Empagliflozin Repurposing for Lafora Disease: A Pilot Clinical Trial and Preclinical Investigation of Novel Therapeutic Targets
Abstract
1. Introduction
2. Experimental Design
2.1. Study Design and Setting
2.2. Eligibility Criteria
- Documented genetic diagnosis of LD (pathogenic mutations in the EPM2A or EPM2B genes);
- Age: 10–22 years;
- Patient able to understand and complete the protocol (even with the help of a caregiver over 18 years of age) and perform neuropsychological tests.
- Undocumented genetic diagnosis of LD;
- Advanced stage of disease (3–4 progression scale) [32];
- Participation in other ongoing therapeutic treatments for LD;
- Pregnancy;
- Allergy or known hypersensitivity to empagliflozin;
- Other conditions, at the discretion of the investigator, that could interfere with participation or completion of the study.
2.3. Discontinuation of Interventions
2.4. Sample Size
2.5. Data Collection and Management
2.6. Statistical Methods
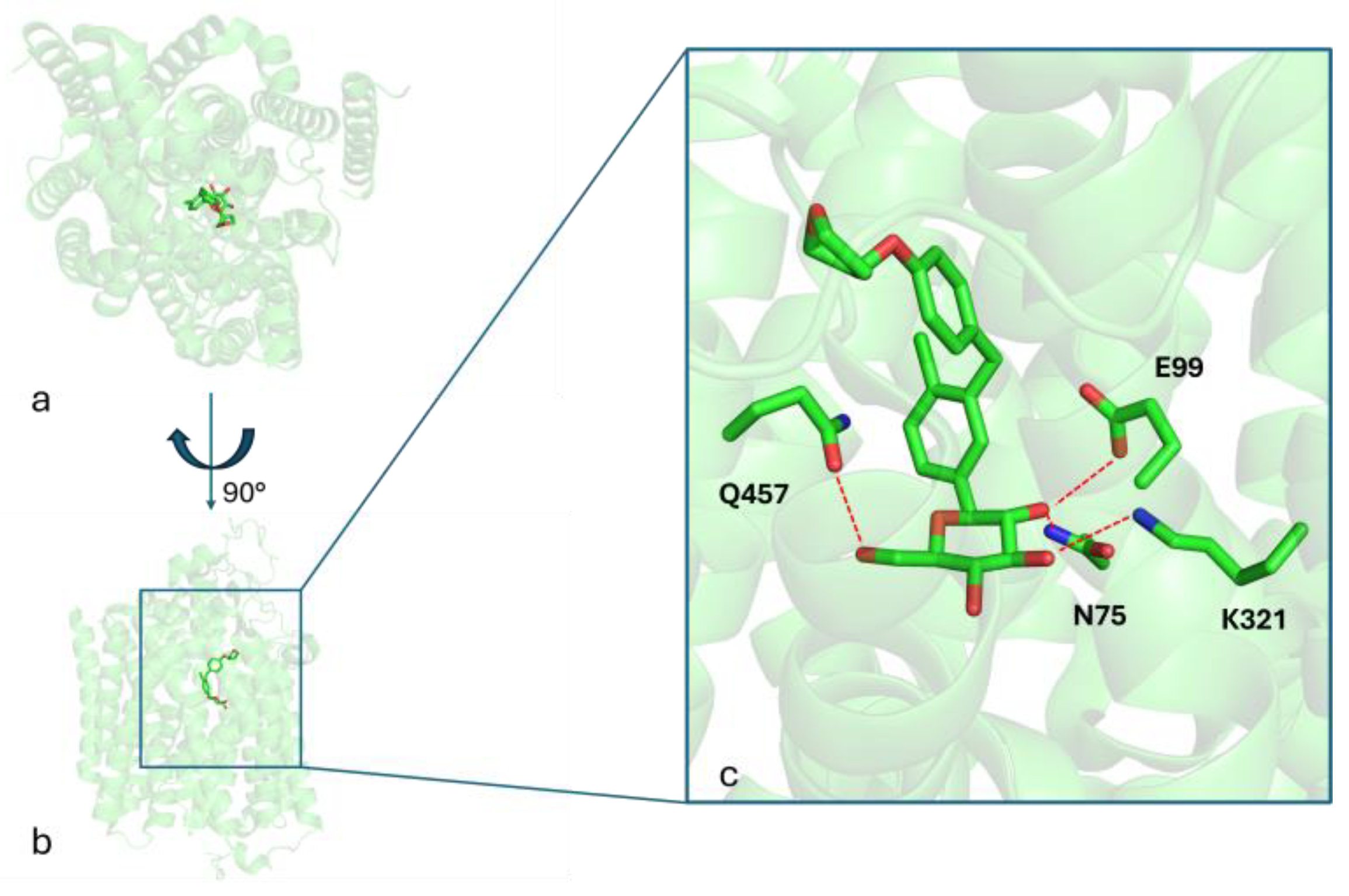
2.7. In Silico Studies on Target Candidates
2.8. Validation in Cell Lines
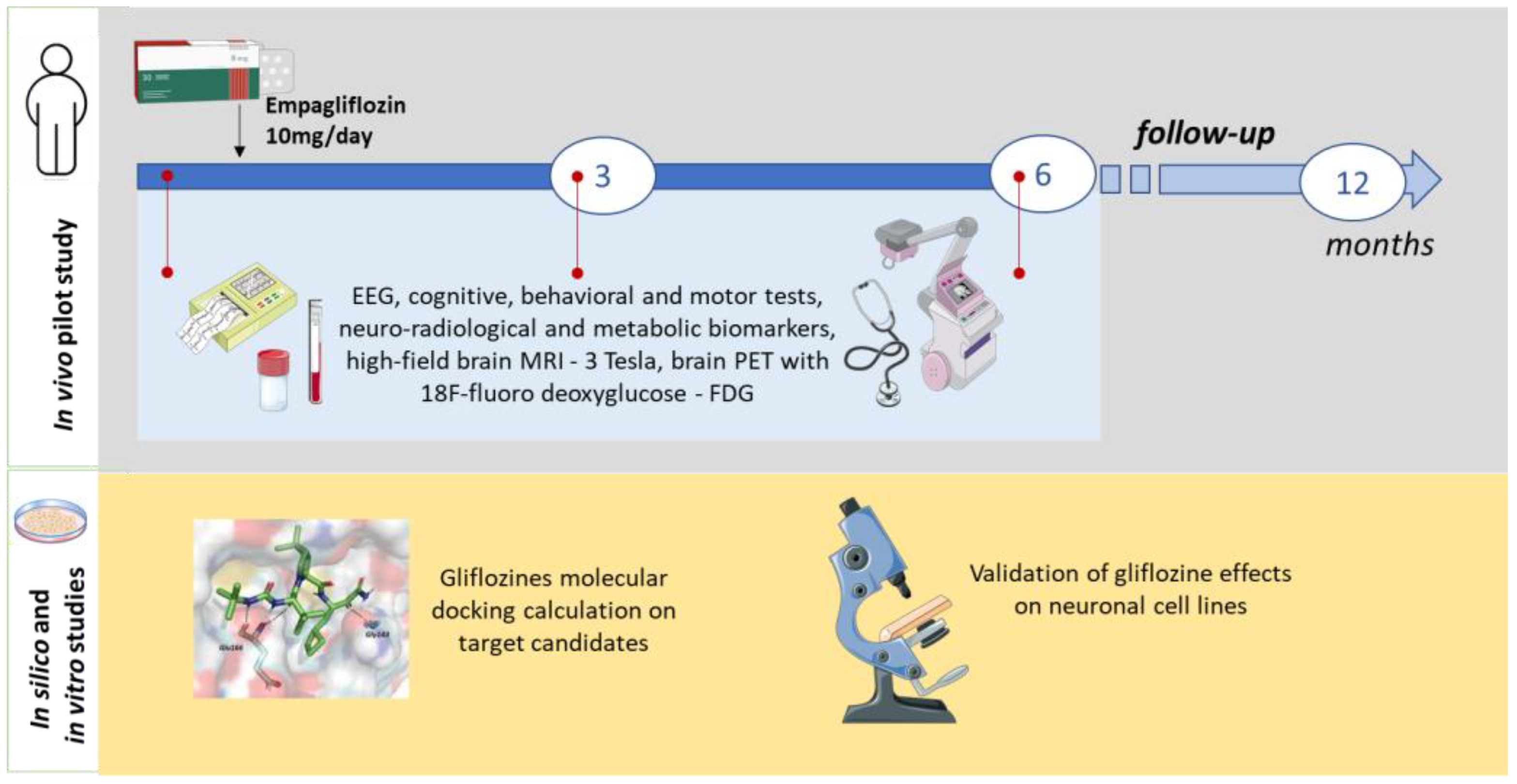
3. Procedure
3.1. Recruitment
3.2. Intervention Description
3.3. Participant Timeline
3.4. Outcomes
- Safety: Type, severity, incidence, and timing of adverse events; incidence of treatment discontinuation due to adverse events; and changes in vital signs, ECG, and laboratory parameters.
