal Ab (64%) | Veselský et al. 1999 [20] |
| Mouse | Caudal epididymal and vas deferens collected Percoll gradient centrifuged mouse sperm | Mouse ZP-intact oocytes | SLIP1 (AS-A) (affinity purified from either whole antiserum or IgG fraction of polyclonal rabbit Ab) | Sperm preincubation (30 min, 1 h) Oocyte preincubation (no effect) | Used dose-dependent response; controls included medium only and the eluate of BSA blots preincubated with anti-SLIP1 of preimmune rabbit serum; only sperm that were in the same focal plane as the diameter of the ZP were counted; two-cell embryos were used as a control for non-specific binding | Anti-SLIP1 antibodies reduced sperm–ZP binding in a dose-dependent manner | Tanphaichitr et al., 1993 [23] |
| Mouse | Caudal epididymal and vas deferens collected Percoll gradient centrifuged mouse sperm | Mouse ZP-intact oocytes | SGG (in-house generated polyclonal Ab affinity purified from the rabbit antiserum IgG) | Sperm preincubation (30 min) | Used dose-dependent response, highlighted glycolipid role | Anti-SGG Ab reduced sperm–ZP binding in a dose-dependent manner, identifying SGG as a key ZP-binding molecule | White et al., 2000 [24] |
| Human | Capacitated human sperm (infertility clinic) | Human ZP-intact oocytes failed fertilization at IVF clinic | SLIP1 (rabbit polyclonal Ab in-house generated against rat testis SLIP1 and purified by SGG affinity chromatography) | Sperm preincubation (30 min) | Controlled for sperm function (motility, sperm acrosome status); species translation of method; normal rabbit serum IgG used as control | Anti-SLIP1 IgG reduced sperm–ZP binding; effect not due to motility or acrosome status | Rattanachaiyanont et al., 2001 [25] |
| Mouse | Caudal epididymal and vas deferens collected Percoll gradient centrifuged mouse sperm | Mouse ZP-intact oocytes | AS-A (IgG, Fab, affinity-purified IgG) | Sperm preincubation (30 min) | Compared IgG vs. Fab to test for steric hindrance (anti-AS-A Fab fragments also showed similar inhibitory effects) | Anti-AS-A IgG and Fab reduced binding up to 70%; similar effects for affinity-purified and Fab | Tantibhedhyang-kul et al., 2002 [10] |
| Porcine | Percoll gradient centrifuged capacitated boar sperm | Porcine ZP-intact oocytes | AS-A (rabbit polyclonal Ab; IgG, Fab) | Sperm preincubation (45 min) | Cross-species validation; compared IgG and Fab effects | Anti-AS-A Antibodies inhibited sperm–ZP binding in a dose-dependent manner; Fab fragments also inhibitory | Carmona et al., 2002 [26] |
| Porcine | Capacitated boar sperm | Porcine ZP-intact oocytes | 55 kDa sperm protein (in-house generated mouse polyclonal Ab) | Sperm preincubation (1 h before completing capacitation period) | Titration of antibody; established threshold for blocking effect | Anti-55 kDa Ab reduced binding in a dose-dependent manner; complete block at 1:100 dilution | Zayas-Pérez et al., 2005 [27] |
| Porcine | Percoll gradient centrifuged capacitated boar sperm | Porcine ZP-intact oocytes | pB1/DQH (in-house generated mouse monoclonal Abs, DQH/G12 and DQH/H6) | Both sperm preincubation (30 min) and sperm/oocyte coincubation (30 min, stopped by NaN3) | Compared sperm vs. sperm/oocyte treatment; spermatozoa preincubated in ascites of myeloma cells Sp2/0 and without any pretreatment were used for controls | DQH antibody blocked sperm–ZP binding in both experiments (sperm and sperm/oocytes coincubation) | Maňásková et al., 2007 [29] |
