Integrating Cartilage Explant Culture with Simulated Digestion and Hepatic Biotransformation Refines In Vitro Screening of Joint Care Nutraceuticals
Abstract
1. Introduction
2. Materials and Methods
2.1. Nutraceutical
2.2. Simulated Biological Extract
2.3. Explant Culture
2.4. Sample Analyses
2.5. Cell Viability
2.6. Nitric Oxide
2.7. PGE2
2.8. Media Glycosaminoglycan
2.9. Tissue Glycosaminoglycan
2.10. Glycosaminoglycan Retention Index
2.11. Statistical Analyses
3. Results
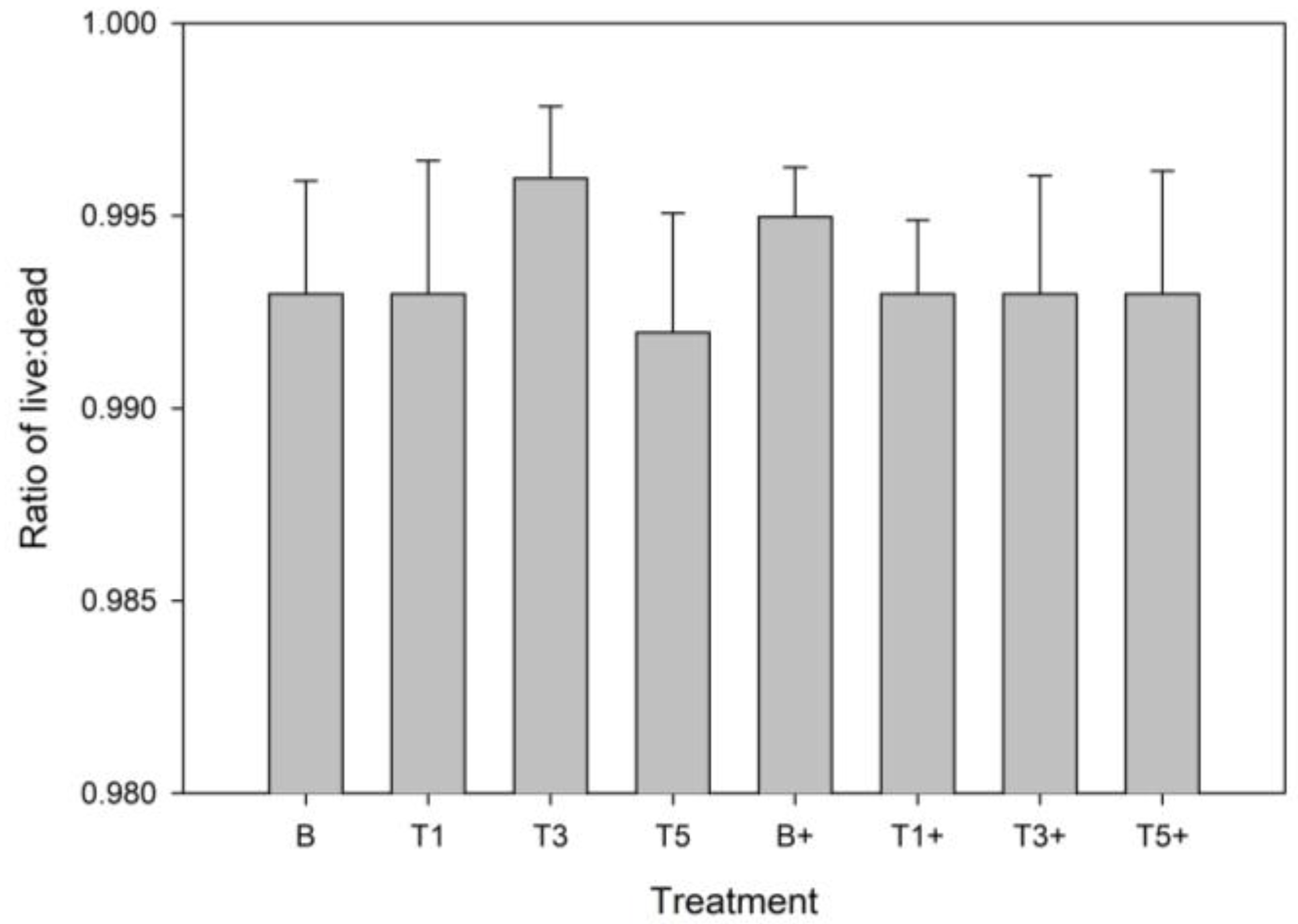
3.1. Cell Viability
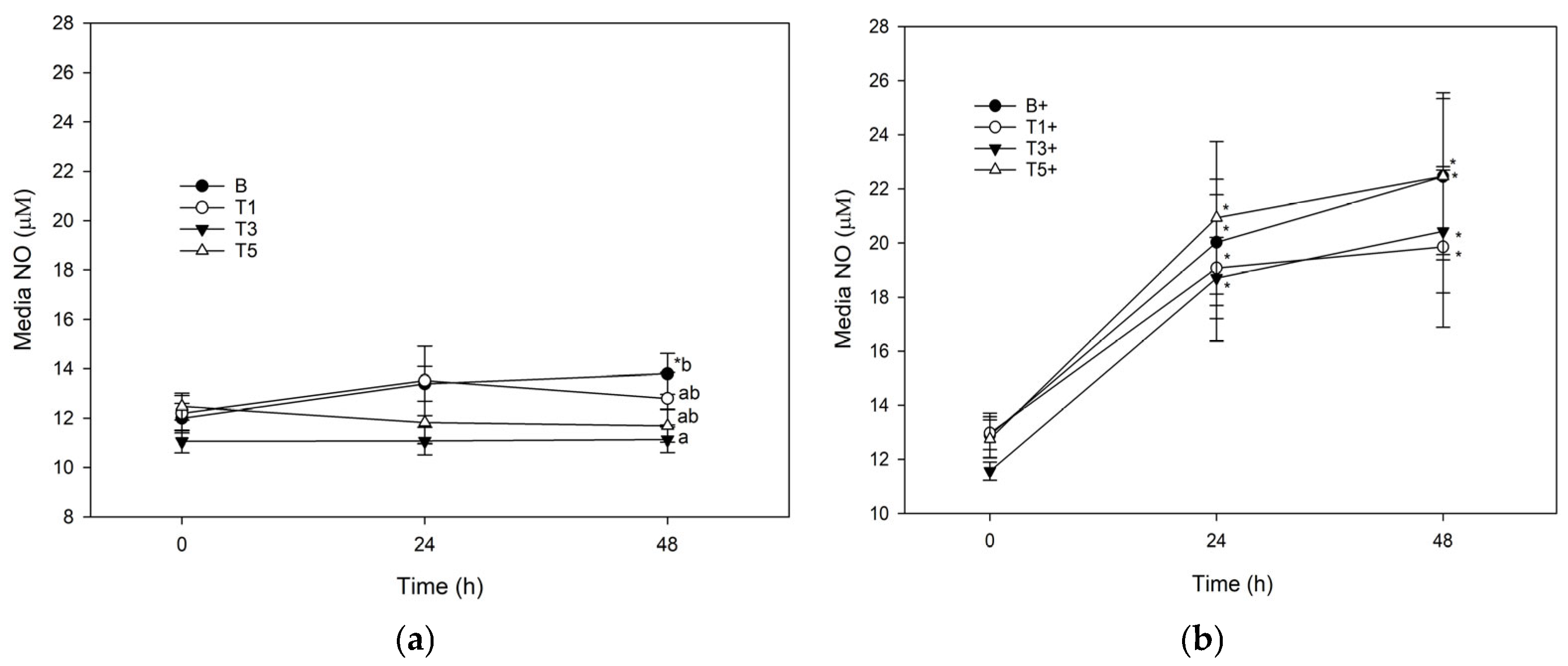
3.2. Nitric Oxide
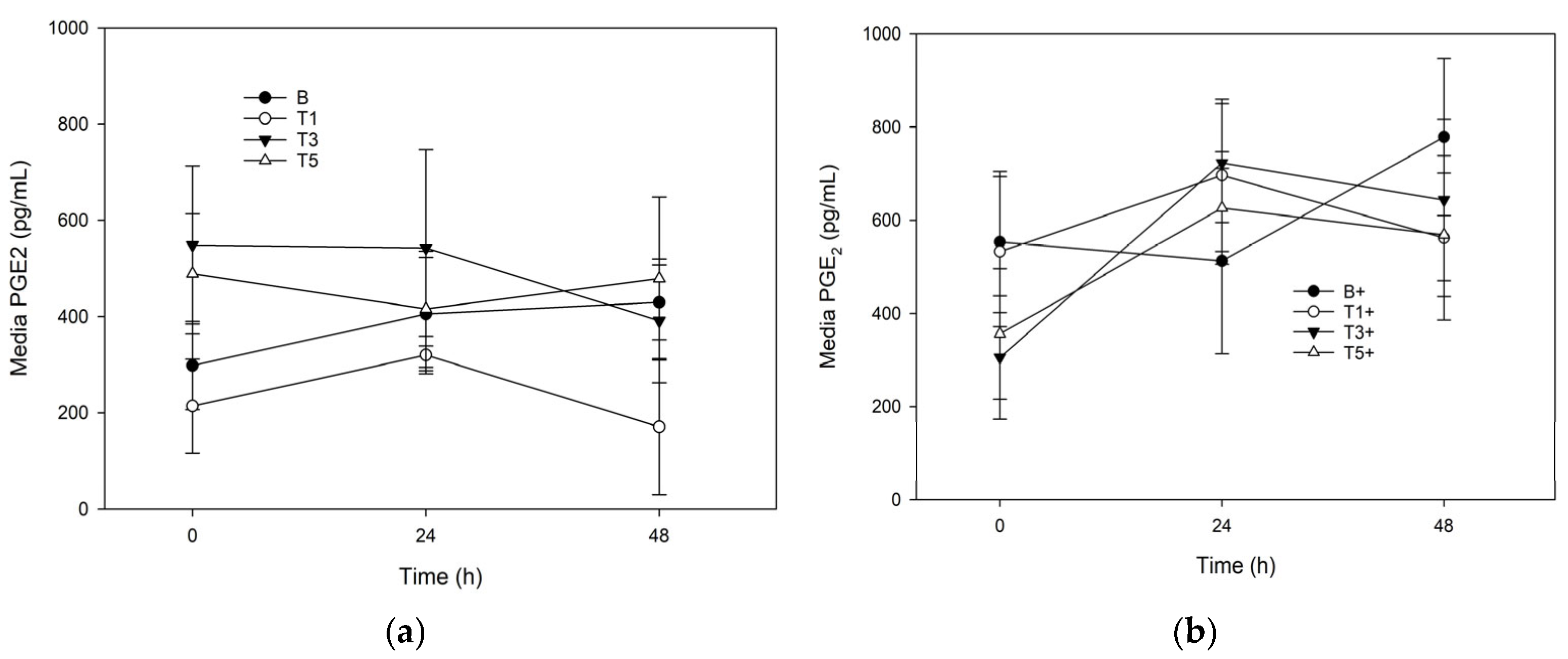
3.3. PGE2
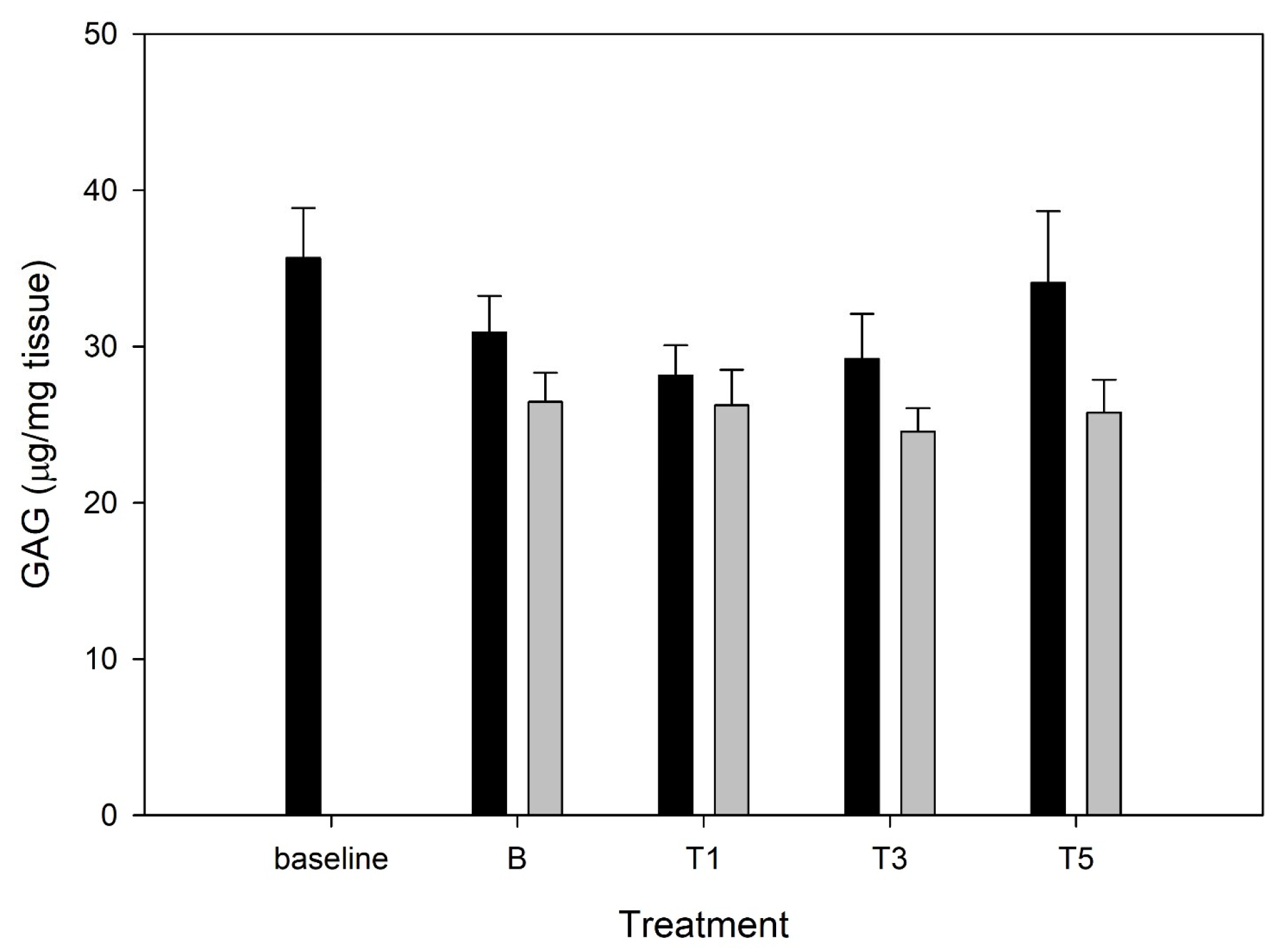
3.4. Media Glycosaminoglycan
3.5. Tissue Glycosaminoglycan
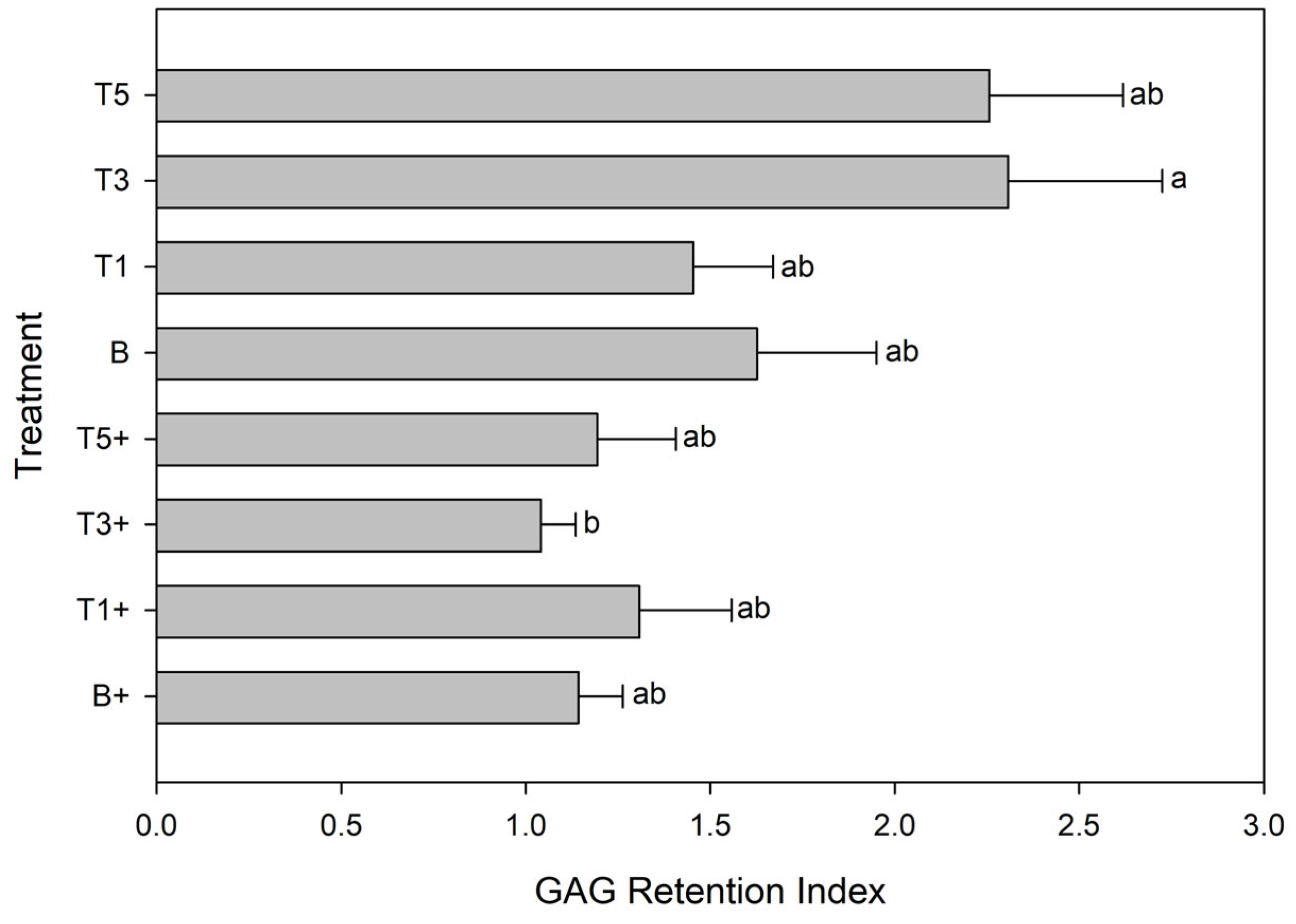
3.6. Glycosaminoglycan Rention Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C-AM | Calcein-AM |
| EthD-1 | Ethidium homodimer-1 |
| GAG | Glycosaminoglycan |
| GIT | Gastrointestinal tract |
| LPS | Lipopolysaccharide |
| NO | Nitric oxide |
| PGE2 | Prostaglandin E2 |
| TCM | Tissue culture media |
References
- Kawcak, C.E.; Frisbie, D.D.; Werpy, N.M.; Park, R.D.; McIlwraith, C.W. Effects of exercise vs experimental osteoarthritis on imaging outcomes. Osteoarthr. Cartil. 2008, 16, 1519–1525. [Google Scholar] [CrossRef]
