Abstract
Early initiation of palliative care in patients with advanced chronic conditions significantly improves their quality of care; however, variability in disease trajectories complicates such interventions’ timing. The NECPAL CCOMS-ICO prognostic tool was developed as a straightforward instrument to help healthcare providers in all clinical settings promptly identify patients with advanced chronic conditions who require palliative care, thereby enhancing service planning and delivery. Its latest version, 4.0, 2021, for the first time, incorporates a patient survival estimation. Nevertheless, validation is necessary. This study aims to validate the NECPAL version 4.0 tool in an independent cohort. It is an observational, prospective study involving outpatients and hospitalized non-randomized patients at Hospital Universitario Infanta Leonor–Virgen de la Torre in Madrid, Spain, all of whom have at least one advanced chronic condition. The study is scheduled to last 6 years, including a recruitment period of 30 months starting 1 February 2024, followed by a 12-month follow-up period for each patient. This is the first prospective study designed to validate the NECPAL version 4.0 instrument. Implementing this tool would allow the identification of patients with advanced chronic conditions and unmet palliative care needs and determine the more appropriate care pathway at the proper moment.
1. Introduction
In economically developed countries, most people now die from complex long-term illnesses such as cancer, respiratory and heart diseases, stroke, and dementia []. These conditions are characterized by gradual progression, prolonged duration, and prognostic uncertainty. Identification of patients with a life-limiting illness when they are starting to need a change in their goals of care contributes to end-of-life care planning.
The World Health Organization (WHO) underlines the importance of providing palliative care (PC) early in the disease trajectory of patients suffering from a life-limiting illness []. Evidence shows earlier access to PC promotes quality of life, reduces hospitalizations, and even prolongs survival [,,,,,,,,]. However, the WHO estimates that only 14% of patients with life-limiting diseases who need PC receive it []. In this setting, patients should not only be treated at the final stages of their disease but before. The concepts of “first transition”, “second transition”, and “third transition” have been developed to address the evolving needs of patients with advanced chronic illnesses (Figure 1). The “first transition” emphasizes integrating PC at the onset of a chronic or life-limiting diagnosis, aiming to manage symptoms and enhance life quality from the beginning. The “second transition” arises as the patient’s condition becomes more complex, necessitating specialized PC services for advanced symptom control and intensive psychosocial support. The “third transition” represents the final stage, centering on end-of-life care focusing on comfort, dignity, and emotional and spiritual support, ensuring a peaceful closure for patients and their families [].
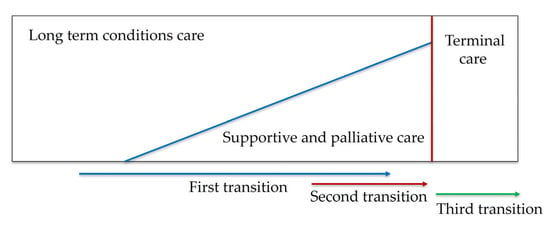
Figure 1.
Early integration model of palliative care.
A systematic method could facilitate the early identification of patients with advanced progressive illnesses likely to have unmet PC needs and facilitate end-of-life care planning.
The Surprise Question (SQ) has been proposed as a prompt for the early identification of patients who may benefit from PC. It involves a single reflective query: “Would you be surprised if this patient were to die within the next 6 to 12 months?” Given its simplicity and lack of reliance on complex scoring systems, the SQ has been widely adopted in clinical practice to help healthcare professionals identify patients with potential PC needs. Nevertheless, its subjective nature means that its accuracy may vary depending on both the characteristics of the patient population and the clinician’s judgment. In contrast, the Palliative Care Screening Tool (PCST) offers a more structured approach. It integrates clinical variables—such as functional status and comorbidities—into a scoring algorithm that estimates the patient’s expected survival time. While both the SQ and PCST are established instruments used to support clinical decision-making in the context of PC, comparative data regarding their prognostic performance remain limited. Emerging evidence suggests that using these tools in combination may yield greater predictive accuracy than employing either tool independently.
