Comparing the Impact of Pre-Operative Antibiotics on the Outcomes of Immediately Placed Dental Implants: A Retrospective Multi-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brånemark, P.-I. Osseointegrated implants in the treatment of the edentulous jaw: Experience from a 10-year period. Scand. J. Plast. Reconstr. Surgery Suppl. 1977, 16, 1–132. [Google Scholar]
- Borges, H.; Correia, A.R.M.; Castilho, R.M.; de Oliveira Fernandes, G.V. Zirconia Implants and Marginal Bone Loss: A Systematic Review and Meta-Analysis of Clinical Studies. Int. J. Oral Maxillofac. Implant. 2020, 35, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.R.E.; Otero, A.I.P.; Fernandes, J.C.H.; Nassani, L.M.; Castilho, R.M.; de Oliveira Fernandes, G.V. Clinical Performance Comparing Titanium and Titanium-Zirconium or Zirconia Dental Implants: A Systematic Review of Randomized Controlled Trials. Dent. J. 2022, 10, 83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Remísio, M.J.D.S.; Borges, T.; Castro, F.; Gehrke, S.A.; Fernandes, J.C.H.; Fernandes, G.V.O. Histologic Osseointegration Level Comparing Titanium and Zirconia Dental Implants: Meta-analysis of Preclinical Studies. Int. J. Oral Maxillofac. Implant. 2023, 38, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Morena, D.; Leitão-Almeida, B.; Pereira, M.; Resende, R.; Fernandes, J.C.H.; Fernandes, G.V.O.; Borges, T. Comparative Clinical Behavior of Zirconia versus Titanium Dental Implants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 4488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Derks, J.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Larsson, M.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Early and late implant loss. J. Dent. Res. 2015, 94 (Suppl. S1), 44S–51S. [Google Scholar] [CrossRef]
- Shibli, J.A.; Naddeo, V.; Cotrim, K.C.; Kalil, E.C.; de Avila, E.D.; Faot, F.; Faverani, L.P.; Souza, J.G.S.; Fernandes, J.C.H.; Fernandes, G.V.O. Osteoporosis’ effects on dental implants osseointegration and survival rate: A systematic review of clinical studies. Quintessence Int. 2025, 56, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Van Assche, N.; Botticelli, D.; Berglundh, T. How does the timing of implant placement to extraction affect outcome? Int. J. Oral Maxillofac. Implant. 2007, 22, 203–223. [Google Scholar]