| Porcine | Capacitated boar spermatozoa | Porcine ZP-intact oocytes | Acrosin (monoclonal Ab ACR.2); Human intra-acrosomal protein (in-house generated mouse monoclonal Ab Hs-8) | Both sperm preincubation (30 min) and sperm/oocyte coincubation (30 min) | Timing-specific inhibition analysis; showed inhibition only during co-incubation, not pre-treatment; probed AR-released proteins; spermatozoa after induction of AR were used as a negative control for the binding test | Both antibodies reduce the secondary sperm–ZP-binding. Hs-8 blocked sperm–ZP binding only when present during incubation, not during sperm pre-treatment | Peknicova et al., 2001 [30] |
| Porcine | Capacitated boar sperm | Porcine ZP-intact oocytes | GAPDHS (in-house generated monoclonal Ab Hs-8: undiluted hybridoma supernatant; anti-GAPDHS: commercial monoclonal Ab) | Sperm/oocyte coincubation (30 min—stopped by NaN3) | Used both lab-produced and commercial Ab; monoclonal Ab against progesterone, acrosin (GAPDHS is an intra-acrosomal protein), or medium only used as a control group | Both antibodies reduced sperm–ZP binding | Margaryan et al., 2015 [21] |
| Mouse | Capacitated mouse sperm | Mouse ZP-intact oocytes | CRISP1 (rabbit polyclonal Ab) | Sperm preincubation (30 min) | Dilution series; used normal rabbit IgG and medium only as controls | Anti-CRISP1 Ab reduced sperm–ZP binding in a dilution-dependent manner | Busso et al., 2007 [31] |
| Mouse | Capacitated mouse sperm | Mouse ZP-intact oocytes | SED1 (Purified anti-SED1 IgG) | Sperm/oocyte coincubation (30 min) | Used preimmune IgG as a control, and two-cell embryos as negative controls | Anti-SED1 drastically reduced sperm–ZP adhesion | Ensslin & Shur, 2003 [32] |
| Bovine | Fresh bull sperm | Bovine ZP-intact oocytes | GalTase (rabbit antiserum) | Sperm preincubation (1 h) | Used antiserum against recombinant protein; SEM for quantification, quantification of sperm bound to the upper hemisphere of the oocyte | Anti-GalTase antiserum reduced sperm binding to oocytes (SEM quantification) | Tengowski et al., 2001 [34] |
| Human, Mouse, Porcine | Swim up selected capacitated human sperm; Percoll gradient centrifuged capacitated boar sperm; caudal epididymal swim up selected capacitated mouse sperm | Human, mouse, and porcine ZP-intact oocytes | Human acrosomal antigen (1A1—monoclonal Ab) | Sperm/oocyte coincubation (up to 12 h for IVF) | Cross-species application; tested IVF impact; medium only or the supernatants of other antibodies (e.g., anti-ZP, anti-hCG) used as controls | 1A1 Ab reduced sperm binding to ZP and IVF rates across species. | Dubova-Mihailova et al., 1991 [35] |
| Bovine | Pooled frozen-thawed Percoll gradient centrifuged bull sperm, capacitated during sperm/egg interaction | Bovine ZP-intact oocytes | SPAM1 (monoclonal antibody (203–7D10): N-terminal domain-specific Ab; or custom-made rabbit polyclonal C-terminal domain-specific Ab) | Sperm/oocyte coincubation (6 h) | Domain-specific blocking; mapped functional region of SPAM1; mouse or rabbit IgG used as controls | Blocking C-terminal domain reduced binding by 85%, N-terminal by 30% | Morin et al., 2010 [36] |
| Guinea pig | Acrosome-reacted guinea pig sperm | Guinea pig ZP-intact oocytes | PH-20 (in-house generated mouse monoclonal Ab) | Sperm preincubation during the induction of AR | Focused on secondary ZP receptors; used in vitro AR prior to assay; medium only used as a control; sperm counted in a single plane of focus | Anti-PH-20 antibody blocked binding of acrosome-reacted sperm to ZP | Primakoff et al., 1985 [37] |