- Bascoul-Colombo, C.; Garaiova, I.; Plummer, S.F.; Harwood, J.L.; Caterson, B.; Hughes, C.E. Glucosamine hydrochloride but not chondroitin sulfate prevents cartilage degradation and inflammation induced by interleukin-1α in bovine cartilage explants. Cartilage 2016, 7, 70–81. [Google Scholar] [CrossRef]
- de Mattei, M.; Pellati, A.; Pasello, M.; de Terlizzi, F.; Massari, L.; Gemmati, D.; Caruso, A. High doses of glucosamine-HCl have detrimental effects on bovine articular cartilage explants cultured in vitro. Osteoarthr. Cartil. 2002, 10, 816–825. [Google Scholar] [CrossRef]
- Mello, D.M.; Nielsen, B.D.; Peters, T.L.; Caron, J.P.; Orth, M.W. Comparison of inhibitory effects of glucosamine and mannosamine on bovine articular cartilage degradation in vitro. Am. J. Vet. Res. 2004, 65, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.I.; Chlebek-Brown, K.A.; Peters, T.L.; Caron, J.P.; Orth, M.W. Glucosamine HCl reduces equine articular cartilage degradation in explant culture. Osteoarthr. Cartil. 2000, 8, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Ortved, K.F.; Begum, L.; Mohammed, H.O.; Nixon, A.J. Glucosamine hydrochloride and chondroitin sulfate inhibit IL-1-induced gene expression and protein activity in cartilage explants. Osteoarthr. Cartil. 2005, 13, 894–902. [Google Scholar]
- Uitterlinden, E.J.; Jahr, H.; Koevoet, J.L.; Jenniskens, Y.M.; Bierma-Zeinstra, S.M.; Degroot, J.; Verhaar, J.A.; Weinans, H.; van Osch, G.J. Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthr. Cartil. 2006, 14, 250–257. [Google Scholar] [CrossRef]