- Esposito, M.A.B.; Koukoulopoulou, A.; Coulthard, P.; Worthington, H.V. Interventions for replacing missing teeth: Dental implants in fresh extraction sockets. Cochrane Database Syst. Rev. 2006, 2006, CD005968. [Google Scholar]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontol. 2000 2015, 68, 122–134. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Marzola, A.; Bozzi, L.; Liljenberg, B.; Lindhe, J. Modeling and remodeling of human extraction sockets. J. Clin. Periodontol. 2008, 35, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Chappuis, V.; Belser, U.C.; Chen, S. Implant placement post extraction in esthetic single tooth sites: When immediate, when early, when late? Periodontol. 2020 2017, 73, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Ickroth, A.; Seyssens, L.; Christiaens, V.; Pitman, J. Immediate versus early implant placement for single tooth replacement in the aesthetic area: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2024, 35, 585–597. [Google Scholar] [CrossRef]
- Ragucci, G.M.; Elnayef, B.; Criado-Cámara, E.; Del Amo, F.S.-L.; Hernández-Alfaro, F. Immediate implant placement in molar extraction sockets: A systematic review and meta-analysis. Int. J. Implant. Dent. 2020, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Weinstein, T.; Scutellà, F.; Wang, H.-L.; Zucchelli, G. Implant placement in the esthetic area: Criteria for positioning single and multiple implants. Periodontol. 2000 2018, 77, 176–196. [Google Scholar] [CrossRef]
- Chu, S.J.; A Salama, M.; Salama, H.; A Garber, D.; Saito, H.; O Sarnachiaro, G.; Tarnow, D.P. The dual-zone therapeutic concept of managing immediate implant placement and provisional restoration in anterior extraction sockets. Compend. Contin. Educ. Dent. 2012, 33, 524–534. [Google Scholar]
- Yuenyongorarn, P.; Kan, J.Y.K.; Rungcharassaeng, K.; Matsuda, H.; Roe, P.; Lozada, J.L.; Caruso, J. Facial gingival changes with and without socket gap grafting following single maxillary anterior immediate tooth replacement: One-year results. J. Oral Implantol. 2020, 46, 496–505. [Google Scholar] [CrossRef]
- Romanos, G.E. Wound healing in immediately loaded implants. Periodontol. 2000. 2015, 68, 153–167. [Google Scholar] [CrossRef]
- Campi, M.; Leitão-Almeida, B.; Pereira, M.; Shibli, J.A.; Levin, L.; Fernandes, J.C.H.; Fernandes, G.V.O.; Borges, T. Immediate implant placement in damaged extraction sockets: A systematic review and meta-analysis of randomized controlled trials. Quintessence Int. 2025, 56, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.C.R.; Marques, M.D.C.; Vidal, M.G.; Tolentino, P.H.M.P.; Dinelli, R.G.; Fernandes, G.V.O.; Shibli, J.A. Is the facial bone wall critical to achieving esthetic outcomes in immediate implant placement with immediate restoration? A systematic review. Adv. Clin. Exp. Med. 2024, 33, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Klokkevold, P.R.; Han, T.J. How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? Int. J. Oral Maxillofac. Implants. 2007, 22, 173–202. [Google Scholar] [PubMed]
