Comparative Analysis of a Rapid Quantitative Immunoassay to the Reference Methodology for the Measurement of Blood Vitamin D Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Volunteers
2.2. Capillary Blood Vitamin D Test—Rapi-D and IgLoo Reader
2.3. Dried Blood Spot Vitamin D Testing—Laboratory Reference Method
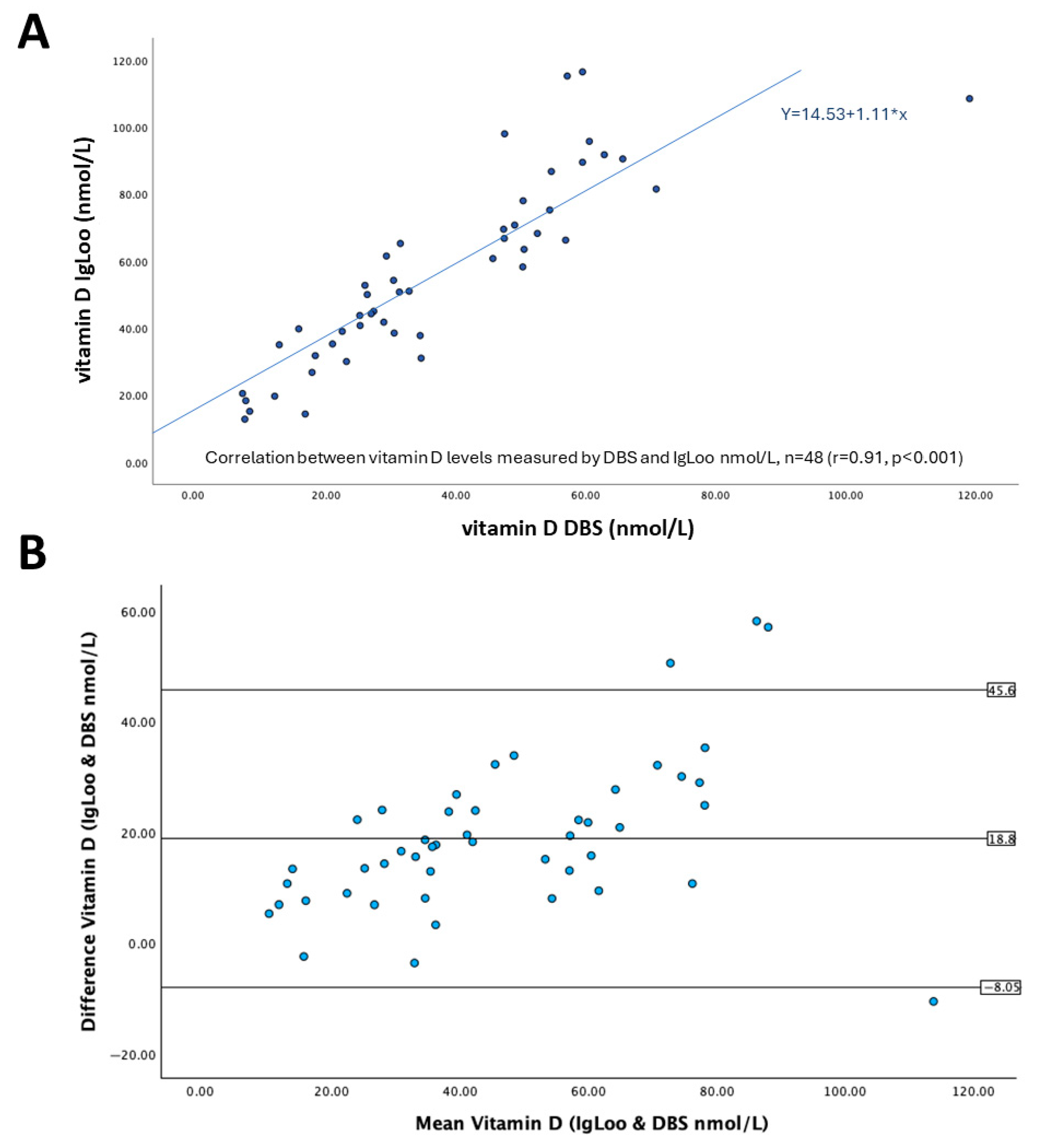
2.4. Data Analysis and Statistics
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLuca, H.F. Vitamin D: Historical Overview. Vitam. Horm. 2016, 100, 1–20. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D and Its Potential Benefit for the COVID-19 Pandemic. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2021, 27, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Voltan, G.; Cannito, M.; Ferrarese, M.; Ceccato, F.; Camozzi, V. Vitamin D: An Overview of Gene Regulation, Ranging from Metabolism to Genomic Effects. Genes 2023, 14, 1691. [Google Scholar] [CrossRef]
- Schmidt-Gayk, H.; Bouillon, R.; Roth, H.J. Measurement of vitamin D and its metabolites (calcidiol and calcitriol) and their clinical significance. Scand. J. Clin. Lab. Investig. Suppl. 1997, 227, 35–45. [Google Scholar] [CrossRef]
- Holick, M.F.; Smith, E.; Pincus, S. Skin as the site of vitamin D synthesis and target tissue for 1,25-dihydroxyvitamin D3. Use of calcitriol (1,25-dihydroxyvitamin D3) for treatment of psoriasis. Arch. Dermatol. 1987, 123, 1677–1683a. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Lin, L.Y.; Smeeth, L.; Langan, S.; Warren-Gash, C. Distribution of vitamin D status in the UK: A cross-sectional analysis of UK Biobank. BMJ Open 2021, 11, e038503. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Xiao, P.; Ma, Y.; Fan, Z.; Zhou, F.; Zheng, J.; Zhang, L. Prevalence, trend, and predictor analyses of vitamin D deficiency in the US population, 2001–2018. Front. Nutr. 2022, 9, 965376. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Brannon, P.M.; Rosen, C.J.; Taylor, C.L. Vitamin D Deficiency—Is There Really a Pandemic? N. Engl. J. Med. 2016, 375, 1817–1820. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Auluck, J.; Ng, L.L.; Jones, D.J. Improved analysis of vitamin D metabolites in plasma using liquid chromatography tandem mass spectrometry, and its application to cardiovascular research. Biomed. Chromatogr. BMC 2014, 28, 913–917. [Google Scholar] [CrossRef]
- Zhan, Z.; Quan, F.; Zhao, N.; Mai, L.; Li, Z.; Li, Y.; Sun, T.; Zeng, X. Evaluating vitamin D status in Chinese pre-school children using dried blood spots coupled with liquid chromatography-tandem mass spectrometry. J. Paediatr. Child Health 2025, 61, 20–25. [Google Scholar] [CrossRef]