- Gouze, J.N.; Bianchi, A.; Bécuwe, P.; Dauça, M.; Netter, P.; Magdalou, J.; Terlain, B.; Bordji, K. Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF-κB pathway. FEBS Lett. 2002, 510, 166–170. [Google Scholar] [CrossRef]
- Largo, R.; Alvarez-Soria, M.A.; Díez-Ortego, I.; Calvo, E.; Sánchez-Pernaute, O.; Egido, J.; Herrero-Beaumont, G. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 2003, 11, 290–298. [Google Scholar] [CrossRef]
- Varghese, S.; Theprungsirikul, P.; Sahani, S.; Hwang, N.; Yarema, K.J.; Elisseeff, J.H. Glucosamine modulates chondrocyte proliferation, matrix synthesis, and gene expression. Osteoarthr. Cartil. 2007, 15, 59–68. [Google Scholar] [CrossRef]
- Piperno, M.; Reboul, P.; Hellio Le Graverand, M.P.; Peschard, M.J.; Annefeld, M.; Richard, M.; Vignon, E. Glucosamine sulfate modulates dysregulated activities of human osteoarthritic chondrocytes in vitro. Osteoarthr. Cartil. 2000, 8, 207–212. [Google Scholar] [CrossRef]
- Gulihar, A.; Shaunak, S.; Novak, P.L.; Vinayakam, P.; Dhinsa, B.; Taylor, G. Glucosamine reduces the inhibition of proteoglycan metabolism caused by local anaesthetic solution in human articular cartilage: An in vitro study. J. Exp. Orthop. 2017, 4, 37. [Google Scholar] [CrossRef]
- Phitak, T.; Pothacharoen, P.; Kongtawelert, P. Comparison of glucose derivatives effects on cartilage degradation. BMC Musculoskelet. Disord. 2010, 11, 162. [Google Scholar] [CrossRef]