- Efficacy: Change in the frequency of generalized tonic–clonic seizures compared to the 6 months prior to the study; EEG: changes in posterior background activity and frequency of epileptiform abnormalities; cognitive function: changes (no worsening or improvement) in neuropsychological assessments; motor function: changes (no worsening or improvement) in motor function assessments; global function and autonomy: changes (no worsening or improvement) in LDS, LESS, ADL, IADL, Barthel Index, Vineland-II, and CGI-I and PGI-CaGI-C scales.
- Biomarkers: Brain FDG-PET: changes (no worsening) in areas of cerebral hypometabolism. High-field brain MRI (3 Tesla): changes (no worsening) in measures of cerebral atrophy. Direct measurement of CSF polyglucosan levels, although potentially informative, is not feasible within the constraints of this pilot study. Thus, brain FDG-PET imaging is included as a surrogate biomarker to assess changes in cerebral metabolism. This choice was primarily due to the significant logistical challenges associated with sample collection and processing for this ultra-rare disease, as well as to the absence of widely available, fully validated assays for the quantitative analysis of CSF polyglucosan levels.
3.5. Ethics and Dissemination
3.5.1. Ethics Approval and Consent to Participate
3.5.2. Competing Interest
3.5.3. Protocol Amendments
3.5.4. Confidentiality
3.5.5. Access to Data
3.5.6. Dissemination Policy
3.6. In Silico Screening
3.7. In Vitro Cell Models Assays
4. Expected Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LD | Lafora disease; |
| LBs | Lafora bodies; |
| AILA | Italian Lafora Association; |
| AMPK | AMP-activated protein kinase; |
| rhGAA | Recombinant human α-glucosidase; |
| SGLT2 | Sodium-glucose cotransporter 2; |
| EEG | Electroencephalogram; |
| GLUT1 | Glucose transporter type 1; |
| GLUT3 | Glucose transporter type 3; |
| GSD | Glycogen storage disorder; |
| LESS | Lafora Epilepsy Severity Scale; |
| ADL | Activities of Daily Living; |
| IADL | Instrumental Activities of Daily Living; |
| LDS | Lafora Disease Performance Scale. |
References
- Turnbull, J.; Tiberia, E.; Striano, P.; Genton, P.; Carpenter, S.; Ackerley, C.A.; Minassian, B.A. Lafora disease. Epileptic Disord. 2016, 18 (Suppl. 2), S38–S62. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, F.; Ahonen, S.J.; Nitschke, S.; Mitra, S.; Minassian, B.A. Lafora disease—From pathogenesis to treatment strategies. Nat. Rev. Neurol. 2018, 14, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Minassian, B.A. Lafora’s disease: Towards a clinical, pathologic, and molecular synthesis. Pediatr. Neurol. 2001, 25, 21–29. [Google Scholar] [CrossRef] [PubMed]
- D’orsi, G.; Lalla, A.; Palumbo, O.; Di Claudio, M.T.; Valenzano, A.; Sabetta, A.; Lopopolo, A.; Di Muro, E.; Palumbo, P.; Copetti, M.; et al. The presenting symptoms of Lafora disease: An electroclinical and genetic study in five Apulian (Southern Italy) families. Seizure 2020, 83, 145–153. [Google Scholar] [CrossRef]
- Pondrelli, F.; Muccioli, L.; Licchetta, L.; Mostacci, B.; Zenesini, C.; Tinuper, P.; Vignatelli, L.; Bisulli, F. Natural history of Lafora disease: A prognostic systematic review and individual participant data meta-analysis. Orphanet J. Rare Dis. 2021, 16, 362. [Google Scholar] [CrossRef]
- d’Orsi, G.; Di Claudio, M.T.; Palumbo, O.; Carella, M. on behalf of the Lafora Multidisciplinary Team 2010–2021. Electro-clinical features and management of the late stage of the Lafora disease. Front. Neurol. 2022, 13, 969297. [Google Scholar]