- Altieri, B.; Cavalier, E.; Bhattoa, H.P.; Pérez-López, F.R.; López-Baena, M.T.; Pérez-Roncero, G.R.; Chedraui, P.; Annweiler, C.; Della Casa, S.; Zelzer, S.; et al. Vitamin D testing: Advantages and limits of the current assays. Eur. J. Clin. Nutr. 2020, 74, 231–247. [Google Scholar] [CrossRef]
- Tseneva, K.; Perić Kačarević, Ž. Challenges in vitamin D measurement and its role on bone regeneration. Int. J. Dent. Biomater. Res. 2023, 1, 36–45. [Google Scholar] [CrossRef]
- Ford, L.; Graham, V.; Wall, A.; Berg, J. Vitamin D concentrations in an UK inner-city multicultural outpatient population. Ann. Clin. Biochem. 2006, 43, 468–473. [Google Scholar] [CrossRef]
- van den Ouweland, J.M.; Beijers, A.M.; Demacker, P.N.; van Daal, H. Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandem-mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 1163–1168. [Google Scholar] [CrossRef]
- Enko, D.; Fridrich, L.; Rezanka, E.; Stolba, R.; Ernst, J.; Wendler, I.; Fabian, D.; Hauptlorenz, S.; Halwachs-Baumann, G. 25-hydroxy-Vitamin D status: Limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin. Lab. 2014, 60, 1541–1550. [Google Scholar] [CrossRef]
- Chen, X.; Sun, S.; Liu, Q.; Ren, F.; Bai, Z.; Wang, C. A Rapid Chemiluminescence Immunoassay for Total Vitamin D Status Assessment in Fingertip Blood. Clin. Lab. 2020, 66, 1479. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, K.; Lotz, J.; Frommer, L.; Lackner, K.J.; Kahaly, G.J. A rapid point-of-care assay accurately measures vitamin D. J. Endocrinol. Investig. 2021, 44, 2485–2492. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Wang, F.; Cui, Y.; Li, Z.; Lin, J. Innovative strategies for enhancing AuNP-based point-of-care diagnostics: Focus on coronavirus detection. Talanta 2025, 285, 127362. [Google Scholar] [CrossRef] [PubMed]
- Park, J. Lateral Flow Immunoassay Reader Technologies for Quantitative Point-of-Care Testing. Sensors 2022, 22, 7398. [Google Scholar] [CrossRef]
- Park, J. Smartphone based lateral flow immunoassay quantifications. J. Immunol. Methods 2024, 533, 113745. [Google Scholar] [CrossRef]
- Pieri, M.; Nicolai, E.; Nuccetelli, M.; Sarubbi, S.; Tomassetti, F.; Pelagalli, M.; Minieri, M.; Terrinoni, A.; Bernardini, S. Validation of a quantitative lateral flow immunoassay (LFIA)-based point-of-care (POC) rapid test for SARS-CoV-2 neutralizing antibodies. Arch. Virol. 2022, 167, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.A.; Cavalier, É.; Lukas, P.; Peeters, S.; Le Goff, C.; Briggs, L.E.; Williams, E.L.; Mineva, E.; Pfeiffer, C.M.; Vesper, H.; et al. Commutability assessment of new standard reference materials (SRMs) for determining serum total 25-hydroxyvitamin D using ligand binding and liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays. Anal. Bioanal. Chem. 2025, 417, 2539–2561. [Google Scholar] [CrossRef] [PubMed]