Identifying patients likely to have unmet PC needs should focus on anticipating their needs and predicting functional decline to create a plan proactively. In this setting, several screening tools have been developed (Table 1). Most tools use either prediction of death or deterioration or both to identify patients. Some tools are applicable in both primary care and hospital settings. Moreover, some utilize the SQ alongside other indicators for a thorough evaluation, while others are tailored to specific patient conditions and do not have a fixed cutoff value. Nevertheless, evaluating these tools is limited because of a lack of a valid comparator, so their actual clinical utility is still being determined [,,]. Further research on effective strategies for identifying patients with potential PC needs would be beneficial.

Table 1.
Tools for identifying patients with potential palliative care needs.
As the early identification of patients in need of PC could meet patients’ treatment goals and improve their quality of life, it is imperative to screen these patients using a highly accurate screening tool. The NECPAL CCOMS-ICO tool, developed by the Catalan Institute of Oncology in Spain, aims to identify early patients with advanced chronic conditions requiring PC across the healthcare system []. This instrument can be used in outpatient and in-hospital environments. It combines a simple SQ, ‘Would you be surprised if this patient died within the next 12 months?’, with six parameters. The parameters of the NECPAL instrument include (1) Palliative Needs Identified, where healthcare professionals determine if the patient requires PC; (2) Functional Decline, assessing the patient’s ability to perform daily activities; (3) Nutritional Decline; (4) Multi-Morbidity, indicating the presence of more than two chronic diseases added to the principal condition; (5) Use of Resources, relating to the frequency of emergency admissions or increased demand for medical interventions within six months; and (6) Specific Disease Criteria, which assesses the severity or progression of the specific chronic conditions. A patient is NECPAL+ if the SQ is answered positively (SQ+) and at least one parameter is met. Its latest version, 4.0, 2021, for the first time, incorporates a patient survival estimation (Supplementary Materials). NECPAL+ patients are categorized into three prognostic stages to estimate survival: Stage I (SQ+ and 1–2 parameters), with an estimated survival of 38 months; Stage II (SQ+ and 3–4 parameters), with 17.2 months; and Stage III (SQ+ and 5–6 parameters), with 3.6 months (Supplementary Material S1) []. Incorporating mortality and prognosis assessments into the tool could more accurately pinpoint the optimal time to initiate PC. However, external validation is essential to improve their clinical performance.
2. Aims and Objectives
This prospective observational cohort study aims to investigate the accuracy of the NECPAL version 4.0 (2021) tool—developed by the NECesidades PALiativas, a Collaborating Centre of the World Health Organization at the Catalan Institute of Oncology—in identifying patients with advanced chronic conditions who require early PC and analyzes its effectiveness as a mortality predictor.
2.1. Primary Objectives
Two primary objectives of the research are considered:
- a.
- To explore, in an accessible population of patients assisted within the Community of Madrid, Spain public health system, the proportion of patients with advanced chronic illnesses who require PC using the NECPAL version 4.0 (2021) tool.
- b.
- To investigate the instrument’s accuracy in predicting mortality in this population.
2.2. Secondary Objectives
- a.
- To describe in this population the functional and nutritional deterioration, the prevalent advanced chronic diseases, the demand and need for PC by the patient, their family, and the professional.
- b.
- To compare the perceived quality of life among NECPAL+ patients receiving PC, those not receiving it, and NECPAL- patients using the EuroQol 5D tool (EQ-5D).
3. Methods
3.1. Study Design
We are conducting a longitudinal, prospective, observational study based on a cohort of non-randomized patients, following the STROBE recommendations for observational studies [].
The goal is to identify existing screening tools for the identification of patients with advanced progressive diseases who are likely to have PC needs in primary healthcare and evaluate their accuracy.
This study analyzes the predictive capacity of the latest version, 4.0, of the NECPAL instrument for identifying patients with advanced progressive diseases who are likely to have PC needs. Moreover, it evaluates the accuracy of mortality predictions at three time points (3, 17, and 38 months), focusing on specific clinical outcomes and patient-reported measures. The study commenced on 1 February 2024, with an anticipated completion date of 1 May 2030.