| Bovine | Frozen-thawed Percoll gradient centrifuged bull sperm, capacitated and acrosome reacted during coincubation with oocytes | Bovine ZP-intact oocytes | SP-10 (monoclonal Ab cocktail containing monoclonal antibodies MHS-10, 6C12, and 3C12 targeting human SP-10, and rabbit polyclonal antisera raised against a recombinant SP-10 fusion protein) | Sperm/oocyte coincubation (18 h) | Used staged washing (pipetting, vortex) to distinguish superficial vs. stable binding; control antibodies or medium only as controls | SP-10 antibodies interfered with stable secondary sperm–ZP binding; only superficial attachment observed | Coonrod et al., 1996 [38] |
| Human | Capacitated human sperm | Human ZP-intact oocytes | CCT6A, ZPBP2 (Ab not specified) | Sperm preincubation (30 min) | Assayed protein complexes, not just single proteins; compared native lysate preincubation | Ab block of protein complexes reduced sperm–ZP binding | Redgrove et al., 2011 [39] |
| Mouse | Caudal epididymal capacitated mouse sperm | Mouse ZP-intact oocytes | sp56 (Ab not specified) | Sperm preincubation (15 min), then sperm/oocyte coincubation (additional 30 min) Alternatively, oocytes preincubated with sperm membrane vesicles (15 min) | Used sperm membrane vesicles; compared vesicle and whole-cell blocking—sperm vesicles were used for antibody block, and their preincubation with anti-sp56 also showed a 6–7-fold reduction in their ability to block sperm–egg binding; medium only and two-cell embryos used as a control | Anti-sp56 reduced sperm–ZP binding by 70–80%; vesicle block confirmed functional role | Cohen & Wassarman, 2001 [40] |
| Human | Capacitated swim-up selected human sperm (infertility clinic) | Human ZP-intact oocytes | C5F10 (anti-proacrosin/acrosin, monoclonal Ab) | Sperm pretreatment (15 min) and then gamete coincubation (additional 3 h) | Used functional controls to rule out non-specific detrimental effects of the antibody on spermatozoa (fertilization in hamster eggs) | C5F10 inhibited sperm–ZP binding; the effect was not due to sperm dysfunction | Moreno et al., 1998 [41] |
| Mouse | Caudal epididymal mouse spermatozoa | Mouse ZP-intact oocytes | HABP (polyclonal antiserum) | Sperm preincubation (30 min) | Standard antibody-blocking assay; functional validation-tested for agglutination caused by the antiserum; preimmune rabbit serum used as a control | Ab block reduced sperm–ZP binding, implicating HABP role in the binding | Ranganathan et al., 1994 [42] |
| Porcine | Percoll gradient centrifuged boar sperm | Porcine ZP-intact oocytes | ACRBP (Ab not specified) | Sperm preincubation and sperm/oocyte coincubation | Recent application; standard functional block; medium only, and pre-immune rabbit IgG used as controls | Ab block reduced sperm–ZP binding, implicating ACRBP role in the binding; anti-ACRBP Ab also impeded capacitation and the AR | Kato et al., 2021 [43] |
| Human | Percoll gradient centrifuged human sperm | Unfertilized human ZP-intact oocytes | P34H/P26h (antiserum) | Sperm preincubation (1 h) | Cross-species validation; standard Ab-blocking, preimmune serum used as a control; tested for the effect of the antibody on the sperm acrosome status and motility | Ab block reduced sperm–ZP binding. The inhibition was not due to the induction of premature AR nor to an effect on the motility of the spermatozoa | Boue et al., 1994 [44] |
| Hamster | Caudal epididymal capacitated hamster sperm | Cumulus cell oocyte complexes | P34H/P26h (preimmune serum) | Sperm/oocyte coincubation (35 min) | Sperm selected based on hyperactivation. COCs used, cumuli removed after gamete coincubation | Ab block reduced sperm–ZP binding | Berube & Sullivan, 1994 [45] |