- McCulloch, D.R.; Wylie, J.D.; Longpre, J.M.; Leduc, R.; Apte, S.S. 10mM glucosamine prevents activation of proADAMTS5 (aggrecanase-2) in transfected cells by interference with post-translational modification of furin. Osteoarthr. Cartil. 2010, 18, 455–463. [Google Scholar] [CrossRef][Green Version]
- Sumantran, V.N.; Chandwaskar, R.; Joshi, A.K.; Boddul, S.; Patwardhan, B.; Chopra, A.; Wagh, U.V. The relationship between chondroprotective and antiinflammatory effects of Withania somnifera root and glucosamine sulphate on human osteoarthritic cartilage in vitro. Phytother. Res. 2008, 22, 1342–1348. [Google Scholar] [CrossRef]
- Chan, P.S.; Caron, J.P.; Orth, M.W. Effects of glucosamine and chondroitin sulfate on bovine cartilage explants under long-term culture conditions. Am. J. Vet. Res. 2007, 68, 709–715. [Google Scholar] [CrossRef]
- Chan, P.S.; Caron, J.P.; Orth, M.W. Short-term gene expression changes in cartilage explants stimulated with interleukin beta plus glucosamine and chondroitin sulfate. J. Rheumatol. 2006, 33, 1329–1340. [Google Scholar]
- Garland, A.; Wierenga, C.; McCrae, P.; Pearson, W. Cartilage-Sparing Properties of Equine Omega Complete in an Organ Culture Model of Cartilage Inflammation. J. Equine Vet. Sci. 2023, 121, 104165. [Google Scholar] [CrossRef]
- Pearson, W.; Kott, L.S. A biological extract of turmeric (Curcuma longa) modulates response of cartilage explants to lipopolysaccharide. BMC Complement. Altern. Med. 2019, 19, 252. [Google Scholar] [CrossRef]
