Optimal Reference Gene Selection and Potential Target Gene Identification During Xanthomonas phaseoli pv. dieffenbachiae–Anthurium andreanum Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Plant Materials
2.2. Selection of Reference Genes, Target Genes, and Primer Design
2.3. Experiment Design and RNA Extractions
2.4. Reverse-Transcription and Quantitative RT-PCR (qPCR)
2.5. Expression Stability of Candidate Reference Genes
2.6. Validation of Reference Genes
3. Results and Discussion
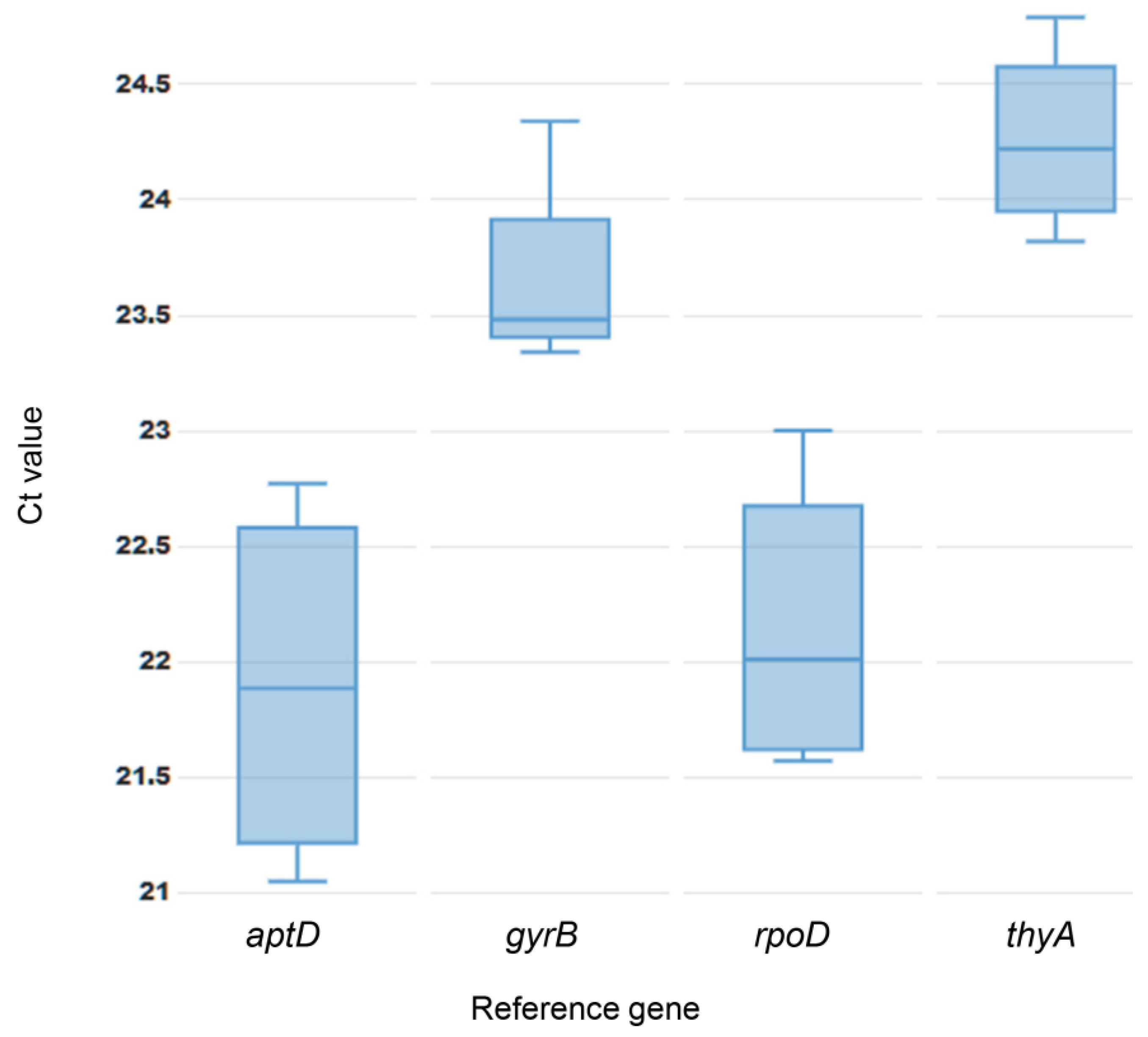
3.1. Primer Specificity and Amplification Efficiency of Candidate Reference Genes