| Hamster Mouse | Caudal epididymal capacitated hamster and mouse sperm | Hamster and mouse ZP-intact oocytes | P34H/P26h (IgG and Fab fragments of polyclonal Ab) | sperm/oocyte coincubation (35 min) | Compared cross-reactivity of mouse and hamster sperm proteins recognized by the antibody against hamster P34H (P26h) | IgG reduced sperm–ZP binding in both hamster and mouse, although the reduction was less significant in mouse, Fab fragments only in hamster | Bégin et al., 1995 [46] |
| Mouse | Caudal epididymal capacitated mouse sperm | Mouse ZP-intact oocytes | α-L-fucosidase (Ab not specified) | Sperm preincubation (30 or 60 min) | Functional block; 30 and 60 min of gamete co-incubation—data were plotted as average number of sperm tightly bound to the ZP per oocyte versus length of gamete co-incubation for each group; IgG used as a control | Ab block reduced sperm–ZP binding | Phopin et al., 2013 [47] |
| Mouse | Caudal epididymal and vas deferens Percoll gradient centrifuged mouse sperm | Mouse ZP-intact oocytes | SLIP1/AS-A (polyclonal Ab-Fab fragments) | Sperm preincubation (30 min) | Functional block; confirmed previous findings. Nonimmune rabbit serum Fab, and two-cell embryos were used as a control | Ab block reduced sperm–ZP binding, implicating SLIP1/AS-A | Moase et al., 1997 [48] |
| Competitive Binding Assay | |||||||
|---|---|---|---|---|---|---|---|
| Species | Sperm Type and Preparation | ZP Source | Competitor | Competitor Co-Incubation Timing | Methodological Advances | Key Findings | Study |
| Porcine | Capacitated boar sperm | Porcine ZP-intact oocytes | Isolated purified AQN-1 (15 kDa protein from boar ejaculated spermatozoa) | Oocyte preincubation (1 h) | Introduced concentration-dependence; used native protein receptor; medium only as a control | AQN-1 reduced sperm binding in a dose-dependent manner | Veselský et al., 1992 [51] |
| Porcine | Capacitated boar sperm | Porcine ZP-intact oocytes | Isolated purified AWN-1,2/AQN-1 (from boar ejaculated spermatozoa) | Oocyte preincubation (1 h) | Established minimum inhibitory concentration protocol; compared multiple spermadhesins | All tested spermadhesins significantly inhibited sperm binding in a dose-dependent manner, suggesting critical roles in ZP recognition (at ≥50 µg/mL) | Sanz et al., 1992a,b [19,52] |
| Porcine | Percoll gradient centrifuged capacitated boar sperm | Porcine ZP-intact oocytes | pB1/DQH antigen isolated from boar seminal plasma | Sperm/oocyte coincubation | Co-incubation was stopped by the addition of 50 μL of 10% NaN3 solution into the droplets containing the gametes | Sperm binding was strongly reduced, the percentage of ZP-bound spermatozoa decreased to 14% | Maňásková et al., 2007 [29] |
| Mouse | Percoll-gradient centrifuged sperm | Mouse ZP-intact oocytes and isolated ZP | Native SLIP1 (AS-A, isolated from rat testis, and mouse epididymal and vas deferens sperm) | Both oocyte pre-incubation (30 min) and sperm/oocyte coincubation (30 min) Sperm/isolated ZP coincubation (10 min) | Biotinylated SLIP1 was used for verification of the binding to the ZP. SLIP1 denaturation experiments to confirm loss of function | Inhibition required native SLIP1 conformation; SLIP1 binds ZP; the antibody blocked AR | Tanphaichitr et al., 1993 [23] |
| Bovine | Pooled frozen-thawed Percoll gradient centrifuged bull sperm, capacitated during sperm/egg interaction | Bovine ZP-intact oocytes | Purified SPAM1 (from bull sperm, deglycosylated vs. native) | Oocyte preincubation (2 h) | Dose-dependent inhibition; glycosylation status analysis, used native, total, N-deglycosylated, or O-deglycosylated bull sperm SPAM1, or deglycosylation buffer and enzymes as controls | Dose-dependent reduction in sperm binding. Deglycosylation of SPAM1 did not alter its inhibitory effect on sperm–ZP binding, demonstrating that SPAM1-mediated binding is independent of its glycosylation status | Morin et al., 2010 [36] |