- Sanz, P.; Serratosa, J.M.; Sánchez, M.P. Beneficial Effects of Metformin on the Central Nervous System, with a Focus on Epilepsy and Lafora Disease. Int. J. Mol. Sci. 2021, 22, 5351. [Google Scholar] [CrossRef]
- Burgos, D.F.; Machío-Castello, M.; Iglesias-Cabeza, N.; Giráldez, B.G.; González-Fernández, J.; Sánchez-Martín, G.; Sánchez, M.P.; Serratosa, J.M. Early Treatment with Metformin Improves Neurological Outcomes in Lafora Disease. Neurotherapeutics 2023, 20, 230–244. [Google Scholar] [CrossRef]
- Bisulli, F.; Muccioli, L.; D’orsi, G.; Canafoglia, L.; Freri, E.; Licchetta, L.; Mostacci, B.; Riguzzi, P.; Pondrelli, F.; Avolio, C.; et al. Treatment with metformin in twelve patients with Lafora disease. Orphanet J. Rare Dis. 2019, 14, 149. [Google Scholar] [CrossRef]
- Israelian, L.; Wang, P.; Gabrielian, S.; Zhao, X.; Minassian, B.A. Ketogenic diet reduces Lafora bodies in murine Lafora disease. Neurol. Genet. 2020, 6, e533. [Google Scholar] [CrossRef]
- Cardinali, S.; Canafoglia, L.; Bertoli, S.; Franceschetti, S.; Lanzi, G.; Tagliabue, A.; Veggiotti, P. A pilot study of a ketogenic diet in patients with Lafora body disease. Epilepsy Res. 2006, 69, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, S.; Nitschke, S.; Grossman, T.R.; Kordasiewicz, H.; Wang, P.; Zhao, X.; Guisso, D.R.; Kasiri, S.; Nitschke, F.; A Minassian, B. Gys1 antisense therapy rescues neuropathological bases of murine Lafora disease. Brain 2021, 144, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Varea, O.; Guinovart, J.J.; Duran, J. Malin restoration as proof of concept for gene therapy for Lafora disease. Brain Commun. 2022, 4, fcac168. [Google Scholar] [CrossRef]
- Della Vecchia, S.; Ogi, A.; Licitra, R.; Abramo, F.; Nardi, G.; Mero, S.; Landi, S.; Battini, R.; Sicca, F.; Ratto, G.M.; et al. Trehalose Treatment in Zebrafish Model of Lafora Disease. Int. J. Mol. Sci. 2022, 23, 6874. [Google Scholar] [CrossRef]
- Duran, J.; Gruart, A.; García-Rocha, M.; Delgado-García, J.M.; Guinovart, J.J. Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease. Hum. Mol. Genet. 2014, 23, 3147–3156. [Google Scholar] [CrossRef]
- Brewer, M.K.; Uittenbogaard, A.; Austin, G.L.; Segvich, D.M.; DePaoli-Roach, A.; Roach, P.J.; McCarthy, J.J.; Simmons, Z.R.; Brandon, J.A.; Zhou, Z.; et al. Targeting pathogenic lafora bodies in Lafora disease using an antibody-enzyme fusion. Cell Metab. 2019, 30, 689–705.e6. [Google Scholar] [CrossRef]
- Roessler, H.I.; Knoers, N.V.A.M.; van Haelst, M.M.; van Haaften, G. Drug Repurposing for Rare Diseases. Trends Pharmacol. Sci. 2021, 42, 255–267. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, R.; Guan, C.; Zhang, Y.; Chen, Z.; Hoerer, S.; Nar, H.; Chen, L. Structural basis of inhibition of the human SGLT2–MAP17 glucose transporter. Nature 2022, 601, 280–284. [Google Scholar] [CrossRef]
- Imbrici, P.; d’Orsi, G.; Carella, M.; Nicolotti, O.; De Luca, A.; Altomare, C.D.; Liantonio, A. Sodium-glucose cotransporter-2 inhibitors: A potential novel treatment for Lafora disease? Pharmacol. Res. 2024, 199, 107012. [Google Scholar] [CrossRef]
- Atiya, A.; Das Gupta, D.; Alsayari, A.; Alrouji, M.; Alotaibi, A.; Sharaf, S.E.; Al Abdulmonem, W.; Alorfi, N.M.; Abdullah, K.M.; Shamsi, A. Linagliptin and Empagliflozin Inhibit Microtubule Affinity Regulatory Kinase 4: Repurposing Anti-Diabetic Drugs in Neurodegenerative Disorders Using In Silico and In Vitro Approaches. ACS Omega 2023, 8, 6423–6430. [Google Scholar] [CrossRef]