The study was approved by the Ethics Committee of the Hospital Universitario Clínico San Carlos (code 22/202–E) and conforms to the Declaration of Helsinki, alongside complying with the Good Clinical Practices (GCP) of the International Conference of Harmonization (ICH). Written informed consent is obtained from all participants, with provisions for adapted communication strategies for patients with limited cognitive capacity whose legally authorized representatives provide consent.
3.2. Setting
The study is planned to span six years, including a recruitment period of 30 months followed by a 12-month follow-up period for each patient. Patients will be recruited from both outpatient consultations and hospital admissions.
3.3. Participants
Patients with advanced chronic conditions will be invited to take part in the study. Oral and written information will be given.
3.3.1. Inclusion Criteria
Patients are eligible for inclusion in the study if they fulfill the following criteria: adult (at least 18 years of age) with cancer or non-oncological advanced chronic condition. Non-cancer advanced chronic conditions are defined as progressive and incurable diseases with no reasonable possible therapeutic response by the criteria established by the Spanish Society of Palliative Care (SECPAL) []. These conditions include target organ damage, such as heart, pulmonary, liver, and kidney diseases; neurodegenerative disorders, dementia, and cerebrovascular disease; and peripheral vascular illnesses (Table 2). Cancer describes neoplasms that cannot be cured [,].

Table 2.
Criteria for advanced chronic conditions.
3.3.2. Exclusion Criteria
- a.
- Patients who are likely to be transferred to other facilities or expected to move out of the hospital’s geographic area during the study period.
- b.
- Patients with a projected life expectancy of less than 3 months. Patients with an estimated life expectancy of less than three months are excluded from this study, as the NECPAL version 4.0 tool is designed for the early identification of patients with advanced chronic conditions who may benefit from anticipatory palliative care planning. The prognostic stratification provided by the tool begins at a minimum horizon of three months (Stage III), and including patients with a shorter expected survival could impair the assessment of its predictive performance and clinical applicability in earlier stages of disease. This criterion ensures that the study remains focused on the population for whom proactive palliative interventions may still have a meaningful impact.
3.4. Patients Recruitment
Investigators will identify potential participants through electronic health records in various settings, including outpatient consultations, hospitalized patients, or follow-up visits. Principal Investigators will complete an eligibility criteria checklist for all considered cases. Clinical information will be used to assess eligibility. To ensure a fair and objective study, patients will be stratified based on age, gender, underlying chronic conditions, and NECPAL tool results (positive or negative) to create balanced groups.
At the time of writing this manuscript, 105 patients were included in the study.
3.5. Principal Outcome Measures
- Predictive accuracy of NECPAL version 4.0 (2021) tool for identifying patients who need PC.
- Mortality prediction accuracy at 3, 17, and 38 months.
- Quality of life.
3.6. Data Collection
The data for each study participant will be incorporated into the electronic Case Report Form (eCRF) using REDCap [] designed specifically for this purpose. The data recorded in the eCRF will be directly imported from the computerized medical records.
3.7. Data Variables
3.7.1. Primary Dependents Variables
- Positive NECPAL identification.
- Mortality at 3, 17, and 38 months.
3.7.2. Independent Variables
- NECPAL instrument: SQ (+/−) and number of positive parameters.
- The patient receives PC (Yes/No).
- Quality of Life: assessed using the EuroQol 5D-5L questionnaire.
- Demographics: age, gender.
- Chronic conditions: cancer and non-oncological advanced chronic conditions.
- Setting: outpatients and hospitalized patients.
- Functional status: Barthel Index score
- Health variables: primary healthcare center visits, home visits by primary care, hospital emergency visits, number of active specialist consultations, number of daily medications consumed.
- Nutritional variables: albumin, lymphocytes, prealbumin, retinol-binding protein, transferrin, cholesterol, body mass index (BMI), dietary intake record.