| Water buffalo | Frozen-thawed Percoll gradient centrifuged capacitated water buffalo sperm | Water buffalo ZP-intact oocytes | Recombinant HABP1 (E. coli expressed) + DMA (synthetic) | Sperm/oocyte coincubation (2 h) | Competitor reversal protocol used—DMA considered a substitute for ZP | Sperm surface HABP1 may serve as mannose-binding sites for zona recognition | Ghosh & Datta, 2003 [53] |
| Mouse | Caudal epididymal mouse sperm | Mouse ZP-intact oocytes | Mannose analogs | Sperm/oocyte coincubation (1 h) | Inhibition study using various sugars; tested for deleterious effects of the mannose analogs on sperm motility and acrosome status, none were observed | Dose-dependent inhibition of sperm–egg binding and mannosidase activity implicated the involvement of a sperm surface mannosidase in ZP recognition | Cornwall et al., 1991 [54] |
| Porcine | Capacitated boar sperm | Porcine ZP-intact oocytes | 55 kDa protein (mouse testis, unlabeled, ion-exchange purified) | Oocyte preincubation (1 h) | Medial plane quantification method; controls included no protein, non-ZP-binding proteins, or elution buffer | 55 kDa protein caused dose-dependent inhibition; Complete inhibition at ≥50 µg | Zayas-Pérez et al., 2005 [27] |
| Mouse | Capacitated mouse sperm | Mouse ZP-intact oocytes | Recombinant SED1 (His-tagged expressed in E. coli/GST-fusion expressed in insect cells, purified) | Addition during sperm–ZP interaction (30 min) | Dual expression system validation | Both recombinant protein forms showed dose-dependent inhibition | Ensslin & Shur, 2003 [32] |
| Mouse | Capacitated mouse sperm | Mouse ZP-intact oocytes | Purified native rat CRISP1; bacterially expressed recombinant CRISP1 | Oocyte preincubation (30 min) | Cross-species protein testing; maltose-binding protein or medium alone used as a control | Conserved CRISP1–ZP interaction across rodents. Native rat CRISP1, but not recombinant CRISP1, interfered with the sperm–ZP interaction | Busso et al., 2007 [31] |
| Mouse | Caudal epididymal mouse sperm | Mouse ZP-intact oocytes | Recombinant ZP3R/sp56 | Oocyte preincubation (45 min) | Two-cell embryos used as negative controls | Specific inhibition of sperm binding to ZP-intact oocytes | Buffone et al., 2008 [55] |
| Mouse | Caudal epididymal and vas deferens Percoll gradient centrifuged pre-capacitated mouse sperm | Isolated ovarian ZP | SGG-liposomes (synthetic, cholesterol:SGG 1:1) | Sperm/oocyte co-incubation (10 min) | Artificial membrane system development | Free SGG-containing liposomes competed with sperm-bound SGG for the same binding sites on the ZP, thereby inhibiting sperm binding | White et al., 2000 [24] |
| Bovine | Frozen-thawed Percoll gradient centrifuged | Isolated bovine ZP | Sialic acid-specific lectins (LFA, MAA, and SNA) | Oocyte preincubation (1 h) | Lectin specificity profiling; combined binding competition assays and IVF in combination with different lectins, and neuraminidase digestion | MAA lectin recognizing a-2,3-linked sialic acid and neuraminidase with catalytic activity for a-2,3-linked sialic acid, demonstrated a high inhibitory effect on the sperm–ZP binding, indicating the presence of a sperm plasma membrane-specific protein for the sialic acid | Velásquez et al., 2007 [56] |
| Buffalo | Pooled swim-up selected buffalo sperm | Buffalo ZP-intact oocytes | MAA/ABL lectins (commercial, FITC-conjugated) | Oocyte preincubation (1 h) | Sperm selection via density gradient | Treatment of ZPs with 25 mg/mL lectin concentration significantly reduced the number of sperm that attached to the ZP | Kashyap et al., 2023 [57] |