- Klinc, A.; Groselj, U.; Mlinaric, M.; Homan, M.; Markelj, G.; Novak, A.M.; Campa, A.S.; Sikonja, J.; Battelino, T.; Tansek, M.Z.; et al. Case report: The success of empagliflozin therapy for glycogen storage disease type 1b. Front. Endocrinol. 2024, 15, 1365700. [Google Scholar] [CrossRef] [PubMed]
- Grünert, S.C.; Gautschi, M.; Baker, J.; Boyer, M.; Burlina, A.; Casswall, T.; Corpeleijn, W.; Çıki, K.; Cotter, M.; Crushell, E.; et al. Empagliflozin for treating neutropenia and neutrophil dysfunction in 21 infants with glycogen storage disease 1b. Mol. Genet. Metab. 2024, 142, 108486. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Chen, H.; Zeng, H.; Wu, J.; Wang, Y.; Ma, N.; Lan, J.; Zhang, Y.; Niu, H.; et al. Empagliflozin in children with glycogen storage disease-associated inflammatory bowel disease: A prospective, single-arm, open-label clinical trial. Sci. Rep. 2024, 14, 8630. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-X.; Liang, C.-L.; Su, Y.-Y.; Lin, Y.-T.; Lu, Z.-K.; Lin, R.-Z.; Zhou, Z.-Z.; Zeng, C.-H.; Tao, C.-Y.; Liu, Z.-C.; et al. Clinical spectrum, over 12-year follow-up and experience of SGLT2 inhibitors treatment on patients with glycogen storage disease type Ib: A single-center retrospective study. Orphanet J. Rare Dis. 2024, 19, 155. [Google Scholar] [CrossRef]
- Kaczor, M.; Malicki, S.; Folkert, J.; Dobosz, E.; Bryzek, D.; Chruscicka-Smaga, B.; Greczan, M.; Kucharska, D.W.; Piątosa, B.; Samborowska, E.; et al. Neutrophil functions in patients with neutropenia due to glycogen storage disease type 1b treated with empagliflozin. Blood Adv. 2024, 8, 2790–2802. [Google Scholar] [CrossRef]
- Grünert, S.C.; Derks, T.G.; Mundy, H.; Dalton, R.N.; Donadieu, J.; Hofbauer, P.; Jones, N.; Uçar, S.K.; LaFreniere, J.; Contreras, E.L.; et al. Treatment recommendations for glycogen storage disease type IB- associated neutropenia and neutrophil dysfunction with empagliflozin: Consensus from an international workshop. Mol. Genet. Metab. 2024, 141, 108144. [Google Scholar] [CrossRef]
- Derks, T.G.J.; Venema, A.; Köller, C.; Bos, E.; Overduin, R.J.; Stolwijk, N.N.; Hofbauer, P.; Bolhuis, M.S.; van Eenennaam, F.; Groen, H.; et al. Repurposing empagliflozin in individuals with glycogen storage disease Ib: A value-based healthcare approach and systematic benefit-risk assessment. J. Inherit. Metab. Dis. 2024, 47, 244–254. [Google Scholar] [CrossRef]
- Grünert, S.C.; Venema, A.; LaFreniere, J.; Schneider, B.; Contreras, E.; Wortmann, S.B.; Derks, T.G.J. Patient-reported outcomes on empagliflozin treatment in glycogen storage disease type Ib: An international questionnaire study. JIMD Rep. 2023, 64, 252–258. [Google Scholar] [CrossRef]
- Della Vecchia, S.; Imbrici, P.; Liantonio, A.; Naef, V.; Damiani, D.; Licitra, R.; Bernardi, S.; Marchese, M.; Santorelli, F.M. Dapagliflozin ameliorates Lafora disease phenotype in a zebrafish model. Biomed. Pharmacother. 2025, 183, 117800. [Google Scholar] [CrossRef]
- Iancu, C.V.; Bocci, G.; Ishtikhar, M.; Khamrai, M.; Oreb, M.; Oprea, T.I.; Choe, J.-Y. GLUT3 Inhibitor Discovery through in Silico Ligand Screening and in Vivo Validation in Eukaryotic Expression Systems. Sci. Rep. 2022, 12, 1429. [Google Scholar] [CrossRef]