4. Study Procedures
The study is being conducted at Hospital Universitario Infanta Leonor-Virgen de la Torre, a secondary-level hospital in Madrid, Spain, involving outpatients and inpatients. Eligible participants are adults aged 18 years or older with at least one advanced chronic condition or cancer, identified by physicians through electronic medical records. Advanced chronic conditions are defined, according to the criteria established by SECPAL guidelines (Table 2). Cancer describes neoplasms that cannot be cured. Physicians apply the NECPAL tool to each case, beginning with the SQ, which asks, “Would you be surprised if this patient were to die in the next 12 months?”. If the healthcare professional answers “no” to this question, the patient is considered SQ+. To be considered NECPAL+, an SQ+ patient must also meet at least one parameter from the NECPAL tool. If the professional answers “yes” to the SQ (SQ−), or if an SQ+ patient does not meet any additional NECPAL parameters, the patient is classified as NECPAL−. All patients classified as NECPAL+ are deemed to need PC. It is recorded whether the NECPAL+ patient is receiving PC or not. Mortality status (alive or deceased) is assessed and recorded at 3 (NECPAL stage I), 17 (NECPAL stage II), and 38 months (NECPAL stage III), including the date and cause of death, if applicable. Mortality data are collected through telephone follow-ups and chart reviews. The quality of life of the study population is assessed using the EuroQol-5D-5L instrument (EuroQol Research Foundation, Rotterdam, The Netherlands). The questionnaire is administered at inclusion, 3 (NECPAL stage I), 17 (NECPAL stage II), and 38 months (NECPAL stage III), if applicable. Quality of life is compared among NECPAL+ patients receiving PC, those not receiving it, and NECPAL− patients. No assessments beyond those required for usual care are conducted.
Although the study protocol does not include scheduled re-assessments of NECPAL- patients during follow-up, these individuals will continue to receive routine clinical care within the healthcare system. Any significant changes in their clinical status may prompt re-evaluation by the attending medical team, including the potential re-application of the NECPAL tool if deemed appropriate. While these additional assessments are not captured prospectively in the study database, they reflect the clinical reality and the dynamic nature of patients’ needs over time. This approach ensures that patients who may not initially meet criteria for early palliative care are not excluded from appropriate care if their condition evolves.
5. Statistical Analysis
5.1. Sample Size Calculation
To determine the appropriate sample size for our study, we considered a population of approximately 350,000 inhabitants. Employing a 95% confidence level (Z = 1.96), a 5% margin of error (E = 0.05), and assuming maximum variability (p = 0.5), the initial sample size was calculated to be 384 participants. We applied a 10% oversampling rate to account for potential non-responses, increasing the target sample size to approximately 420 participants.
5.2. Analysis
Quantitative variables will be expressed as means with standard deviations, and categorical variables as absolute frequencies and percentages. To evaluate the association between NECPAL+/– and mortality at 3, 17, and 38 months, we will analyze sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Mortality rates will be compared using the chi-square test for equality of proportions. The Kaplan–Meier method will be used to estimate non-parametric survival curves, and the log-rank test will be applied to assess their differences. Additionally, a semi-parametric Cox proportional hazards regression model will be performed to predict the risk of death. Statistical analyses will be performed using the Statistical Package for Social Science version 29.0 (SPSS Inc., Chicago, IL, USA). A value of p < 0.05 will be defined as statistically significant.
6. Ethical Considerations
This study has been designed by the Helsinki Declaration and the current Spanish legislation on medical research (Biomedical Research Act 17/2007 of July 3) and data protection (Organic Law 3/2018 of December 5 on the Protection of Personal Data and Guarantee of Digital Rights and the General Data Protection Regulation). It will follow Good Clinical Practice Standards and current legal regulations.
6.1. Information and Obtaining Patient Informed Consent
Each patient or their legal representative will be informed orally and in writing through the Patient Information Sheet, detailing the study’s objectives, methods, and duration. The patient or their legal representative will be given sufficient time to read the Patient Information Sheet and will have the opportunity to ask questions. The researcher will obtain written Informed Consent from the patient or their legal representative, including the date and the signatures of both the researcher and the patient/legal representative. An electronic signature (e-consent) option will also be offered. This signing procedure will be optional and will only be carried out if the patient or legal representative consents. Researchers will implement robust security measures for the electronic signature process. Once the signing procedure is completed, the e-consent will be securely stored on the REDCap data collection platform with the same patient ID number in the study, and a copy will be given to the signer. In no case will study procedures be initiated before signature.