3.2. Expression Stability
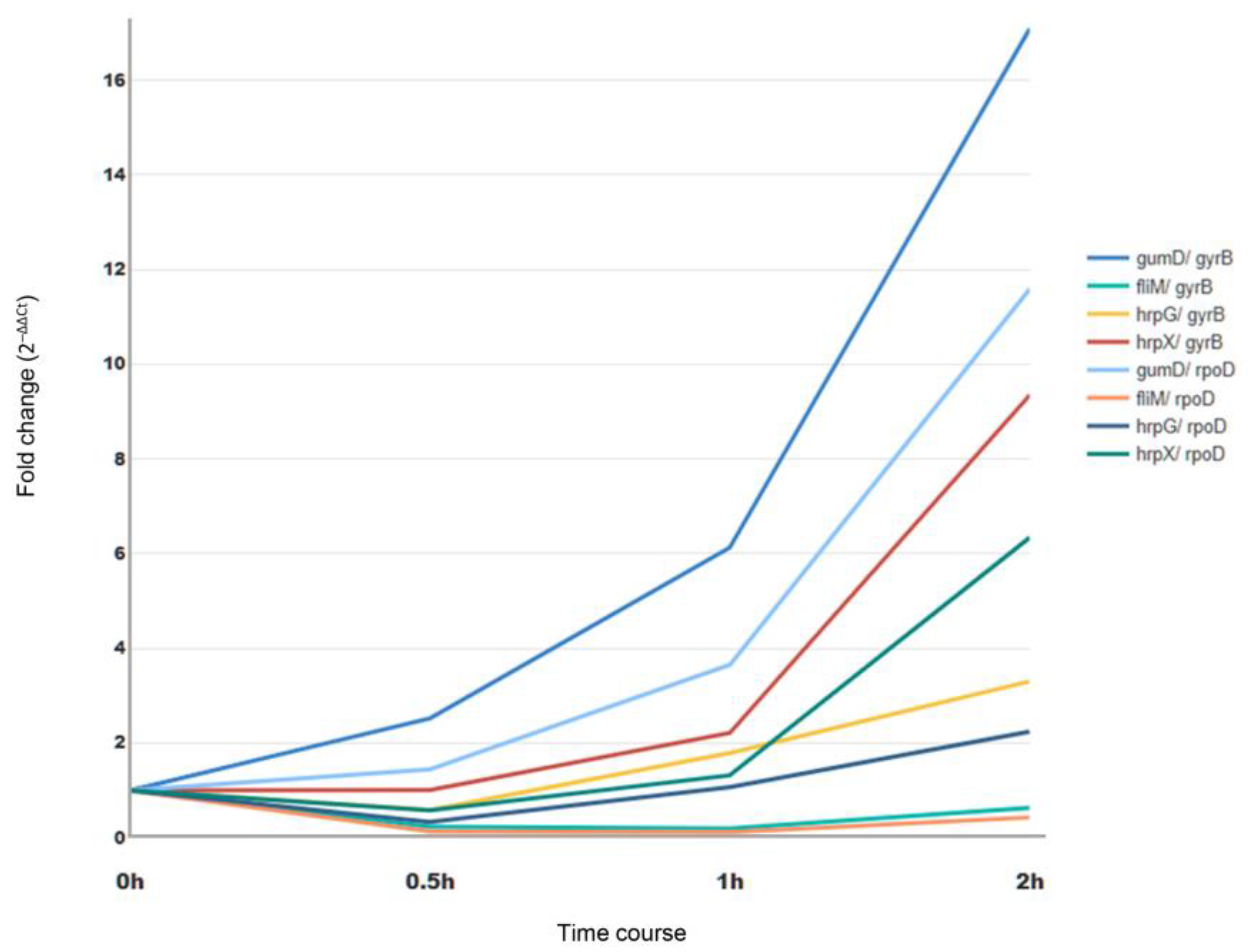
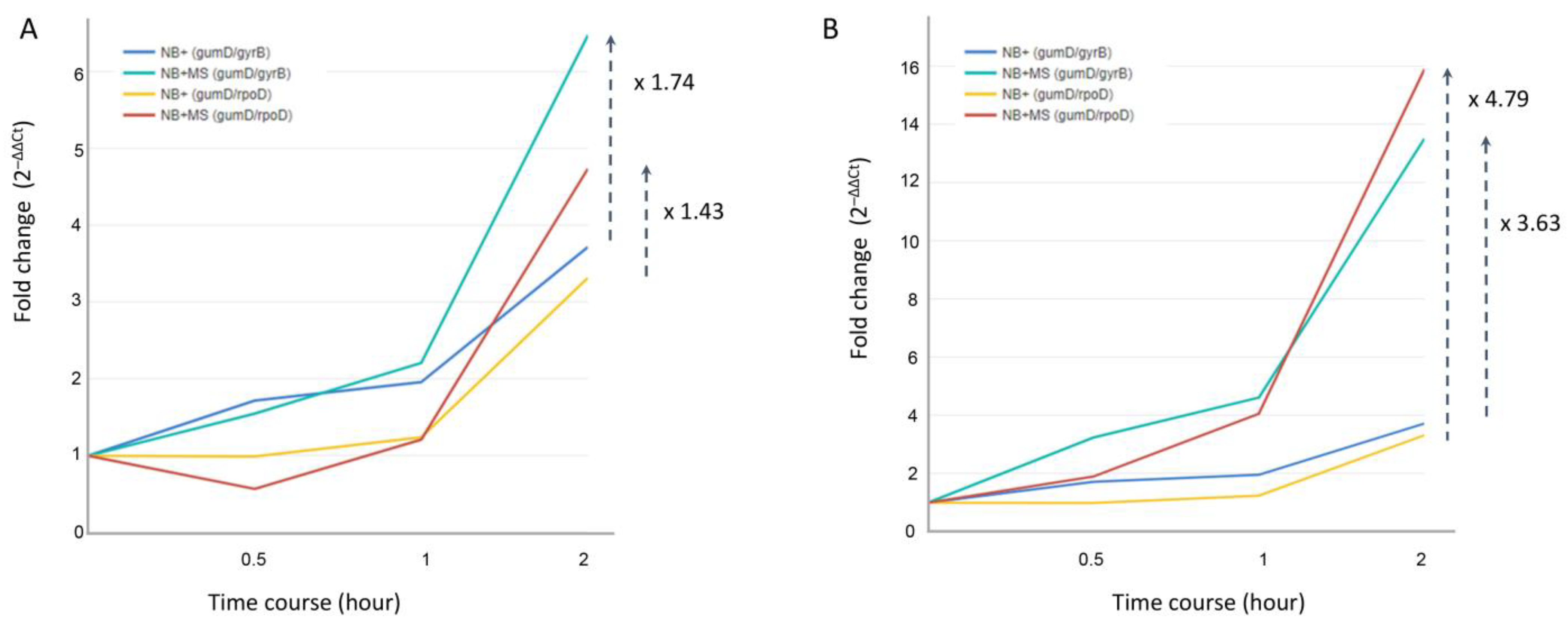
3.3. Expression Profile of Target Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCulloch, L.; Pirone, P.P. Bacterial leaf spot of Dieffenbachia. Phytopathology 1939, 29, 956–962. [Google Scholar]
- Constantin, E.C.; Cleenwerck, I.; Maes, M.; Baeyen, S.; Van Malderghem, C.; De Vos, P.; Cottyn, B. Genetic characterization of strains named as Xanthomonas axonopodis pv. dieffenbachiae leads to a taxonomic revision of the X. axonopodis species complex. Plant Pathol. 2016, 65, 792–806. [Google Scholar] [CrossRef]
- EPPO. Xanthomonas axonopodis pv. dieffenbachiae: Diagnostic protocols. Bull. OEPP 2004, 34, 183–186. [Google Scholar] [CrossRef]
- EPPO. PM 7/23 (2): Xanthomonas axonopodis pv. dieffenbachiae. Bull. OEPP 2009, 39, 393–402. [Google Scholar] [CrossRef]
- Boyce, P.C.; Croat, T.B. (2011 Onwards) The Überlist of Araceae, Totals for Published and Estimated Number of Species in Aroid Genera. International Aroid Society. Available online: http://www.aroid.org/genera/180211uberlist.pdf (accessed on 28 June 2025).