| Total Number of Participants, n | 48 |
|---|---|
| Males (%) | 21 (44) |
| Females (%) | 27 (56) |
| Mean age in years (SD) | 34.2 (8.99) |
| Vitamin D supplementation: number of volunteers that report taking dietary supplements within the last month prior to sampling (%) | 24 (48) |
| Ethnicity, self-reported, n (%) | Asian 19 (40) |
| Black 12 (25) | |
| White 13 (27) | |
| Hispanic 1 (2) | |
| Arab 1 (2) | |
| Mixed 2 (4) | |
| Mean body mass index kg/m2 (SD) | 24.38 (4.57) |
| Mean vitamin D status nmol/L * | |
| Rapid test with IgLoo reader (SD) | 56.2 (27.4) |
| DBS and LC/MS-MS (SD) | 37.4 (21.4) |
| Subject ID | IgLoo Vitamin D Levels from Reader (ng/mL) | IgLoo Vitamin D Levels (nmol/L) | IgLoo Vitamin D Levels Interpretation a | DBS Vitamin D Levels (nmol/L) | DBS Vitamin D levels Interpretation a |
|---|---|---|---|---|---|
| 0001 | 12.4 | 31 | Insufficient | 34.65 | Insufficient |
| 0002 | 36.2 | 90.5 | Sufficient | 65.7 | Sufficient |
| 0003 | 7.86 | 19.65 | Deficient | 12.1 | Deficient |
| 0004 | 17.5 | 43.75 | Insufficient | 25.2 | Insufficient |
| 0005 | 20.3 | 50.75 | Sufficient | 31.3 | Insufficient |
| 0006 | 7.32 | 18.3 | Deficient | 7.65 | Deficient |
| 0007 | 24.3 | 60.75 | Sufficient | 45.7 | Insufficient |
| 0008 | 39.2 | 98 | Sufficient | 47.5 | Insufficient |
| 0009 | 35.8 | 89.5 | Sufficient | 59.5 | Sufficient |
| 0010 | 16.3 | 40.75 | Insufficient | 25.25 | Insufficient |
| 0011 | 18 | 45 | Insufficient | 27.35 | Insufficient |
| 0012 | 26.7 | 66.75 | Sufficient | 47.45 | Insufficient |
| 0013 | 8.18 | 20.45 | Deficient | 7.15 | Deficient |
| 0014 | 31.2 | 78 | Sufficient | 50.35 | Sufficient |
| 0015 | 25.4 | 63.5 | Sufficient | 50.5 | Sufficient |
| 0016 | 36.7 | 91.75 | Sufficient | 62.85 | Sufficient |
| 0017 | 5.73 | 14.325 | Deficient | 16.8 | Deficient |
| 0018 | 10.7 | 26.75 | Insufficient | 17.85 | Deficient |
| 0019 | 21.7 | 54.25 | Sufficient | 30.4 | Insufficient |
| 0020 | 15.1 | 37.75 | Insufficient | 34.5 | Insufficient |
| 0021 | 23.3 | 58.25 | Sufficient | 50.3 | Sufficient |
| 0022 | 32.6 | 81.5 | Sufficient | 70.85 | Sufficient |
| 0023 | 21.1 | 52.75 | Sufficient | 26 | Insufficient |
| 0024 | 5.11 | 12.775 | Deficient | 7.5 | Deficient |
| 0025 | 14.1 | 35.25 | Insufficient | 21 | Deficient |
| 0026 | 15.4 | 38.5 | Insufficient | 30.5 | Insufficient |
| 0027 | 12 | 30 | Insufficient | 23.15 | Deficient |
| 0028 | 38.3 | 95.75 | Sufficient | 60.55 | Sufficient |
| 0029 | 46.6 | 116.5 | Sufficient | 59.5 | Sufficient |
