Development of an Ex Vivo Platform to Model Urethral Healing †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Model Creation
- Phosphate-buffered saline (PBS; Gibco, Waltham, MA, USA; ThermoFisher Cat. no.: 10010023).
- Penicillin–Streptomycin (Corning, Glendale, AZ, USA; Fisher Scientific Cat. no.: MT30001CI).
- Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Waltham, MA, USA; ThermoFisher Cat. no.: 11965118).
- Fetal Bovine Serum (FBS) (Corning, Glendale, AZ, USA; Fisher Scientific Cat. no.: MT35016CV).
2.2. Equipment for Model Creation
- Large sharp scissors, forceps, small sharp scissors, and a needle driver.
- Disposable centrifuge tubes with a 15mL flat cap (Fisherbrand, Waltham, MA, USA; Fisher Scientific Cat. no.: 07-200-886).
- An original Prusa i3 3D printer (Prusa Research, Prague, Czech Republic).
- Prusament PLA Pristine White, 1 kg (Prusa Research, Prague, Czech Republic).
- PDS II Suture, Size 4-0, SH, 27” (Ethicon, Cincinnati, OH, USA; Fisher Scientific Cat. no.: 50-118-0707).
- #15 protected disposable scalpel stainless steel blade (Bard-Parker, Caledonia, MI, USA; Fisher Scientific Cat. No.: 02-688-80).
2.3. Materials for Growth Factor Testing
- Prostate Epithelial Basal Medium (ATCC, Manassas, VA, USA; ATCC Cat. no.: PCS-440-030).
- Corneal Epithelial Cell Growth Kit (ATCC, Manassas, VA, USA; ATCC Cat. No.: PCS-700-040).