- Pearson, W.; Fletcher, R.S.; Kott, L.S.; Hurtig, M.B. Protection against LPS-induced cartilage inflammation and degradation provided by a biological extract of Mentha spicata. BMC Complement. Altern. Med. 2010, 10, 19. [Google Scholar] [CrossRef]
- Forro, M.; Cieslar, S.; Ecker, G.L.; Walzak, A.; Hahn, J.; Lindinger, M.I. Total Body Water and ECFV Measured Using Bioelectrical Impedance Analysis and Indicator Dilution in Horses. J. Appl. Physiol. 2000, 89, 663–671. [Google Scholar] [CrossRef]
- Chan, P.S.; Caron, J.P.; Rosa, G.J.; Orth, M.W. Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E(2) in articular cartilage explants. Osteoarthr. Cartil. 2005, 13, 387–394. [Google Scholar] [CrossRef]
- Pearson, W. In Vitro and In Vivo Methods to Evaluate Putative Anti-Inflammatory Nutraceuticals. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, December 2007. [Google Scholar]
- Jiang, H.; Ji, P.; Shang, X.; Zhou, Y. Connection between Osteoarthritis and Nitric Oxide: From Pathophysiology to Therapeutic Target. Molecules 2023, 28, 1683. [Google Scholar] [CrossRef]
- Abramson, S.B. Nitric Oxide in Inflammation and Pain Associated with Osteoarthritis. Arthritis Res. Ther. 2008, 10, S2. [Google Scholar] [CrossRef]
- Buca, B.R.; Mititelu-Tartau, L.; Lupusoru, R.V.; Popa, G.E.; Rezus, C.; Lupusoru, C.E. New Nitric Oxide Donors with Therapeutic Potential. Med.-Surg. J. 2016, 120, 942–946. [Google Scholar]
- Meininger, C.J.; Kelly, K.A.; Li, H.; Haynes, T.E.; Wu, G. Glucosamine Inhibits Inducible Nitric Oxide Synthesis. Biochem. Biophys. Res. Commun. 2000, 279, 234–239. [Google Scholar] [CrossRef]