| Bull | Frozen-thawed and Percoll gradient centrifuged bull sperm | Bovine ZP-intact oocytes (some with cumulus cells) | Neuraminidase (C. perfringens, 0.1 U/mL) | Sperm preincubation (1 h) | Viability-controlled enzyme treatment; two groups of oocytes—one was stripped of cumulus cells, and the second group was placed in fertilization media without removing their cumulus cells; combined with IVF experiments | Treatment with neuraminidase increased the number of sperm bound to the ZP but also the rate of polyspermic fertilization | Fernandez-Fuertes et al., 2018 [58] |
| Mouse | Caudal epididymal capacitated mouse sperm | Mouse ZP-intact oocytes | Purified human liver a-L-fucosidase | Oocyte preincubation (30 min) | Use of the enzyme as a competitor | Mouse oocytes preincubated with purified human liver a-L-fucosidase presented a reduced number of sperm tightly bound to the ZP | Phopin et al., 2013 [47] |
| Hemizona Binding Assay | |||||||
|---|---|---|---|---|---|---|---|
| Species | Hemizona Source and Preparation | Sperm Source and Preparation | Studied Protein | Gamete Pretreatment Details | Methodological Advances/Differences | Key Finding | Study |
| Human | Unfertilized oocytes from the assisted reproduction program | Motile sperm from fertile donors | UBAP2L (ubiquitin-associated protein-2 like) | Sperm preincubated with Ab against UBAP2L (4 h) | Used BSA and control immunoglobulins as controls | Anti-UBAP2L antibody inhibited sperm–ZP binding | Naz & Dhandapani, 2010 [70] |
| Human | Unfertilized oocytes from assisted reproduction program | Capacitated human sperm | Galectin-3 | Sperm preincubation with anti-galectin 3 (1 h) | Irrelevant mouse IgG or mannotriose (a glycan without binding affinity to galectin-3) was used as a control | Ab block reduced sperm–ZP binding, proposing galectin-3 as a sperm–ZP receptor | Mei et al., 2019 [71] |
| Human | Unfertilized oocytes | Swim up selected capacitated spermatozoa | C1orf56 (chromosome 1 open reading frame 56—SLeX-binding protein) | Sperm preincubated with anti-C1orf56 antibody (1 h) | Non-specific rabbit IgG and medium were used only as controls; linked receptor inhibition to AR; checked for the effects of the antibody on the viability, motility, and acrosomal status | Anti-C1orf56 reduced sperm binding to ZP and the AR | Wang et al., 2021 [72] |
| Human | Immature oocytes | Swim up selected capacitated spermatozoa | SPACA4 (Sperm Acrosome Associated 4) | Sperm preincubated with anti-SPACA4 antibody | Demonstrated functional inhibition by anti-SPACA4 antibody; checked for the effects of the Ab on the viability, motility, and acrosomal status | After the Ab pretreatment, sperm binding to hemizona was reduced by ~60% | Chen et al., 2023 [60] |
| Human | Unfertilized oocytes | Swim up selected motile sperm from fertile men | SP-10 (acrosomal protein) | Anti-SP-10 antibody applied | Used an antibody against intra-acrosomal protein | Anti-SP-10 had no effect on sperm–ZP binding | Hamatani et al., 2000 [73] |
| Human | Unclassified oocytes | Capacitated human sperm | ZRK (zona receptor kinase, 95 kDa protein) | Hemizonae preincubated with synthetic peptides corresponding to various regions of ZRK subsequently incubated with sperm (30 min) | Used competition peptides to evaluate functional receptor role; medium alone was used as a control | Synthetic peptides inhibited sperm binding | Burks et al., 1995 [11] |