- Recombinant Human EGF (PreproTech, Cranbury, NJ, USA; ThermoFisher Cat. No.: AF-100-15-500UG).
- Recombinant Human FGF-basic (PreproTech, Cranbury, NJ, USA; ThermoFisher Cat. No.: AF-100-18B-50UG).
- Recombinant Human IGF-1 (PreproTech, Cranbury, NJ, USA; ThermoFisher Cat. No.: AF-100-11-500UG).
- Recombinant Human PDGF-BB (PreproTech, Cranbury, NJ, USA; ThermoFisher Cat. No.: AF-100-14B-10UG).
- Recombinant Human TGF- β1 (PreproTech, Cranbury, NJ, USA; ThermoFisher Cat. No.: AF-100-21C-10UG).
- Recombinant Human VEGF (PreproTech, Cranbury, NJ, USA; ATCC Scientific Cat. No.: AF-100-20-10UG).
- Calcein AM (Invitrogen, Waltham, MA, USA; ThermoFisher Cat. No.: C3100MP
- Ethidium Homodimer I (Biotium, Fremont, CA, USA; Fisher Scientific Cat. No. 50-196-4627).
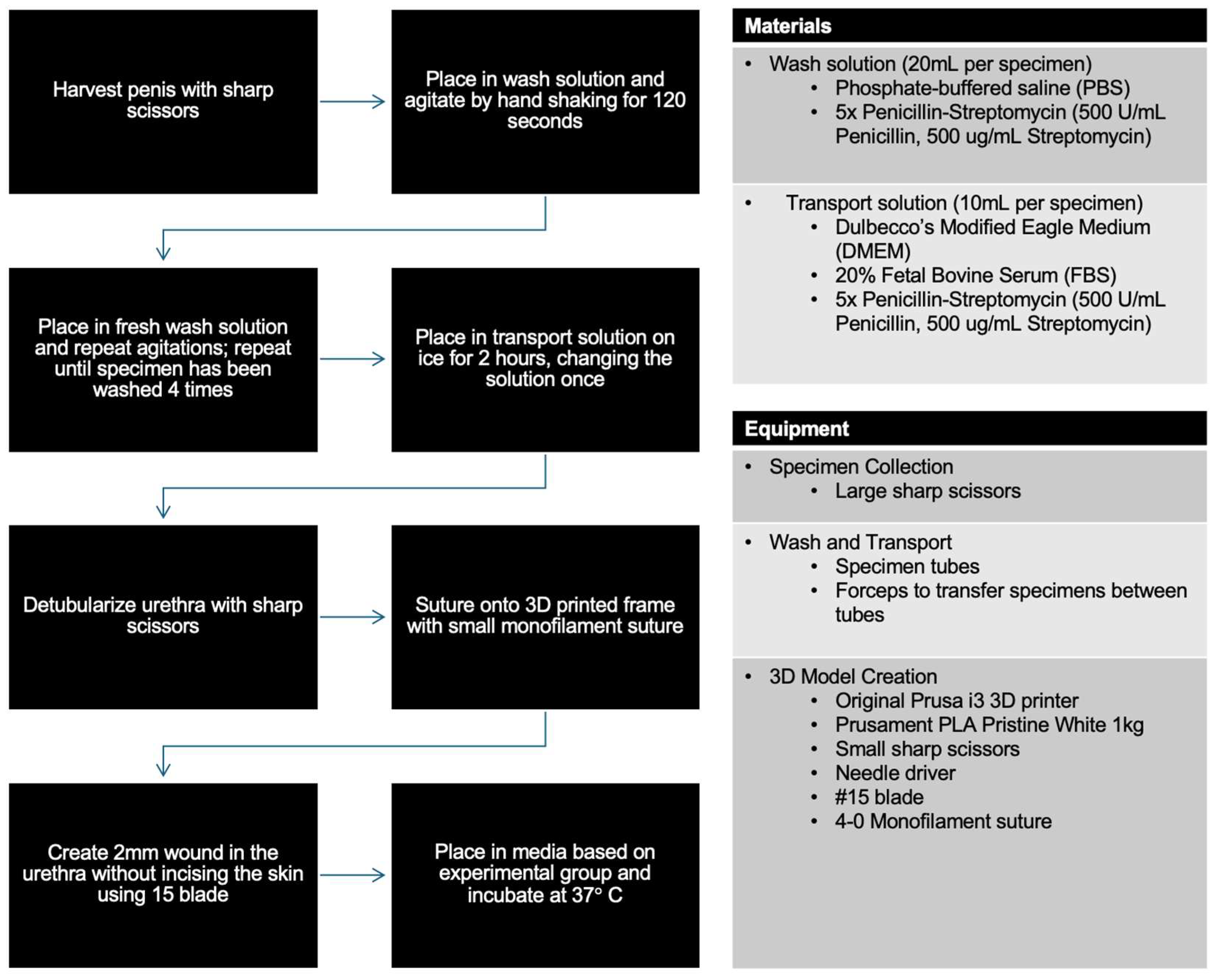
2.4. Specimen Collection
2.5. Wash and Transport
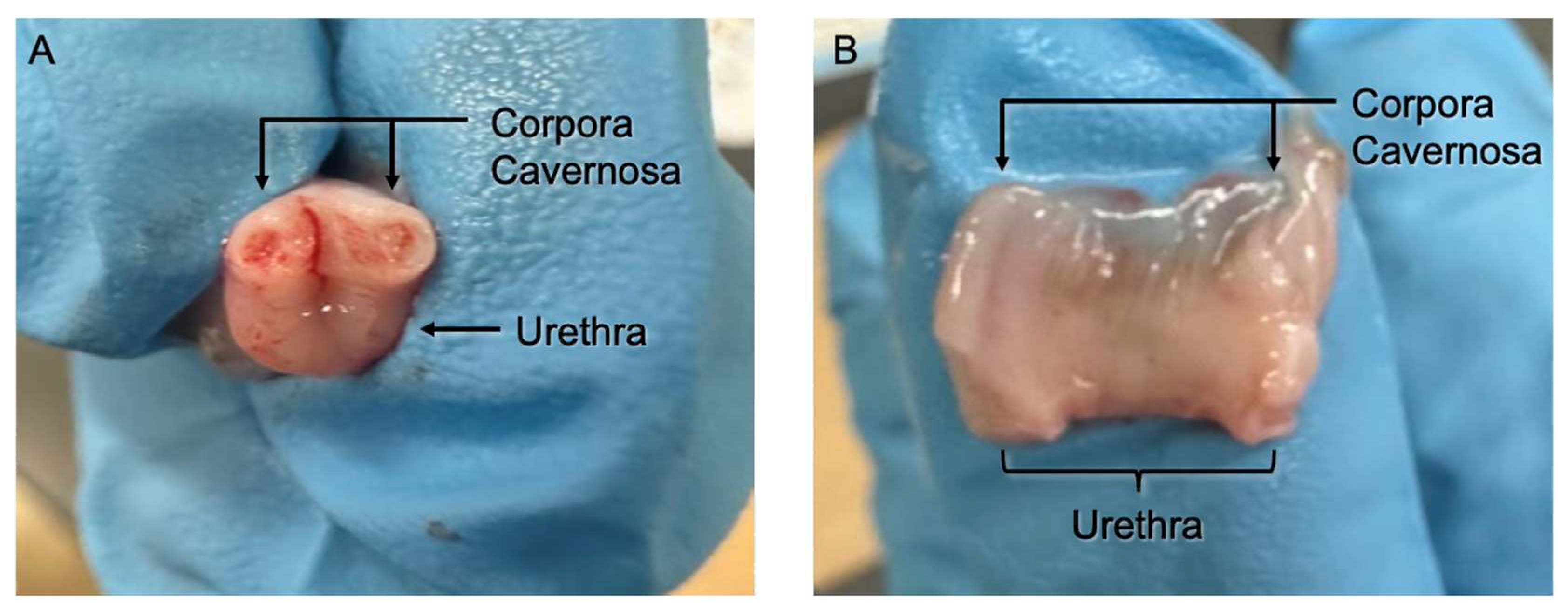
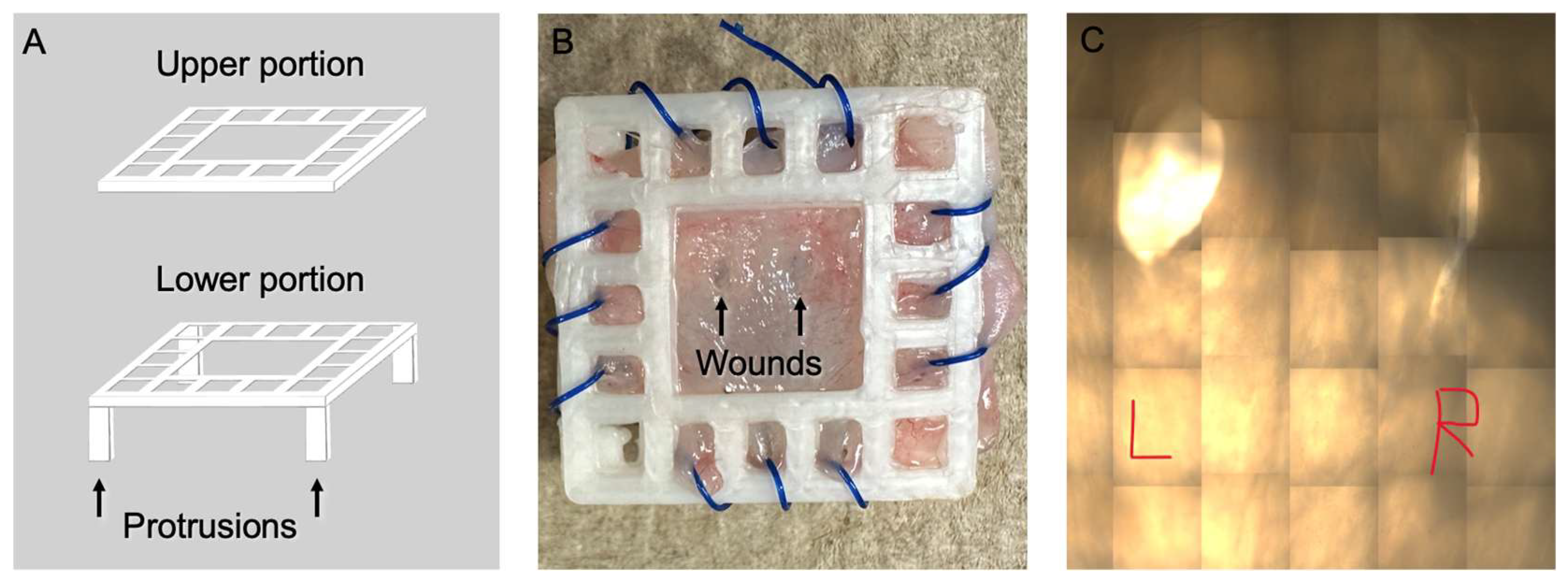
2.6. 3D Model and Wound Creation
2.7. Controls and Evaluated GFs
2.8. Imaging and Analysis
2.9. Cell Viability
3. Results
3.1. 3D Wound Model
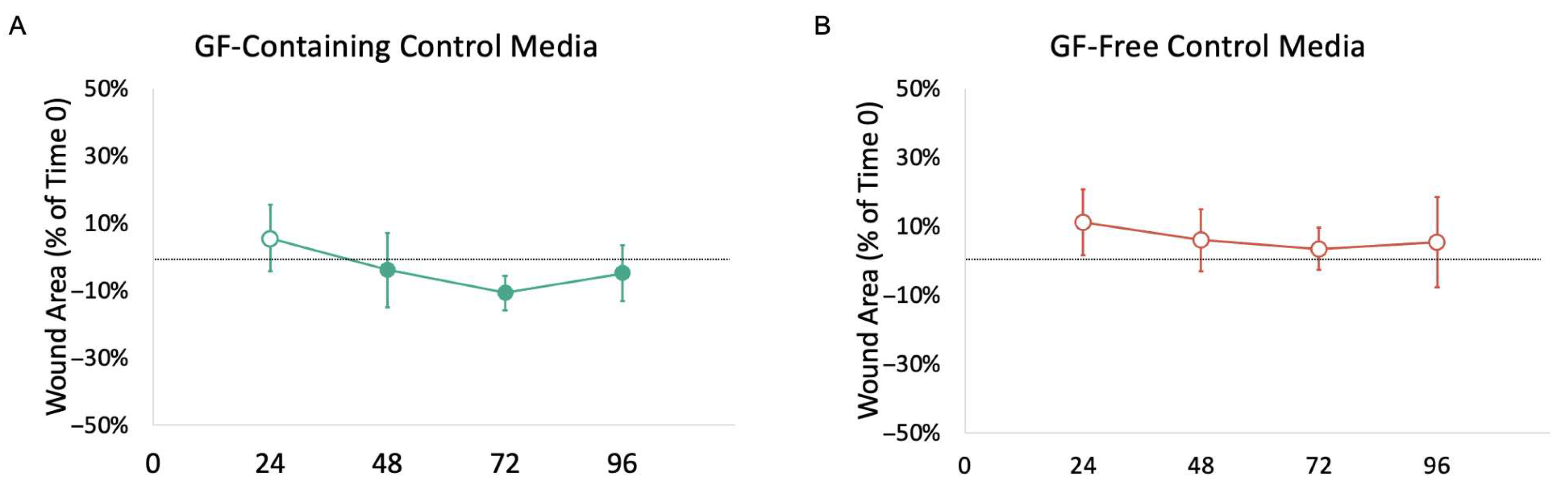
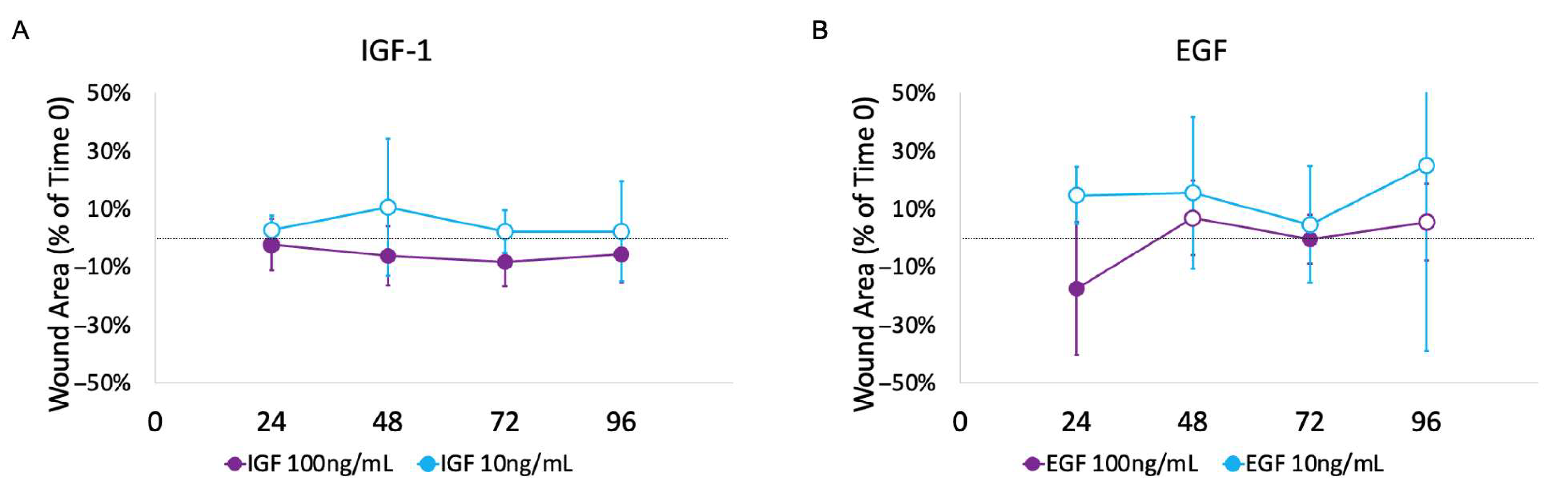
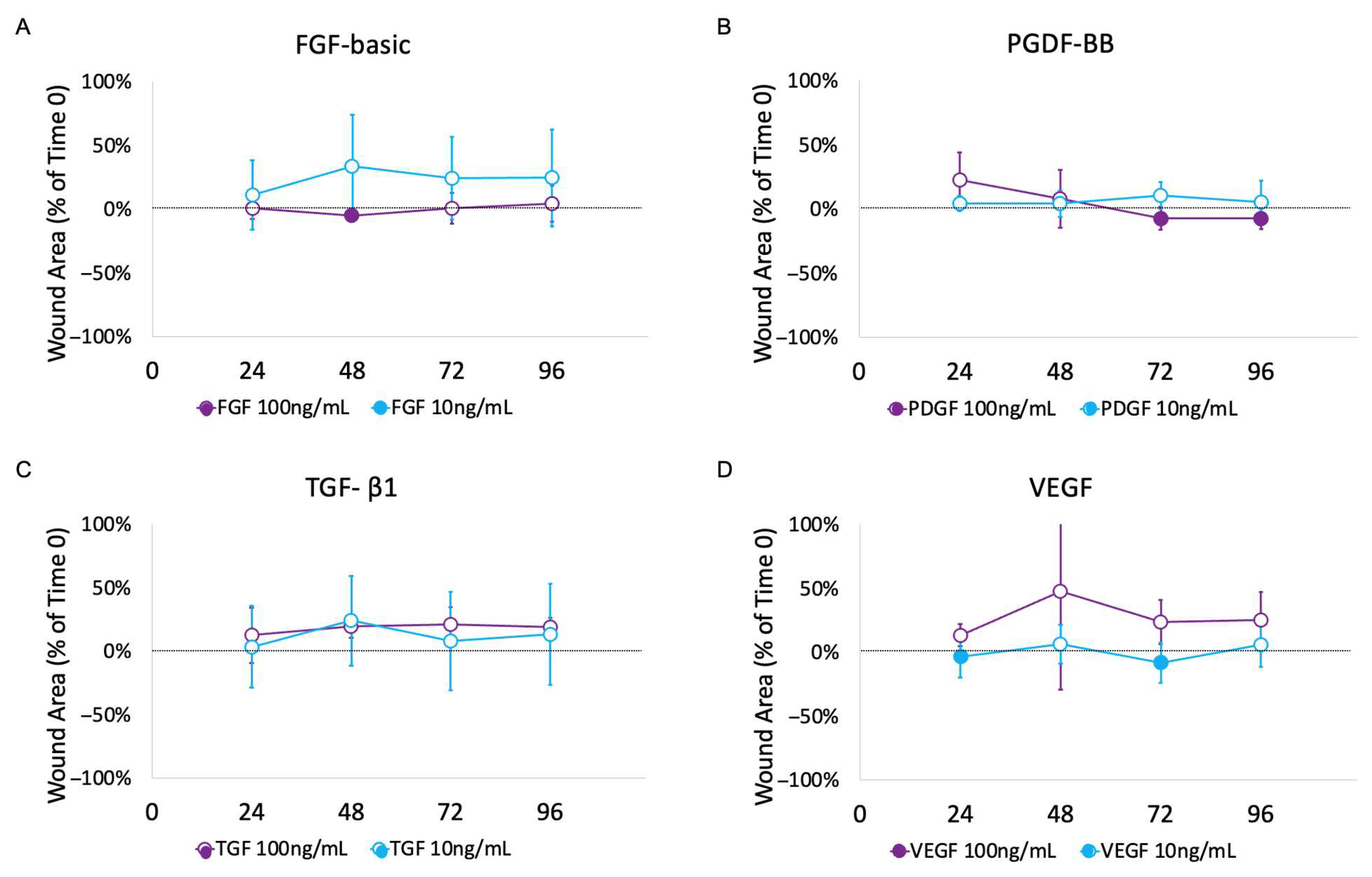
3.2. Impact of Growth Factors
3.3. Cell Viability
4. Discussion
4.1. Benefit of This Ex Vivo Model over Other In Vitro Urethra Models
4.2. Impact of GFs on Ex Vivo Urethral Model
4.3. Other Uses for the Described Model
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GF | Growth factor |
| EGF | Epidermal growth factor |
| FGF-basic | Fibroblast growth factor-basic |
| IGF-1 | Insulin-like growth factor-1 |
| PDGF | Platelet-derived growth factor |
| TGF-β1 | Transforming growth factor beta |
| VEGF | Vascular endothelial growth factor |
References
- Santucci, R.A.; Joyce, G.F.; Wise, M. Male urethral stricture disease. J. Urol. 2007, 177, 1667–1674. [Google Scholar] [CrossRef]
- Abdeen, B.M.; Leslie, S.W.; Badreldin, A.M. Urethral Strictures, in StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564297/ (accessed on 23 April 2025).