- Lund, B.; Hultin, M.; Tranaeus, S.; Naimi-Akbar, A.; Klinge, B. Complex systematic review: Perioperative antibiotics in conjunction with dental implant placement. Clin. Oral Implant. Res. 2015, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Masucci, L.; Quaranta, G.; Patini, R.; Caponio, V.C.A.; Pesce, P.; Ravidà, A.; Penarrocha-Oltra, D.; Penarrocha-Diago, M. Culturomic and quantitative real-time polymerase chain reaction analyses for early contamination of abutments with different surfaces: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 568–578. [Google Scholar] [CrossRef]
- Duval, X.; Millot, S.; Tubiana, S.; Iung, B. Prévention de l’endocardite infectieuse. Presse Med. 2019, 48, 556–562. [Google Scholar] [CrossRef]
- Salgado-Peralvo, A.O.; Kewalramani, N.; Peña-Cardelles, J.F.; Mateos-Moreno, M.V.; Monsalve-Guil, L.; Jiménez-Guerra, Á.; Ortiz-García, I.; Velasco-Ortega, E. Preventive antibiotic prescribing habits among professionals dedicated to oral implantology: An observational study. Antibiotics 2021, 10, 301. [Google Scholar] [CrossRef]
- Sánchez, F.R.; Arteagoitia, I.; Teughels, W.; Andrés, C.R.; Quirynen, M.; Kielbassa, A.M. Antibiotic dosage prescribed in oral implant surgery: A meta-analysis of cross-sectional surveys. PLoS ONE 2020, 15, e0236981. [Google Scholar] [CrossRef]
- Cunha, B.A. Antibiotic side effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Anitua, E.; Aguirre, J.J.; Gorosabel, A.; Barrio, P.; Errazquin, J.M.; Román, P.; Pla, R.; Carrete, J.; De Petro, J.; Orive, G. A multicentre placebo-controlled randomised clinical trial of antibiotic prophylaxis for placement of single dental implants. Eur. J. Oral Implantol. 2009, 2, 283–292. [Google Scholar]
- Abu-Ta’A, M.; Quirynen, M.; Teughels, W.; Van Steenberghe, D. Asepsis during periodontal surgery involving oral implants and the usefulness of peri-operative antibiotics: A prospective, randomized, controlled clinical trial. J. Clin. Periodontol. 2008, 35, 58–63. [Google Scholar] [CrossRef]
- Salgado-Peralvo, Á.; Kewalramani, N.; Pérez-Jardón, A.; Pérez-Sayáns, M.; Mateos-Moreno, M.; Arriba-Fuente, L. Antibiotic prescribing patterns in the placement of dental implants in Europe: A systematic review of survey-based studies. Med. Oral Patol. Oral Cir. Bucal. 2024, 29, e1–e10. [Google Scholar] [CrossRef]
- Salgado-Peralvo, A.-O.; Peña-Cardelles, J.-F.; Kewalramani, N.; Mateos-Moreno, M.-V.; Jiménez-Guerra, Á.; Velasco-Ortega, E.; Uribarri, A.; Moreno-Muñoz, J.; Ortiz-García, I.; Núñez-Márquez, E.; et al. Preventive antibiotic therapy in the placement of immediate implants: A systematic review. Antibiotics 2021, 11, 5. [Google Scholar] [CrossRef]