- Fenton, J.I.; Chlebek-Brown, K.A.; Caron, J.P.; Orth, M.W. Effect of glucosamine on interleukin-1-conditioned articular cartilage. Equine Vet. J. Suppl. 2002, 34, 219–223. [Google Scholar] [CrossRef]
- Frisbie, D.D.; McIlwraith, C.W.; Kawcak, C.E.; Werpy, N.M. Efficacy of intravenous administration of hyaluronan, sodium chondroitin sulfate, and N-acetyl-d-glucosamine for prevention or treatment of osteoarthritis in horses. Am. J. Vet. Res. 2016, 77, 1064–1070. [Google Scholar] [CrossRef]
- Koenig, T.J.; Dart, A.J.; McIlwraith, C.W.; Horadagoda, N.; Bell, R.J.; Perkins, N.; Dart, C.; Krockenberger, M.; Jeffcott, L.B.; Little, C.B. Treatment of experimentally induced osteoarthritis in horses using an intravenous combination of sodium pentosan polysulfate, N-acetyl glucosamine, and sodium hyaluronan. Vet. Surg. 2014, 43, 612–622. [Google Scholar] [CrossRef]
- Meulyzer, M.; Vachon, P.; Beaudry, F.; Vinardell, T.; Richard, H.; Beauchamp, G.; Laverty, S. Comparison of pharmacokinetics of glucosamine and synovial fluid levels following administration of glucosamine sulphate or glucosamine hydrochloride. Osteoarthr. Cartil. 2008, 16, 973–979. [Google Scholar] [CrossRef]
- Laverty, S.; Sandy, J.D.; Celeste, C.; Vachon, P.; Marier, J.F.; Plaas, A.H. Synovial fluid levels and serum pharmacokinetics in a large animal model following treatment with oral glucosamine at clinically relevant doses. Arthritis Rheum. 2005, 52, 181–191. [Google Scholar] [CrossRef]