- Kapoor, K.; Finer-Moore, J.S.; Pedersen, B.P.; Caboni, L.; Waight, A.; Hillig, R.C.; Bringmann, P.; Heisler, I.; Müller, T.; Siebeneicher, H.; et al. Mechanism of Inhibition of Human Glucose Transporter GLUT1 Is Conserved between Cytochalasin B and Phenylalanine Amides. Proc. Natl. Acad. Sci. USA 2016, 113, 4711–4716. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, S.; Gambardella, A.; Canafoglia, L.; Striano, P.; Lohi, H.; Gennaro, E.; Ianzano, L.; Veggiotti, P.; Sofia, V.; Biondi, R.; et al. Clinical and genetic findings in 26 Italian patients with Lafora disease. Epilepsia 2006, 47, 640–643. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, G.; Farolfi, A.; Muccioli, L.; Palumbo, O.; Palumbo, P.; Modoni, S.; Allegri, V.; Garibotto, V.; Di Claudio, M.T.; Di Muro, E.; et al. Association of CSF and PET markers of neurodegeneration with electroclinical progression in Lafora disease. Front. Neurol. 2023, 14, 1202971. [Google Scholar] [CrossRef]
- Ciriaco, F.; Gambacorta, N.; Trisciuzzi, D.; Nicolotti, O. PLATO: A Predictive Drug Discovery Web Platform for Efficient Target Fishing and Bioactivity Profiling of Small Molecules. Int. J. Mol. Sci. 2022, 23, 5245. [Google Scholar] [CrossRef]
- Rho, J.M.; Boison, D. The metabolic basis of epilepsy. Nat. Rev. Neurol. 2022, 18, 333–347. [Google Scholar] [CrossRef]
- Wiciński, M.; Wódkiewicz, E.; Górski, K.; Walczak, M.; Malinowski, B. Perspective of SGLT2 Inhibition in Treatment of Conditions Connected to Neuronal Loss: Focus on Alzheimer’s Disease and Ischemia-Related Brain Injury. Pharmaceuticals 2020, 13, 379. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Di Meo, I.; Polito, R.; Auriemma, M.C.; Gambardella, A.; di Mauro, G.; Capuano, A.; Paolisso, G. Cognitive impairment and type 2 diabetes mellitus: Focus of SGLT2 inhibitors treatment. Pharmacol. Res. 2022, 176, 106062. [Google Scholar] [CrossRef]
- D’Acierno, M.; Resaz, R.; Iervolino, A.; Nielsen, R.; Sardella, D.; Siccardi, S.; Costanzo, V.; D’Apolito, L.; Suzumoto, Y.; Segalerba, D.; et al. Dapagliflozin Prevents Kidney Glycogen Accumulation and Improves Renal Proximal Tubule Cell Functions in a Mouse Model of Glycogen Storage Disease Type 1b. J. Am. Soc. Nephrol. 2022, 33, 1864–1875. [Google Scholar] [CrossRef]
- Trepiccione, F.; Iervolino, A.; D’acierno, M.; Siccardi, S.; Costanzo, V.; Sardella, D.; De La Motte, L.R.; D’apolito, L.; Miele, A.; Perna, A.F.; et al. The SGLT2 inhibitor dapagliflozin improves kidney function in glycogen storage disease XI. Sci. Transl. Med. 2023, 15, eabn4214. [Google Scholar] [CrossRef]
- Tomlinson, B.; Hu, M.; Zhang, Y.; Chan, P.; Liu, Z.-M. Evaluation of the pharmacokinetics, pharmacodynamics and clinical efficacy of empagliflozin for the treatment of type 2 diabetes. Expert Opin. Drug Metab. Toxicol. 2017, 13, 211–223. [Google Scholar] [CrossRef]
- Frampton, J.E. Empagliflozin: A Review in Type 2 Diabetes. Drugs 2019, 78, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L.M.B.; Tamborlane, W.V.; Yver, A.; Simons, G.; Wu, J.; Nock, V.; Hobson, D.; Hughan, K.S.; Kaspers, S.; Marquard, J. Pharmacokinetic and pharmacodynamic profile of the sodium-glucose co-transporter-2 inhibitor empagliflozin in young people with Type 2 diabetes: A randomized trial. Diabet. Med. 2018, 35, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Köse, E.; Özçay, F.; Aydın, H.İ.; Kasapkara, Ç.S.; İnci, A.; Yavaş, A.K.; Tümer, L.; Eminoğlu, F.T. Effect of empagliflozin treatment on laboratory and clinical findings of patients with glycogen storage disease type Ib: First study from Türkiye. J. Pediatr. Endocrinol. Metab. 2025, 38, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2023-4: Protein Preparation Wizard; Epik, Schrödinger, LLC: New York, NY, USA; Impact, Schrödinger, LLC: New York, NY, USA; Prime, Schrödinger, LLC: New York, NY, USA, 2023.