- Lang, N.P.; Pun, L.; Lau, K.Y.; Li, K.Y.; Wong, M.C. A systematic review on survival and success rates of implants placed immediately into fresh extraction sockets after at least 1 year. Clin. Oral Implant. Res. 2012, 23 (Suppl. S5), 39–66. [Google Scholar] [CrossRef]
- Wagenberg, B.; Froum, S.J. A retrospective study of 1925 consecutively placed immediate implants from 1988 to 2004. Int. J. Oral Maxillofac. Implant. 2006, 21, 71–80. [Google Scholar]
- Wagenberg, B.D.; Froum, S.J.; Eckert, S.E. Long-term bone stability assessment around 1,187 immediately placed implants with 1- to 22-year follow-up. Int. J. Oral Maxillofac. Implant. 2013, 28, 605–612. [Google Scholar] [CrossRef]
- Wagenberg, B.; Froum, S.J. A retrospective study of bone level stability around 441 mandibular and 350 maxillary molar implants placed with an immediate implant protocol. Int. J. Periodontics Restor. Dent. 2020, 40, 635–643. [Google Scholar] [CrossRef]
- Miclau, T.; Edin, M.L.; Lester, G.E.; Lindsey, R.W.; Dahners, L.E. Effect of ciprofloxacin on the proliferation of osteoblast-like MG-63 human osteosarcoma cells in vitro. J. Orthop. Res. 1998, 16, 509–512. [Google Scholar] [CrossRef]
- Huddleston, P.M.; Steckelberg, J.M.; Hanssen, A.D.; Rouse, M.S.; Bolander, M.E.; Patel, R. Ciprofloxacin inhibition of experimental fracture healing. J. Bone Jt. Surg. Am. 2000, 82, 161–173. [Google Scholar] [CrossRef]
- Tuncay, I.; Ozbek, H.; Köşem, M.; Unal, O. A comparison of effects of fluoroquinolones on fracture healing: An experimental study in rats. Ulus. Travma Acil Cerrahi Derg. 2005, 11, 17–22. [Google Scholar]
- Zahra, B.; Nicholas, B.; Geoffrey, R.; Dina, Z.; Janal, M.N.; Stuart, F. Dental implant failure rates in patients with self-reported allergy to penicillin. Clin. Implant. Dent. Relat. Res. 2022, 24, 301–306. [Google Scholar] [CrossRef]
- Edibam, N.R.; Lorenzo-Pouso, A.I.; Caponio, V.C.A. Self-reported allergy to penicillin and clindamycin administration may be risk factors for dental implant failure: A systematic review, meta-analysis and delabeling protocol. Clin. Oral Implant. Res. 2023, 34, 651–661. [Google Scholar] [CrossRef]
| Patient-Related Characteristics | Total n = 2391 (100%) | Implant Survival n = 2314 (96.8%) | Implant Failure n = 77 (3.2%) | p-Value | |
|---|---|---|---|---|---|
| Age (mean (SD)) | 59.56 (13.42) | 59.57 (13.48) | 59.25 (11.74) | 0.84 | |
| Gender (%) | Female | 1281 (53.6) | 1235 (53.4) | 46 (59.7) | 0.30 |
| Male | 1110 (46.4) | 1079 (46.6) | 31 (40.3) | ||
| Ethnicity (%) | Non-Hispanic | 2193 (91.7) | 2119 (91.6) | 74 (96.1) | 0.22 |
| Hispanic | 117 (4.9) | 114 (4.9) | 3 (3.9) | ||
| Others | 81 (3.4) | 81 (3.5) | 0 (0.0) | ||
| Race (%) | White | 1814 (75.9) | 1752 (75.7) | 62 (80.5) | 0.26 |
| Asian | 113 (4.7) | 111 (4.8) | 2 (2.6) | ||
| African American | 87 (3.6) | 81 (3.5) | 6 (7.8) | ||