- Navarro, S.L.; Levy, L.; Curtis, K.R.; Lampe, J.W.; Hullar, M.A.J. Modulation of Gut Microbiota by Glucosamine and Chondroitin in a Randomized, Double-Blind Pilot Trial in Humans. Microorganisms 2019, 7, 610. [Google Scholar] [CrossRef]
- Kapoor, M.; Mineau, F.; Fahmi, H.; Pelletier, J.P.; Martel-Pelletier, J. Glucosamine sulfate reduces prostaglandin E(2) production in osteoarthritic chondrocytes through inhibition of microsomal PGE synthase-1. J. Rheumatol. 2012, 39, 635–644. [Google Scholar] [CrossRef]
- Frondoza, C.G.; Heinecke, L.F.; Grzanna, M.W.; Au, A.Y.; Ownby, S.L. Modulation of cytokine-induced prostaglandin E2 production in cultures of articular chondrocytes obtained from carpal joints of camels (Camelus dromedarius). Am. J. Vet. Res. 2011, 72, 51–58. [Google Scholar] [CrossRef]
- Byron, C.R.; Stewart, M.C.; Stewart, A.A.; Pondenis, H.C. Effects of clinically relevant concentrations of glucosamine on equine chondrocytes and synoviocytes in vitro. Am. J. Vet. Res. 2008, 69, 1129–1134. [Google Scholar] [CrossRef]
- Walsh, A.J.; O’neill, C.W.; Lotz, J.C. Glucosamine HCl alters production of inflammatory mediators by rat intervertebral disc cells in vitro. Spine J. 2007, 7, 601–608. [Google Scholar] [CrossRef]
- Clegg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham, C.O., 3rd; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef]