- Alvarez, A.M.; Toves, P.J.; Vowell, T.S. Bacterial blight of Anthuriums: Hawaii’s experience with a global disease. APSnet Features 2006, 2006. [Google Scholar] [CrossRef]
- Duffy, B. Survival of the anthurium blight pathogen, Xanthomonas axonopodis pv. dieffenbachiae, in field crop residues. Eur. J. Plant Pathol. 2000, 106, 291–295. [Google Scholar] [CrossRef]
- Ayin, C.M.; Amore, T.D.; Toves, P.J.; de Silva, A.S.; Alvarez, A.M. Evaluation of anthurium cultivars for resistance to Xanthomonas axonopodis pv. dieffenbachiae, the causal agent of bacterial blight. In Proceedings of the International Symposium on Tropical and Subtropical Ornamentals; Acta Horticulturae: Leuven, Belgium, 2017; Volume 1167, pp. 245–250. [Google Scholar]
- Chuang, S.-C.; Dobhal, S.; Pal, K.; Amore, T.D.; Alvarez, A.M.; Arif, M. Xanthomonas strains isolated from hosts in the family Araceae reveal diverse phylogenetic relationships and origins. Phytopathology 2024, 114, 1963–1974. [Google Scholar] [CrossRef]
- Chuang, S.-C.; Dobhal, S.; Keith, L.M.; Alvarez, A.M.; Arif, M. Xanthomonas spp. infecting Araceae and Araliaceae: Taxonomy, phylogeny, and potential virulence mechanisms. Biology 2025, 14, 766. [Google Scholar] [CrossRef]
- Dobhal, S.; Chuang, S.-C.; Arizala, D.; Keith, L.M.; Alvarez, A.M.; Arif, M. High-quality complete genome sequence of Xanthomonas phaseoli pv. dieffenbachiae outbreak strain D182: The causative agent of Anthurium Bacterial Blight in Hawai’i. PhytoFrontiers 2024, 4, 812–816. [Google Scholar] [CrossRef]
- Fukui, H.; Alvarez, A.M.; Fukui, R. Differential susceptibility of anthurium cultivars to bacterial blight in foliar and systemic infection phases. Plant Dis. 1998, 82, 800–806. [Google Scholar] [CrossRef]
- Constantin, E.C.; Haegeman, A.; Van Vaerenbergh, J.; Baeyen, S.; Van Malderghem, C.; Maes, M.; Cottyn, B. Pathogenicity and virulence gene content of Xanthomonas strains infecting Araceae, formerly known as Xanthomonas axonopodis pv. dieffenbachiae. Plant Pathol. 2017, 66, 1539–1554. [Google Scholar] [CrossRef]
- Mackay, I.M. Real-time PCR in the microbiology laboratory. Clin. Microbiol. Infect. 2004, 10, 190–212. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Wittwer, C.T. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Q.; Zou, J.; He, C.; Tao, J. Selection of optimized reference genes for qRT-PCR normalization in Xanthomonas campestris pv. campestris cultured in different media. Curr. Microbiol. 2019, 76, 613–619. [Google Scholar] [CrossRef]
- Petriccione, M.; Magri, A.; Gaudiano, M.; Scortichini, M. Selection and validation of reference genes for gene expression studies in Xanthomonas arboricola pv. juglandis subjected to abiotic stress. Plant Pathol. 2021, 70, 1455–1466. [Google Scholar] [CrossRef]
- Jacob, T.R.; Laia, M.L.; Ferro, J.A.; Ferro, M.I.T. Selection and validation of reference genes for gene expression studies by reverse transcription quantitative PCR in Xanthomonas citri subsp. citri during infection of Citrus sinensis. Biotechnol. Lett. 2011, 33, 1177–1184. [Google Scholar] [CrossRef]
- Kałużna, M.; Kuras, A.; Puławska, J. mRNA extraction of Xanthomonas fragariae in strawberry and validation of reference genes for RT-qPCR to study bacterial gene expression. Mol. Biol. Rep. 2019, 46, 5723–5733. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Worrall, L.J.; Majewski, D.D.; Strynadka, N.C.J. Structural insights into type III secretion systems of the bacterial flagellum and injectisome. Annu. Rev. Microbiol. 2023, 77, 669–698. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wengelnik, K.; Bonas, U. HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 1996, 178, 3462–3469. [Google Scholar] [CrossRef]