- Fenton, A.S.; Morey, A.F.; Aviles, R.; Garcia, C.R. Anterior urethral strictures: Etiology and characteristics. Urology 2005, 65, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Lumen, N.; Hoebeke, P.; Willemsen, P.; De Troyer, B.; Pieters, R.; Oosterlinck, W. Etiology of urethral stricture disease in the 21st century. J. Urol. 2009, 182, 983–987. [Google Scholar] [CrossRef]
- Lopes, J.F.; Schned, A.; Ellsworth, P.I.; Cendron, M. Histological analysis of urethral healing after tubularized incised plate urethroplasty. J. Urol. 2001, 166, 1014–1017. [Google Scholar] [CrossRef]
- Hafez, A.; Herz, D.; Bägli, D.; Smith, C.; McLorie, G.; Khoury, A. Healing of unstented tubularized incised plate urethroplasty: An experimental study in a rabbit model. BJU Int. 2003, 91, 84–88. [Google Scholar] [CrossRef]
- Hofer, M.D.; Cheng, E.Y.; Bury, M.I.; Park, E.; Xu, W.; Hong, S.J.; Kaplan, W.E.; Sharma, A.K. Analysis of Primary Urethral Wound Healing in the Rat. Urology 2014, 84, 246.e1–246.e7. [Google Scholar] [CrossRef]
- Sun, Z.-L.; Xia, S.-J.; Cao, Y.; Luo, G.-H.; Luo, L.; Yang, X.-S.; Hu, J.-X.; Shi, H.; Huang, P. Re-epithelialization resulted from prostate basal cells in canine prostatic urethra may represent the ideal healing method after two-micron laser resection of the prostate. Asian J. Androl. 2015, 17, 831–838. [Google Scholar] [CrossRef]
- Hyuga, T.; Fujimoto, K.; Hashimoto, D.; Tanabe, K.; Kubo, T.; Nakamura, S.; Ueda, Y.; Fujita-Jimbo, E.; Muramatsu, K.; Suzuki, K.; et al. Wound healing responses of urinary extravasation after urethral injury. Sci. Rep. 2023, 13, 10628. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Y.; Tian, Y.; Luo, G.; Yang, X.; Sun, Z. Urine can speed up the re-epithelialization process of prostatic urethra wounds by promoting the proliferation and migration of prostate epithelial cells. Int. Urol. Nephrol. 2019, 51, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nagele, U.; Maurer, S.; Feil, G.; Bock, C.; Krug, J.; Sievert, K.-D.; Stenzl, A. In vitro investigations of tissue-engineered multilayered urothelium established from bladder washings. Eur. Urol. 2008, 54, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Ou, L.; Hu, G.; Wang, H.; Xu, Y.; Chen, J.; Zhang, J.; Yu, Y.; Kong, D. Tissue Engineering of Urethra Using Human Vascular Endothelial Growth Factor Gene-Modified Bladder Urothelial Cells. Artif. Organs 2008, 32, 91–99. [Google Scholar] [CrossRef]
- El-Tabey, N.; Shokeir, A.; Barakat, N.; El-Refaie, H.; El-Hamid, M.A.; Gabr, M. Cell-seeded tubular acellular matrix for replacing a long circumferential urethral defect in a canine model: Is it clinically applicable? Arab. J. Urol. 2012, 10, 192–198. [Google Scholar] [CrossRef]
- Orabi, H.; AbouShwareb, T.; Zhang, Y.; Yoo, J.J.; Atala, A. Cell-Seeded Tubularized Scaffolds for Reconstruction of Long Urethral Defects: A Preclinical Study. Eur. Urol. 2013, 63, 531–538. [Google Scholar] [CrossRef]
- De Filippo, R.E.; Kornitzer, B.S.; Yoo, J.J.; Atala, A. Penile urethra replacement with autologous cell-seeded tubularized collagen matrices. J. Tissue Eng. Regen. Med. 2015, 9, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, A.; Li, J.; Pan, M.; Han, W.; Xiao, Y. Development of a cell-seeded modified small intestinal submucosa for urethroplasty. Heliyon 2016, 2, e00087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Foster, C.; Jensen, T.; Finck, C.; Rowe, C.K. Development of a Wound-Healing Protocol for In Vitro Evaluation of Urothelial Cell Growth. Methods Protoc. 2023, 6, 64. [Google Scholar] [CrossRef]