- Wandel, S.; Jüni, P.; Tendal, B.; Nüesch, E.; Villiger, P.M.; Welton, N.J.; Reichenbach, S.; Trelle, S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: Network meta-analysis. BMJ 2010, 341, c4675. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care. Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crosbie, M.; Vanderboom, K.; Souccar-Young, J.; Pearson, W. Integrating Cartilage Explant Culture with Simulated Digestion and Hepatic Biotransformation Refines In Vitro Screening of Joint Care Nutraceuticals. Methods Protoc. 2025, 8, 91. https://doi.org/10.3390/mps8040091
Crosbie M, Vanderboom K, Souccar-Young J, Pearson W. Integrating Cartilage Explant Culture with Simulated Digestion and Hepatic Biotransformation Refines In Vitro Screening of Joint Care Nutraceuticals. Methods and Protocols. 2025; 8(4):91. https://doi.org/10.3390/mps8040091
Chicago/Turabian StyleCrosbie, Michelina, Kailey Vanderboom, Jamie Souccar-Young, and Wendy Pearson. 2025. "Integrating Cartilage Explant Culture with Simulated Digestion and Hepatic Biotransformation Refines In Vitro Screening of Joint Care Nutraceuticals" Methods and Protocols 8, no. 4: 91. https://doi.org/10.3390/mps8040091
APA StyleCrosbie, M., Vanderboom, K., Souccar-Young, J., & Pearson, W. (2025). Integrating Cartilage Explant Culture with Simulated Digestion and Hepatic Biotransformation Refines In Vitro Screening of Joint Care Nutraceuticals. Methods and Protocols, 8(4), 91. https://doi.org/10.3390/mps8040091





