Development of an Application Method for Volatile Compounds Derived from Mushroom Fungi Beds as Plant Growth-Promoting Biostimulants
Abstract
1. Introduction
2. Experimental Design
2.1. FBs Types and Sources
- Shiitake (Lentinula edodes): Mori XR1 strain. FBs cultivated under standardized commercial conditions for shiitake mushroom production.
- Enokitake (Flammulina filiformis): Mori No.75 strain. FBs cultivated under standardized commercial conditions for shiitake mushroom production.
- Young shiitake FBs (YSFB)—initial colonization with minimal fruiting;
- Mature shiitake FBs (MSFB)—peak fruiting and harvest stage;
- Waste shiitake FBs (WSFB)—post-harvest substrates considered exhausted but containing residual mycelium.
2.2. Plant Material and Culture Conditions
- Plant material: Rice seeds (Oryza sativa L. cv. Nipponbare) were used in this study. Rice seeds used in this study were harvested under controlled greenhouse conditions during autumn 2021.
- Culture soil: Rice seedlings were grown in Granular Kumiai Synthetic Culture Soil No. 3 (TAKARA Industry Co., Ltd., Tokyo, Japan), characterized by consistent nutrient content (N: 1.5 g, P: 2.7 g, K: 1.5 g per 2.7–3.0 kg soil). To normalize the soil pH, we added calcium silicate (JA ZEN-NOH Co., Ltd., Tokyo, Japan) using the standard 1/4 spoon (0.25 cc capacity, 108 mm overall length; Wadasuke Manufacturing Co., Ltd., Niigata, Japan). The soil was sterilized using an autoclave (HICLAVE HVE-50, HIRAYAMA Manufacturing Co., Ltd., Tokyo, Japan) at 121 °C, 0.20 Mpa for 20 min, and then the soil was kept to room temperature until use. Soil moisture was maintained at 70% capacity using sterile distilled water.
- Growth conditions: For the germination, incubate the Petri dishes with seeds in a growth chamber at 28 °C under a 13 h light/11 h dark photo period without any humidity control (PHCbi MLR-352, PHC Corp., Tokyo, Japan). Maintain a photosynthetic photon flux density (PPFD) of 50 μmol m−2 s−1. For the seedling cultivation, seedlings in a growth chamber were cultivated under a light cycle and temperature of 13 h light at 27 °C/11 h dark at 23 °C, PPFD 123 µmol m−2 s−1, and a relative humidity of 60% (FLI-2000A).
2.3. Chemical Solutions
- Sodium hypochlorite (NaClO), used for surface sterilization of rice seeds;
- Ethanol, used for sterilization of hands, utensils, surfaces and for sterilizing metal textiles;
- 3-octanone (>98.0% (GC) purity, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan);
- 3-octanol (>98.0% (GC) purity, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan);
- 1-octen-3-one (>95.0% (GC) purity, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan);
- 1-octen-3-ol (>98.0% (GC) purity, Tokyo Chemical Industry Co., Ltd., Tokyo, Japan).
2.4. Equipment
- Growth chamber for germination (PHCbi MLR-352, PHC Corp., Tokyo, Japan);
- Growth chamber for VCs exposure test (FLI-2000A; TOKYO RIKAKIKAI Co., Ltd., Tokyo, Japan);
- Alcohol lamp (Maruemu Corp., Osaka, Japan);
- Paper towel, Kim Towel (Nippon Paper Crecia Co., Ltd., Tokyo, Japan);
- Sterile disposable plastic Petri dishes (ϕ90 × 15 mm, Az One Corp., Tokyo, Japan);
- Autoclave (HICLAVE HVE-50, HIRAYAMA Manufacturing Corp., Tokyo, Japan);
- GA-7 Magenta boxes (77 × 77 × 97 mm; Merck KgaA, Darmstadt, Germany);
- LI-250A Light meter (LI-COR Environmental, Lincoln, NE, USA);
- Filter paper (MerkMillipore, Merck KgaA, Burlington, MA, USA, CAT No. RAWP02500);
- Polydimethylsiloxane/Divinylbenzene (PDMS/DVB) fiber assemblies of 65 μm (Pink/plain) (Supelco®, Merck KgaA, Darmstadt, Germany);
- Jeol JMS-T100GCV type GC-TOF Mass Spectrometer (JEOL Co., Ltd., Datum Solution Division MS Service Department, Tokyo Japan);
- Agilent 7890A GC System with FID, serial number CN14333069 (Agilent Technologies, Santa Clara, CA, USA);
- Plastic containers of 3 L (Sanada Seiko Co., Ltd., Osaka, Japan);
- High-density polyethylene (HDPE) film of 10 μm thickness (Fukusuke Kogyo Co., Ltd., Ehime, Japan);
- Polyvinyl chloride (PVC) film of 9 μm thickness (Pack Style, Co., Ltd., Aichi, Japan);
- A 18-8 Extra Thick Measuring Spoon 1/4 Spoon (Wadasuke Co., Ltd., Niigata, Japan);
- Clear glass vial (Supelco®) of 15 mL with a screw cap fitted with a PTFE/silicone septum (Merck KgaA, Darmstadt, Germany).