6.2. Data Confidentiality
The data collected during the study will be kept strictly confidential and accessed only by team members. Researchers must ensure the data’s accuracy, completeness, and legibility; this responsibility is underscored by our strict compliance with the 2018 Data Protection Act. We have put in place regular checks and monitoring to ensure compliance, providing a secure and reassuring data management process. The eCRF meets the requirements for accuracy and reliability. The participants will be assigned an individual-specific number, and their details will be anonymized on the database. At all times, researchers will be responsible for the fidelity and veracity of all recorded data. The study data will be destroyed once their analysis is completed.
7. Discussion
Identifying patients likely to have unmet PC needs is crucial to ensuring they receive the appropriate care at the right time. This identification process should not only lead to a referral to specialist PC services but also guarantee a comprehensive and holistic assessment of patients. This comprehensive assessment is central to improving policy-making, service planning, and care delivery, and we must address all aspects of patient care.
The ability of current screening tools to identify patients with advanced progressive diseases who are likely to have PC needs in primary care is limited. In a systematic review, the performance metrics for the screening tools were generally poor []. Further research is required to identify standardized screening processes. This research, based on predicting mortality and deterioration, anticipating the PC needs, and predicting the rate and course of functional decline, would prompt a comprehensive assessment. This assessment is not just important but crucial to identify and meet their needs on time.
The identification process of these patients should not be based solely on predicting mortality, but it should also focus on anticipating their needs whenever they occur and predicting the rate and course of functional decline in order to make a proactive PC plan. Our study seeks to explore the potential of the NECPAL version 4.0 (2021) tool in our area of care. The unique feature of NECPAL, which allows for the simultaneous establishment of the appropriateness of PC and the prognosis prediction, could significantly improve patient care. Previous studies have evaluated the instrument in predicting mortality using a short cut-off (12 months), showing a moderate capacity [,]. To our knowledge, this study is the first to analyze the predictive capacity of the latest version of the tool that incorporates long-term mortality, offering a more comprehensive understanding of patient needs and providing a solid foundation for future research. The potential impact of our findings could inspire a new wave of research and innovation in the field of PC.
NECPAL is a straightforward instrument based on variables commonly obtained in daily practice, making it easy to incorporate into healthcare systems. The mortality prediction into risk groups could allow classified patients to undergo the three transitions. However, research is needed to evaluate predictive ability and transportability.
8. Conclusions
Implementing validated and standardized screening tools would transform the identification process of people with advanced progressive diseases and improve timely access to PC. NECPAL version 4.0 (2021) instrument could help physicians detect those patients in whom PC should be integrated earlier, with the aim of an integral response to the needs of patients suffering from multiple chronic diseases and to plan care.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/mps8030045/s1, S1: NECPAL 4.0 PROGNOSTIC (2021) [,,,].
Author Contributions
Conceptualization, A.B.-F. and A.F.-M.; methodology, A.B.-F., A.F.-M. and M.B.-V.; validation, A.B.-F., A.F.-M., E.D.-S., N.P.-M. and L.H.; formal analysis, A.F.-M. and J.T.-M.; investigation, A.B.-F., A.F.-M., E.D.-S., N.P.-M., L.H., F.G.-G., H.N.-L. and A.S.-P.; writing—original draft preparation, A.B.-F. and A.F.-M.; writing—review and editing, A.B.-F. and A.F.-M.; visualization, E.D.-S., N.P.-M., L.H., F.G.-G., H.N.-L., A.S.-P., M.B.-V. and J.T.-M.; supervision, A.B.-F. and A.F.-M.; project administration, A.B.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Hospital Universitario Clínico San Carlos (code 22/202-E) for studies involving humans.
Informed Consent Statement
Informed consent is obtained from all subjects involved in the study.
Data Availability Statement
Not applicable, as it is a protocol.