| Human | Oocytes from women without any ovarian abnormality | Swim up selected motile sperm from fertile men | YLP12 (human sperm peptide sequence) | Either sperm or hemizona preincubated with YLP12 peptide or anti-YLP12 Fab’s (both 1 h) | Evaluated both sperm and ZP side interventions with synthetic peptides and Fab’s; checked if peptides or Fab’s caused agglutination of sperm or any apparent deleterious effect on motility; control Fabs from preimmune serum and control animals immunized with the tetanus toxoid (conjugated to peptides) alone used as controls | Both sperm and hemizona treatment with peptide/Fab significantly reduced binding; immunoadsorption of the anti-YLP12 Fabs with purified peptide completely abolished inhibitory activity | Naz et al., 2000 [74] |
| Human | Oocytes that failed to fertilize during IVF | Motile sperm; preincubated with FA-1 antigen, anti-FA-1 Fab’, control Fab’/BSA | FA-1 (fertilization antigen) | Either sperm or hemizona preincubated with FA-1 antigen/anti-FA-1 Fab’ fragments (1 h) | Tested both sperm and hemizona pretreatment; used BSA and control Fab’ for controls | Anti-FA-1 Fab’ blocked sperm–ZP binding; FA-1 antigen on hemizona blocked binding; controls had no effect | Kadam et al., 1995 [68] |
| Human | Unfertilized oocytes from the assisted reproduction program | Capacitated human sperm | FUT5 (α1,3/4-fucosyltransferase 5) | Hemizonae together with sperm coincubated with anti-FUT5 antibody or FUT acceptors (3 h) | Used a panel of FUT ligands and antibodies; examined specificity and reversibility of inhibition; antibody preabsorbed with 1:100 blocking peptide was used as control; ability of different concentrations of FUT acceptors to inhibit spermatozoa-zona pellucida binding was also studied; the effect of an anti-FUT antibody and FUT acceptors on sperm motility, viability, and acrosomal status was determined | Anti-FUT5 antibody inhibited the binding in a dose-dependent manner, and the effects were reversible with blocking peptide; some of the FUT acceptors inhibited sperm–ZP binding | Chiu et al., 2007 [69] |
| Human | Unfertilized or in vitro matured human oocytes | Swim-up selected capacitated sperm | CRISP1 (Cysteine-Rich Secretory Protein 1) | Sperm preincubated with anti-CRISP1 or recombinant human CRISP1 (rec-hCRISP1; 30 min); or hemizonae preincubated with rec-hCRISP1 | MBP and normal rabbit IgG were used as controls; confirmed receptor localization and specificity | Anti-hCRISP1 and rec-hCRISP1 inhibited sperm binding; rec-hCRISP1 specifically bound ZP | Maldera et al., 2014 [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelenkova, N.; Kraus, V.; Maresova, A.; Pilsova, Z.; Pilsova, A.; Klusackova, B.; Chmelikova, E.; Komrskova, K.; Postlerova, P. Functional Methods for Studying Sperm–Zona Pellucida Interaction in Mammals. Methods Protoc. 2025, 8, 95. https://doi.org/10.3390/mps8040095
Zelenkova N, Kraus V, Maresova A, Pilsova Z, Pilsova A, Klusackova B, Chmelikova E, Komrskova K, Postlerova P. Functional Methods for Studying Sperm–Zona Pellucida Interaction in Mammals. Methods and Protocols. 2025; 8(4):95. https://doi.org/10.3390/mps8040095
Chicago/Turabian StyleZelenkova, Natalie, Veronika Kraus, Alexandra Maresova, Zuzana Pilsova, Aneta Pilsova, Barbora Klusackova, Eva Chmelikova, Katerina Komrskova, and Pavla Postlerova. 2025. "Functional Methods for Studying Sperm–Zona Pellucida Interaction in Mammals" Methods and Protocols 8, no. 4: 95. https://doi.org/10.3390/mps8040095
APA StyleZelenkova, N., Kraus, V., Maresova, A., Pilsova, Z., Pilsova, A., Klusackova, B., Chmelikova, E., Komrskova, K., & Postlerova, P. (2025). Functional Methods for Studying Sperm–Zona Pellucida Interaction in Mammals. Methods and Protocols, 8(4), 95. https://doi.org/10.3390/mps8040095