| Hispanic or Latino | 147 (6.1) | 143 (6.2) | 4 (5.2) | ||
| Pacific Islander | 6 (0.3) | 6 (0.3) | 0 (0.0) | ||
| American Indian or Alaskan Native | 6 (0.3) | 6 (0.3) | 0 (0.0) | ||
| Others | 218 (9.1) | 215 (9.3) | 3 (3.9) | ||
| Tobacco use (%) | 187 (7.8) | 182 (7.9) | 5 (6.5) | 0.83 | |
| Hypertension (%) | 385 (16.1) | 371 (16.0) | 14 (18.2) | 0.64 | |
| Marijuana use (%) | 25 (1.0) | 23 (1.0) | 2 (2.6) | 0.19 | |
| Diabetes (%) | 161 (6.7) | 155 (6.7) | 6 (7.8) | 0.64 | |
| Thyroid disorder (%) | 157 (6.6) | 149 (6.4) | 8 (10.4) | 0.16 | |
| HIV (%) | 5 (0.2) | 5 (0.2) | 0 (0.0) | 1.00 | |
| Kidney disease (%) | 107 (4.5) | 101 (4.4) | 6 (7.8) | 0.16 | |
| Arthritis (%) | 267 (11.2) | 259 (11.2) | 8 (10.4) | 1.00 | |
| Osteoporosis (%) | 79 (3.3) | 74 (3.2) | 5 (6.5) | 0.11 | |
| Depression (%) | 149 (6.2) | 143 (6.2) | 6 (7.8) | 0.48 | |
| Seizures/epilepsy (%) | 7 (0.3) | 7 (0.3) | 0 (0.0) | 1.00 | |
| Asthma (%) | 142 (5.9) | 138 (6.0) | 4 (5.2) | 1.00 | |
| Sleep apnea (%) | 55 (2.3) | 52 (2.2) | 3 (3.9) | 0.26 | |
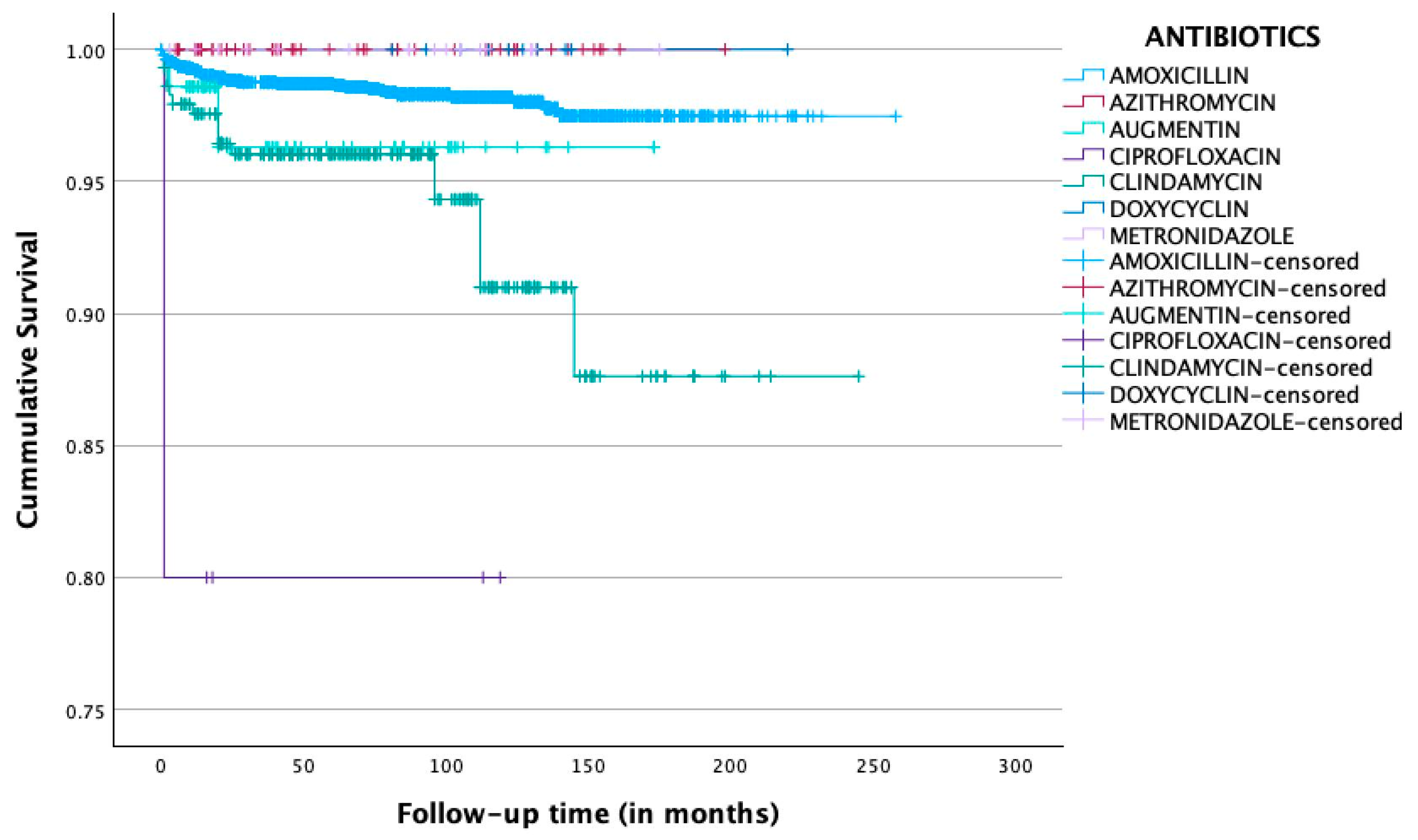
| Antibiotics | Total n = 3351 (100%) | Implant Survival n = 3286 (98.1%) | Implant Failure n = 65 (1.9%) |
|---|---|---|---|
| Amoxicillin (%) | 2894 (86.4) | 2849 (98.4) | 45 (1.6) |
| Azithromycin (%) | 61 (1.8) | 61 (100.0) | 0 (0.0) |
| Amoxicillin and clavulanic acid (Augmentin) (%) | 71 (2.1) | 69 (97.2) | 2 (2.8) |
| Ciprofloxacin (%) | 5 (0.1) | 4 (80.0) | 1 (20.0) |
| Clindamycin (%) | 290 (8.7) | 273 (94.1) | 17 (5.9) |
| Doxycycline (%) | 11 (0.3) | 11 (100.0) | 0 (0.0) |
| Metronidazole (%) | 19 (0.6) | 19 (100.0) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzopoulos, G.S.; Wolff, L.F. Comparing the Impact of Pre-Operative Antibiotics on the Outcomes of Immediately Placed Dental Implants: A Retrospective Multi-Center Study. Methods Protoc. 2025, 8, 69. https://doi.org/10.3390/mps8040069
Chatzopoulos GS, Wolff LF. Comparing the Impact of Pre-Operative Antibiotics on the Outcomes of Immediately Placed Dental Implants: A Retrospective Multi-Center Study. Methods and Protocols. 2025; 8(4):69. https://doi.org/10.3390/mps8040069
Chicago/Turabian StyleChatzopoulos, Georgios S., and Larry F. Wolff. 2025. "Comparing the Impact of Pre-Operative Antibiotics on the Outcomes of Immediately Placed Dental Implants: A Retrospective Multi-Center Study" Methods and Protocols 8, no. 4: 69. https://doi.org/10.3390/mps8040069
APA StyleChatzopoulos, G. S., & Wolff, L. F. (2025). Comparing the Impact of Pre-Operative Antibiotics on the Outcomes of Immediately Placed Dental Implants: A Retrospective Multi-Center Study. Methods and Protocols, 8(4), 69. https://doi.org/10.3390/mps8040069