Acknowledgments
COMPASS Research Group (External validation of the necpal ccoms-ico prognostic tool for early palliative care and mortality prediction in patients with advanced chronic conditions): Eva María Moya-Mateo, María Teresa Bellver-Álvarez, Beatriz Rodríguez-Gómez, Gerardo García-Melcón, María Soledad Acedo-Gutiérrez, and Teresa Blanco-Moya. The authors would like to express their gratitude to Xavier Gómez-Batiste for his immense contributions to the field of palliative care and thanks to the Catalan Institute of Oncology for the authorization to use the NECPAL CCOMS-ICO tool.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NECPAL CCOMS-ICO | NECesidades PALiativas-Centro Colaborador de la Organización Mundial de la Salud-Institut Català d’Oncologia. |
| PC | Palliative Care. |
| SQ | Surprise Question. |
References
- Brennan, P.; Perola, M.; van Ommen, G.J.; Riboli, E.; Onland-Moret, N.C.; Tjonneland, A. Chronic disease research in Europe and the need for integrated population cohorts. Eur. J. Epidemiol. 2017, 32, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, J.; Wolf, J.; Ostgathe, C.; Toepelt, K.; Glossmann, J.-P.; Hallek, M.; Voltz, R. Specifying WHO recommendation: Moving toward disease-specific guidelines. J. Palliat. Med. 2010, 13, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. WHO Definition of Palliative Care. Available online: https://www.who.int/news-room/fact-sheets/detail/palliative-care (accessed on 30 October 2024).
- Vanbutsele, G.; Pardon, K.; Van Belle, S.; Surmont, V.; De Laat, M.; Colman, R.; Eecloo, K.; Cocquyt, V.; Geboes, K.; Deliens, L. Effect of early and systematic integration of palliative care in patients with advanced cancer: A randomised controlled trial. Lancet Oncol. 2018, 19, 394–404. [Google Scholar] [CrossRef]
- Zimmermann, C.; Swami, N.; Krzyzanowska, M.; Hannon, B.; Leighl, N.; Oza, A.; Moore, M.; Rydall, A.; Rodin, G.; Tannock, I.; et al. Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 2014, 383, 1721–1730. [Google Scholar] [CrossRef]
- Hui, D.; Hannon, B.L.; Zimmermann, C.; Bruera, E. Improving patient and caregiver outcomes in oncology: Team-based, timely, and targeted palliative care. CA Cancer J. Clin. 2018, 68, 356–376. [Google Scholar] [CrossRef]
- Gaertner, J.; Siemens, W.; Meerpohl, J.J.; Antes, G.; Meffert, C.; Xander, C.; Stock, S.; Mueller, D.; Schwarzer, G.; Becker, G. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: Systematic review and meta-analysis. BMJ 2017, 357, j2925. [Google Scholar] [CrossRef]
- Gomes, B.; Calanzani, N.; Curiale, V.; McCrone, P.; Higginson, I.J. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst. Rev. 2013, 2013, CD007760. [Google Scholar] [CrossRef]
- Kavalieratos, D.; Corbelli, J.; Zhang, D.; Dionne-Odom, J.N.; Ernecoff, N.C.; Hanmer, J.; Hoydich, Z.P.; Ikejiani, D.Z.; Klein-Fedyshin, M.; Zimmermann, C.; et al. Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA 2016, 316, 2104–2114. [Google Scholar] [CrossRef]
- Allsop, M.J.; Ziegler, L.E.; Mulvey, M.R.; Russell, S.; Taylor, R.; Bennett, M.I. Duration and determinants of hospice-based specialist palliative care: A national retrospective cohort study. Palliat. Med. 2018, 32, 1322–1333. [Google Scholar] [CrossRef]
- Boyd, K.; Murray, S.A. Recognising and managing key transitions in end of life care. BMJ 2010, 341, c4863. [Google Scholar] [CrossRef] [PubMed]
- Maas, E.A.; Murray, S.A.; Engels, Y.; Campbell, C. What tools are available to identify patients with palliative care needs in primary care: A systematic literature review and survey of European practice. BMJ Support. Palliat. Care 2013, 3, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.I.; Mitchell, G.; Francis, L.; van Driel, M.L. What diagnostic tools exist for the early identification of palliative care patients in general practice? A systematic review. J. Palliat. Care 2015, 31, 118–123. [Google Scholar] [CrossRef] [PubMed]
- ElMokhallalati, Y.; Bradley, S.H.; Chapman, E.; Ziegler, L.; Murtagh, F.E.; Johnson, M.J.; Bennett, M.I. Identification of patients with potential palliative care needs: A systematic review of screening tools in primary care. Palliat. Med. 2020, 34, 989–1005. [Google Scholar] [CrossRef]
- Gómez-Batiste, X.; Martínez-Muñoz, M.; Blay, C.; Amblàs, J.; Vila, L.; Costa, X. Identification of people with chronic advanced diseases and need of palliative care in sociosanitary services: Elaboration of the NECPAL CCOMS-ICO© tool. Med. Clin. 2013, 140, 241–245. [Google Scholar] [CrossRef]
- Turrillas, P.; Peñafiel, J.; Tebé, C.; Amblàs-Novellas, J.; Gómez-Batiste, X. NECPAL prognostic tool: A palliative medicine retrospective cohort study. BMJ Support. Palliat. Care 2021, 14, e1909–e1918. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Cabarcos Cazón, A.; Astudillo, W. New Criteria for Health Care Performance in Terminal Illness. Available online: https://paliativossinfronteras.org/wp-content/uploads/02-NUEVOS-CRITERIOS-PARA-LA-ACTUACION-SANITARIA-EN-LA-TERMINALIDAD-Cabarcos_1.pdf (accessed on 15 November 2023).