| 0030 | 17.7 | 44.25 | Insufficient | 26.95 | Insufficient |
| 0031 | 12.7 | 31.75 | Insufficient | 18.35 | Deficient |
| 0032 | 27.8 | 69.5 | Sufficient | 47.35 | Insufficient |
| 0033 | 24.6 | 61.5 | Sufficient | 29.3 | Insufficient |
| 0034 | 15.6 | 39 | Insufficient | 22.5 | Deficient |
| 0035 | >150 | n.d. | High b | 80 | Sufficient |
| 0036 | 28.3 | 70.75 | Sufficient | 49.05 | Insufficient |
| 0037 | 26.1 | 65.25 | Sufficient | 31.45 | Insufficient |
| 0038 | 34.7 | 86.75 | Sufficient | 54.7 | Sufficient |
| 0039 | 43.4 | 108.5 | Sufficient | 119.1 | Sufficient |
| 0040 | 30.1 | 75.25 | Sufficient | 54.45 | Sufficient |
| 0041 | 20.4 | 51 | Sufficient | 32.8 | Insufficient |
| 0042 | 46.1 | 115.25 | Sufficient | 57.15 | Sufficient |
| 0043 | 24.8 | 62 | Sufficient | n.m. | n.m. |
| 0044 | 15.9 | 39.75 | Insufficient | 15.8 | Deficient |
| 0045 | 14 | 35 | Insufficient | 12.8 | Deficient |
| 0046 | 20 | 50 | Sufficient | 26.35 | Insufficient |
| 0047 | 27.3 | 68.25 | Sufficient | 52.55 | Sufficient |
| 0048 | 26.5 | 66.25 | Sufficient | 56.9 | Sufficient |
| 0049 | 16.7 | 41.75 | Insufficient | 28.9 | Insufficient |
| 0050 | 6.05 | 15.125 | Deficient | 8.25 | Deficient |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLean, G.R.; Soyemi, S.; Ajayi, O.P.; Fernando, S.; Sowinski-Mydlarz, W.; Stewart, D.; Illingworth, S.; Atkins, M.; Bhakta, D. Comparative Analysis of a Rapid Quantitative Immunoassay to the Reference Methodology for the Measurement of Blood Vitamin D Levels. Methods Protoc. 2025, 8, 85. https://doi.org/10.3390/mps8040085
McLean GR, Soyemi S, Ajayi OP, Fernando S, Sowinski-Mydlarz W, Stewart D, Illingworth S, Atkins M, Bhakta D. Comparative Analysis of a Rapid Quantitative Immunoassay to the Reference Methodology for the Measurement of Blood Vitamin D Levels. Methods and Protocols. 2025; 8(4):85. https://doi.org/10.3390/mps8040085
Chicago/Turabian StyleMcLean, Gary R., Samson Soyemi, Oluwafunmito P. Ajayi, Sandra Fernando, Wiktor Sowinski-Mydlarz, Duncan Stewart, Sarah Illingworth, Matthew Atkins, and Dee Bhakta. 2025. "Comparative Analysis of a Rapid Quantitative Immunoassay to the Reference Methodology for the Measurement of Blood Vitamin D Levels" Methods and Protocols 8, no. 4: 85. https://doi.org/10.3390/mps8040085
APA StyleMcLean, G. R., Soyemi, S., Ajayi, O. P., Fernando, S., Sowinski-Mydlarz, W., Stewart, D., Illingworth, S., Atkins, M., & Bhakta, D. (2025). Comparative Analysis of a Rapid Quantitative Immunoassay to the Reference Methodology for the Measurement of Blood Vitamin D Levels. Methods and Protocols, 8(4), 85. https://doi.org/10.3390/mps8040085