- Liang, F.-X.; Bosland, M.C.; Huang, H.; Romih, R.; Baptiste, S.; Deng, F.-M.; Wu, X.-R.; Shapiro, E.; Sun, T.-T. Cellular basis of urothelial squamous metaplasia: Roles of lineage heterogeneity and cell replacement. J. Cell Biol. 2005, 171, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, L.; Barry, S.M.; Hope, T.J.; Shattock, R.J. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS 2009, 23, 319–328. [Google Scholar] [CrossRef]
- Lalla, M.; Danielsen, C.C.; Austevoll, H.; Olsen, L.H.; Jørgensen, T.M. Biomechanical and Biochemical Assessment of Properties of the Anterior Urethra After Hypospadias Repair in a Rabbit Model. J. Urol. 2007, 177, 2375–2380. [Google Scholar] [CrossRef]
- Leslie, B.; Jesus, L.E.; El-Hout, Y.; Moore, K.; Farhat, W.A.; Bägli, D.J.; Lorenzo, A.J.; Salle, J.L.P. Comparative Histological and Functional Controlled Analysis of Tubularized Incised Plate Urethroplasty with and Without Dorsal Inlay Graft: A Preliminary Experimental Study in Rabbits. J. Urol. 2011, 186, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Faydacı, G.; Tarhan, F.; Tuncer, M.; Eryıldırım, B.; Celik, O.; Keser, S.H.; Özgül, A. Comparison of two experimental models for urethral stricture in the anterior urethra of the male rabbit. Urology 2012, 80, 225.e7–225.e11. [Google Scholar] [CrossRef]
- Hu, W.-F.; Li, C.-L.; Zhang, H.-P.; Li, T.-T.; Zeng, X.-Y. An Experimental Model of Urethral Stricture in Rabbits Using Holmium Laser under Urethroscopic Direct Visualization. Urol. Int. 2014, 93, 108–112. [Google Scholar] [CrossRef]
- Sawyer, K.N.; Cofield, S.S.; Selph, J.P. Race as a Predictor of Recurrence and Complications After Urethroplasty in Men With Urethral Stricture Disease. Urology 2022, 163, 69–75. [Google Scholar] [CrossRef]
- Tran, R.; Grover, K.; Foster, C.; Rowe, C.K. Development of an Explant Platform to Model Urothelial Healing. In Proceedings of the American Urological Association’s 2025 Annual Meeting, Las Vegas, NV, USA, 26–29 April 2025. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Skonieczna, J.; Madej, J.P.; Pawelska, A.K.; Będziński, R. Histological and morphometric evaluation of the urethra and penis in male New Zealand White rabbits. Anat. Histol. Embryol. 2021, 50, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, M.; Peng, X.; Wang, Y.; Chen, F. Assessing the potential regeneration ability of corpus spongiosum in rabbit models. Andrologia 2021, 53, e13901. [Google Scholar] [CrossRef]
- Muro, S.; Shoji, S.; Suriyut, J.; Akita, K. Anatomy of muscle connections in the male urethra and anorectal canal. BJU Int. 2024, 133, 752–759. [Google Scholar] [CrossRef]
- Mattiasson, A.; Andersson, K.-E.; Sjögren, C. Contractant and relaxant properties of the female rabbit urethral submucosa. J. Urol. 1985, 133, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, B.; van den Broek, L.J.; Bruisten, S.M.; Mullender, M.; de Vries, H.J.C.; Gibbs, S. An Organotypic Reconstructed Human Urethra to Study Chlamydia trachomatis Infection. Tissue Eng. Part A 2018, 24, 1663–1671. [Google Scholar] [CrossRef]
- Casademont-Roca, A.; Xing, Z.; Bernardi, M.; Rookmaker, M.; de Kort, L.; de Graaf, P. A novel vascularized urethra-on-a-chip model. Sci. Rep. 2025, 15, 8062. [Google Scholar] [CrossRef]