- Crawford, G.; Dzierżanowski, T.; Hauser, K.; Larkin, P.; Luque-Blanco, A.; Murphy, I.; Puchalski, C.; Ripamonti, C. Care of the adult cancer patient at the end of life: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100225. [Google Scholar] [CrossRef]
- Sanders, J.J.; Temin, S.; Ghoshal, A.; Alesi, E.R.; Ali, Z.V.; Chauhan, C.; Cleary, J.F.; Epstein, A.S.; Firn, J.I.; Jones, J.A.; et al. Palliative Care for Patients with Cancer: ASCO Guideline Update. J. Clin. Oncol. 2024, 42, 2336–2357. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Gómez-Batiste, X.; Martínez-Muñoz, M.; Blay, C.; Amblàs, J.; Vila, L.; Costa, X.; Espaulella, J.; Villanueva, A.; Oller, R.; Martori, J.C.; et al. Utility of the NECPAL CCOMS-ICO© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: A cohort study. Palliat. Med. 2017, 31, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Spannella, F.; Falzetti, S.; Giulietti, F.; Sarnari, S.; Morichi, V.; Tamburrini, P.; Gattafoni, P.; Mannello, L.; Crippa, M.; Ferrara, L.; et al. Prognostic Role of NECPAL CCOMS-ICO Tool on One-Year Mortality in a Hospitalized Older Population. J. Palliat. Med. 2024, 27, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Amblàs-Novellas, J.; Murray, S.A.; Espaulella, J.; Martori, J.C.; Oller, R.; Gómez-Batiste, X. Identifying patients with advanced chronic conditions for a progressive palliative care approach: A cross-sectional study of indicators related to end-of-life trajectories. BMJ Open 2016, 6, e012340. [Google Scholar] [CrossRef]
- Gómez-Batiste, X.; Blay, C.; Broggi, M.A.; Villa, A.; Costa, X.; Espinosa, J.; Espaulella, J.; Amblàs-Novellas, J.; Martí, C.; Oller, R.; et al. Ethical Challenges of Early Identification of Advanced Chronic Patients in Need of Palliative Care. J. Palliat. Care 2018, 33, 247–251. [Google Scholar] [CrossRef]
- Gomez-Batiste, X.; Martinez-Munoz, M.; Blay, C.; Amblàs-Novellas, J.; Vila, L.; Costa, X.; Espaulella, J.; Espinosa, J.; Constante, C.; Mitchell, G.K. Identifying patients with chronic conditions in need of palliative care in the general population: Development of the NECPAL tool and preliminary prevalence rates in Catalonia. BMJ Support. Palliat. Care 2013, 3, 300–3008. [Google Scholar] [CrossRef]
- Thoonsen, B.; Vissers, K.; Verhagen, S.; Engels, Y.; van Weel, C.; Groot, M.; Vernooij-Dassen, M. Training general practitioners in early identification and anticipatory palliative care planning: A randomized controlled trial. BMC Fam. Pract. 2015, 16, 126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).