- Furutani, A.; Tsuge, S.; Ohnishi, K.; Hikichi, Y.; Oku, T.; Tsuno, K.; Inoue, Y.; Ochiai, H.; Kaku, H.; Kubo, Y. Evidence for HrpXo-dependent expression of type II secretory proteins in Xanthomonas oryzae pv. oryzae. J. Bacteriol. 2004, 186, 1374–1380. [Google Scholar] [CrossRef]
- Guo, Y.; Figueiredo, F.; Jones, J.; Wang, N. HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri. Mol. Plant Microbe Interact. 2011, 24, 649–661. [Google Scholar] [CrossRef]
- Kim, S.; Cho, Y.-J.; Song, E.-S.; Lee, S.H.; Kim, J.-G.; Kang, L.-W. Time-resolved pathogenic gene expression analysis of the plant pathogen Xanthomonas oryzae pv. oryzae. BMC Genom. 2016, 17, 345. [Google Scholar] [CrossRef]
- Katzen, F.; Ferreiro, D.U.; Oddo, C.G.; Ielmini, M.V.; Becker, A.; Pühler, A.; Ielpi, L. Xanthomonas campestris pv. campestris gum mutants: Effects on xanthan biosynthesis and plant virulence. J. Bacteriol. 1998, 180, 1607–1617. [Google Scholar] [CrossRef]
- Chou, F.-L.; Chou, H.-C.; Lin, Y.-S.; Yang, B.-Y.; Lin, N.-T.; Weng, S.-F.; Tseng, Y.-H. The Xanthomonas campestris gumD gene required for synthesis of xanthan gum is involved in normal pigmentation and virulence in causing black rot. Biochem. Biophys. Res. Commun. 1997, 233, 265–269. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, J.-G.; Lee, B.-M.; Cho, J.-Y. Mutational analysis of the gum gene cluster required for xanthan biosynthesis in Xanthomonas oryzae pv. oryzae. Biotechnol. Lett. 2009, 31, 265–270. [Google Scholar] [CrossRef]

| Gene Candidate | Gene Name | Primer Sequence (5′-3′) | Amplicon Size (bp) | Tm (°C) |
|---|---|---|---|---|
| Reference genes (RG) | atpD | atpD_QF: GCTGAGCGAAGAAGACAAGC atpD_QR: GTCCTTCAGCGAGACGTACT | 124 | 62 |
| gyrB | gyrB_QF: TGACCGACGAACAAAACACC gyrB_QR: CATCGCCGATATACATGCCG | 117 | 60 | |
| rpoD | rpoD_QF: ATGAAAATCGCCAAGGAGCC rpoD_QR: TGATGTTGGTGGTGTTGTCG | 124 | 60 | |
| thyA | thyA_QF: AAGCCGTACCTGGAGTTGTT thyA_QR: GGAAAGCCGTCGTTGAGATC | 125 | 60 | |
| Target genes (TG) | fliM | filM_QF: TGGACGTGGACTTCGAGTAC filM_QR: GAATACGGCAGGGTGATGTG | 145 | 60 |
| gumD | gumD_QF: TCCTGAACCATCTGCGTACC gumD_QR: GTTACGGCTCAGGTAGTGGT | 101 | 60 | |
| hrpG | hrpG_QF: ATCGGTGTTTCTGTTGACGC hrpG_QR: GAAGCTCCAGTTCCTCGGAA | 107 | 60 | |
| hrpX | hrpX_QF: ACTGCAACATCTCCAACAGC hrpX_QR: ATACGCATCTTCGGCCTCTT | 133 | 60 |
| Reference Gene | BestKeeper | geNorm | ΔCt | Final Rank | |||||
|---|---|---|---|---|---|---|---|---|---|
| SD [±Ct] | CV [% Ct] | Rank | M Value | CV | Rank | SD* | Rank | ||
| atpD | 0.68 | 3.12 | 4 | 0.52 | 0.28 | 4 | 0.51 | 4 | 4 |
| gyrB | 0.34 | 1.44 | 2 | 0.37 | 0.15 | 2 | 0.37 | 2 | 2 |
| rpoD | 0.53 | 2.38 | 3 | 0.35 | 0.10 | 1 | 0.34 | 1 | 1 |
| thyA | 0.31 | 1.29 | 1 | 0.45 | 0.21 | 3 | 0.45 | 3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, S.-C.; Dobhal, S.; Amore, T.D.; Alvarez, A.M.; Arif, M. Optimal Reference Gene Selection and Potential Target Gene Identification During Xanthomonas phaseoli pv. dieffenbachiae–Anthurium andreanum Infection. Methods Protoc. 2025, 8, 72. https://doi.org/10.3390/mps8040072
Chuang S-C, Dobhal S, Amore TD, Alvarez AM, Arif M. Optimal Reference Gene Selection and Potential Target Gene Identification During Xanthomonas phaseoli pv. dieffenbachiae–Anthurium andreanum Infection. Methods and Protocols. 2025; 8(4):72. https://doi.org/10.3390/mps8040072
Chicago/Turabian StyleChuang, Shu-Cheng, Shefali Dobhal, Teresita D. Amore, Anne M. Alvarez, and Mohammad Arif. 2025. "Optimal Reference Gene Selection and Potential Target Gene Identification During Xanthomonas phaseoli pv. dieffenbachiae–Anthurium andreanum Infection" Methods and Protocols 8, no. 4: 72. https://doi.org/10.3390/mps8040072
APA StyleChuang, S.-C., Dobhal, S., Amore, T. D., Alvarez, A. M., & Arif, M. (2025). Optimal Reference Gene Selection and Potential Target Gene Identification During Xanthomonas phaseoli pv. dieffenbachiae–Anthurium andreanum Infection. Methods and Protocols, 8(4), 72. https://doi.org/10.3390/mps8040072