- Sun, T.-T. Altered phenotype of cultured urothelial and other stratified epithelial cells: Implications for wound healing. Am. J. Physiol. Physiol. 2006, 291, F9–F21. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Shinchi, M.; Kushibiki, T.; Mayumi, Y.; Ito, K.; Asano, T.; Ishihara, M.; Horiguchi, A. Insulin-like growth factor 1 sustained-release collagen on urethral catheter prevents stricture after urethral injury in a rabbit model. Int. J. Urol. 2019, 26, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, C.-S.; Spencerb, E.M.; Lue, T.F. Insulin-like growth factor-I promotes proliferation and migration of cavernous smooth muscle cells. Biochem. Biophys. Res. Commun. 2001, 280, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Howard, P.S.; Macarak, E.J. Recombinant insulin-like growth factor-1 activates satellite cells in the mouse urethral rhabdosphincter. BMC Urol. 2013, 13, 62. [Google Scholar] [CrossRef]
- Sumino, Y.; Yoshikawa, S.; Mimata, H.; Yoshimura, N. Therapeutic effects of IGF-1 on stress urinary incontinence in rats with simulated childbirth trauma. J. Urol. 2014, 191, 529–538. [Google Scholar] [CrossRef]
- Vinter-Jensen, L.; Nielsen, K. The effects of exogenous epidermal growth factor on the developing urinary tract in rats: A stereological description. Urol. Res. 1998, 26, 105–110. [Google Scholar] [CrossRef]
- Kallidonis, P.; Adamou, C.; Castillo, S.V.; Liourdi, D.; Liatsikos, E.; Lange, D. Drug-delivering devices in the urinary tract: A systematic review. Arab. J. Urol. 2021, 19, 191–204. [Google Scholar] [CrossRef]
- Rowe, C.K.; Jamdee, T.; Foster, C.; Burke, K.A. Do the materials matter? A review of the literature and analysis of the materials properties of urethral stents for hypospadias repair. J. Pediatr. Urol. 2022, 18, 160–167. [Google Scholar] [CrossRef]
- Humphreys, O.; Pickering, M.; O’CEarbhaill, E.D.; Flanagan, T.C. A biomimetic urethral model to evaluate urinary catheter lubricity and epithelial micro-trauma. J. Mech. Behav. Biomed. Mater. 2020, 108, 103792. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.N.; Carniel, E.L.; Frigo, A.; Pavan, P.G.; Todros, S.; Pachera, P.; Fontanella, C.G.; Rubini, A.; Cavicchioli, L.; Avital, Y.; et al. Experimental investigation of the biomechanics of urethral tissues and structures. Exp. Physiol. 2016, 101, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Ninan, N.; Thomas, S.; Grohens, Y. Wound healing in urology. Adv. Drug Deliv. Rev. 2015, 82–83, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Furness, P.D., 3rd; Maizels, M.; Han, S.W.; Cohn, R.A.; Cheng, E.Y. Elevated bladder urine concentration of transforming growth factor-beta1 correlates with upper urinary tract obstruction in children. J. Urol. 1999, 162 Pt 2, 1033–1036. [Google Scholar] [CrossRef]
- Ono, K.; Maeshima, A.; Nagayama, I.; Kubo, T.; Yagisawa, T.; Nagata, D. Urinary Epidermal Growth Factor Level as a Noninvasive Indicator of Tubular Repair in Patients with Acute Kidney Injury. Diagnostics 2024, 14, 947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foster, C.; Tran, R.; Grover, K.; Salama, A.; Rowe, C.K. Development of an Ex Vivo Platform to Model Urethral Healing. Methods Protoc. 2025, 8, 96. https://doi.org/10.3390/mps8040096
Foster C, Tran R, Grover K, Salama A, Rowe CK. Development of an Ex Vivo Platform to Model Urethral Healing. Methods and Protocols. 2025; 8(4):96. https://doi.org/10.3390/mps8040096
Chicago/Turabian StyleFoster, Christopher, Ryan Tran, Khushi Grover, Abdullah Salama, and Courtney K. Rowe. 2025. "Development of an Ex Vivo Platform to Model Urethral Healing" Methods and Protocols 8, no. 4: 96. https://doi.org/10.3390/mps8040096
APA StyleFoster, C., Tran, R., Grover, K., Salama, A., & Rowe, C. K. (2025). Development of an Ex Vivo Platform to Model Urethral Healing. Methods and Protocols, 8(4), 96. https://doi.org/10.3390/mps8040096