- Lu, C.; Wu, C.; Ghoreishi, D.; Chen, W.; Wang, L.; Damm, W.; Ross, G.A.; Dahlgren, M.K.; Russell, E.; Von Bargen, C.D.; et al. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. [Google Scholar] [CrossRef]
- Schaubroeck, K.J.; Leitner, B.P.; Perry, R.J. An optimized method for tissue glycogen quantification. Physiol. Rep. 2022, 10, e15195. [Google Scholar] [CrossRef]
- Nagy, J.A.; Semple, C.; Riveros, D.; Sanchez, B.; Rutkove, S.B. Altered electrical properties in skeletal muscle of mice with glycogen storage disease type II. Sci. Rep. 2022, 12, 5327. [Google Scholar] [CrossRef]
- Moreno-Estellés, M.; Campos-Rodríguez, Á.; Rubio-Villena, C.; Kumarasinghe, L.; Garcia-Gimeno, M.A.; Sanz, P. Deciphering the Polyglucosan Accumulation Present in Lafora Disease Using an Astrocytic Cellular Model. Int. J. Mol. Sci. 2023, 24, 6020. [Google Scholar] [CrossRef]
- Muccioli, L.; Vignatelli, L.; Tappatà, M.; Mazzone, S.; Zenesini, C.; Armstrong, D.; DEFEAT-LD study group; Michelucci, R.; Bisulli, F. VAL-1221 for the treatment of patients with Lafora disease: Study protocol for a single-arm, open-label clinical trial. BMJ Open 2024, 14, e085062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Orsi, G.; Liantonio, A.; Imbrici, P.; Gambacorta, N.; Dinoi, G.; Altomare, C.D.; DEFEAT-LD Study Group; Carella, M. Empagliflozin Repurposing for Lafora Disease: A Pilot Clinical Trial and Preclinical Investigation of Novel Therapeutic Targets. Methods Protoc. 2025, 8, 48. https://doi.org/10.3390/mps8030048
d’Orsi G, Liantonio A, Imbrici P, Gambacorta N, Dinoi G, Altomare CD, DEFEAT-LD Study Group, Carella M. Empagliflozin Repurposing for Lafora Disease: A Pilot Clinical Trial and Preclinical Investigation of Novel Therapeutic Targets. Methods and Protocols. 2025; 8(3):48. https://doi.org/10.3390/mps8030048
Chicago/Turabian Styled’Orsi, Giuseppe, Antonella Liantonio, Paola Imbrici, Nicola Gambacorta, Giorgia Dinoi, Cosimo Damiano Altomare, DEFEAT-LD Study Group, and Massimo Carella. 2025. "Empagliflozin Repurposing for Lafora Disease: A Pilot Clinical Trial and Preclinical Investigation of Novel Therapeutic Targets" Methods and Protocols 8, no. 3: 48. https://doi.org/10.3390/mps8030048
APA Styled’Orsi, G., Liantonio, A., Imbrici, P., Gambacorta, N., Dinoi, G., Altomare, C. D., DEFEAT-LD Study Group, & Carella, M. (2025). Empagliflozin Repurposing for Lafora Disease: A Pilot Clinical Trial and Preclinical Investigation of Novel Therapeutic Targets. Methods and Protocols, 8(3), 48. https://doi.org/10.3390/mps8030048