- Analytical balance (Sartorius Entris II BCE124i-1S, Merck KgaA, Darmstadt, Germany).
- Freeze dryer, (EYELA FD-5N, TOKYO RIKAKIKAI Co., Ltd., Tokyo, Japan)
3. Procedure
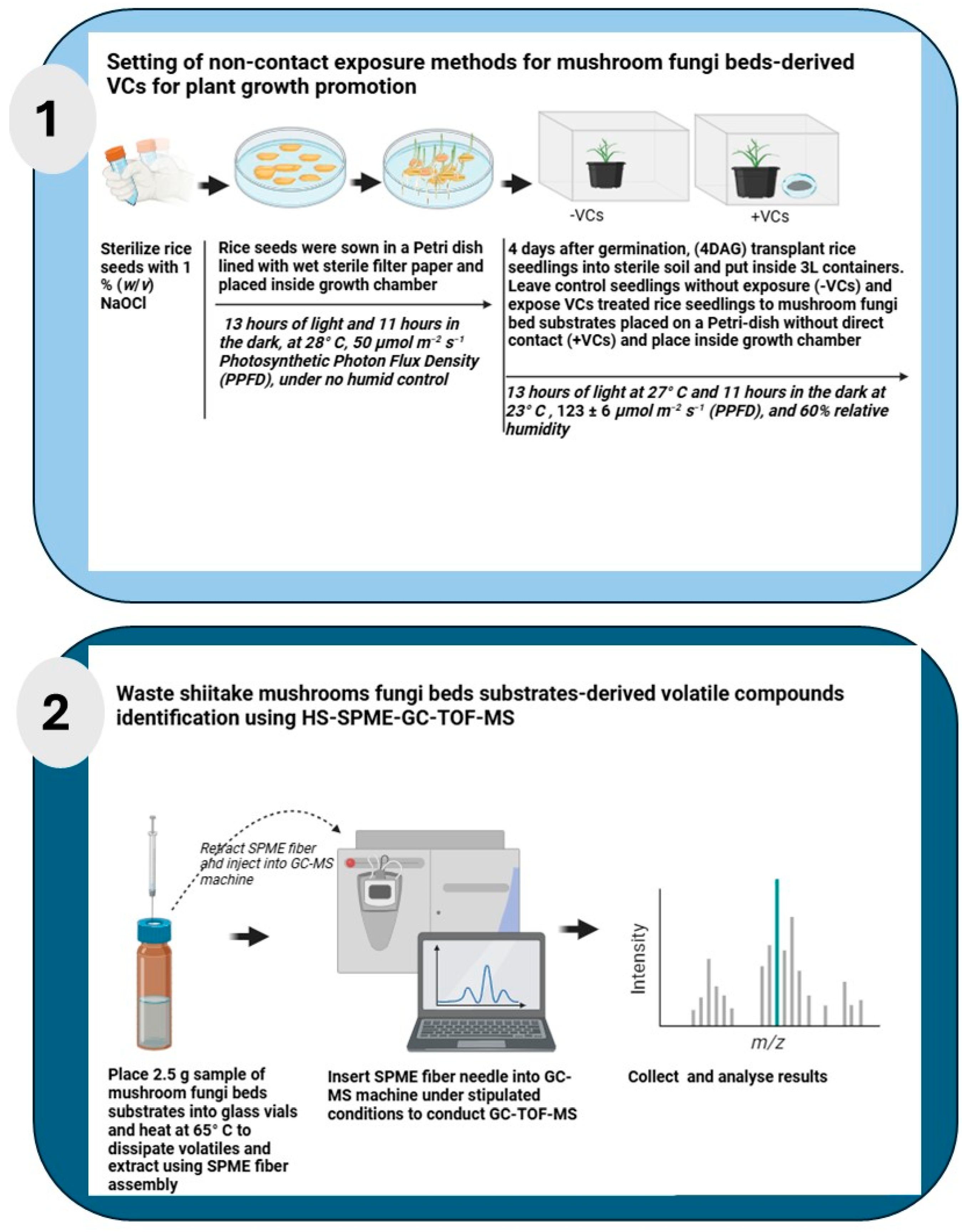
3.1. Setting of Non-Contact Exposure Methods for Mushroom FBs
3.1.1. Preparation of Rice Seedlings
- Sterilize seeds: Surface sterilize rice seeds using 1% (w/v) sodium hypochlorite for 15 min. Rinse the seeds five times with sterile distilled water.
- Germinate seeds: Place sterilized seeds onto four-ply sterile Kim Towel moistened with 10 mL sterile distilled water in a Petri dish and sealed with surgical tape. Incubate the Petri dishes with seeds in a growth chamber for 4 days.
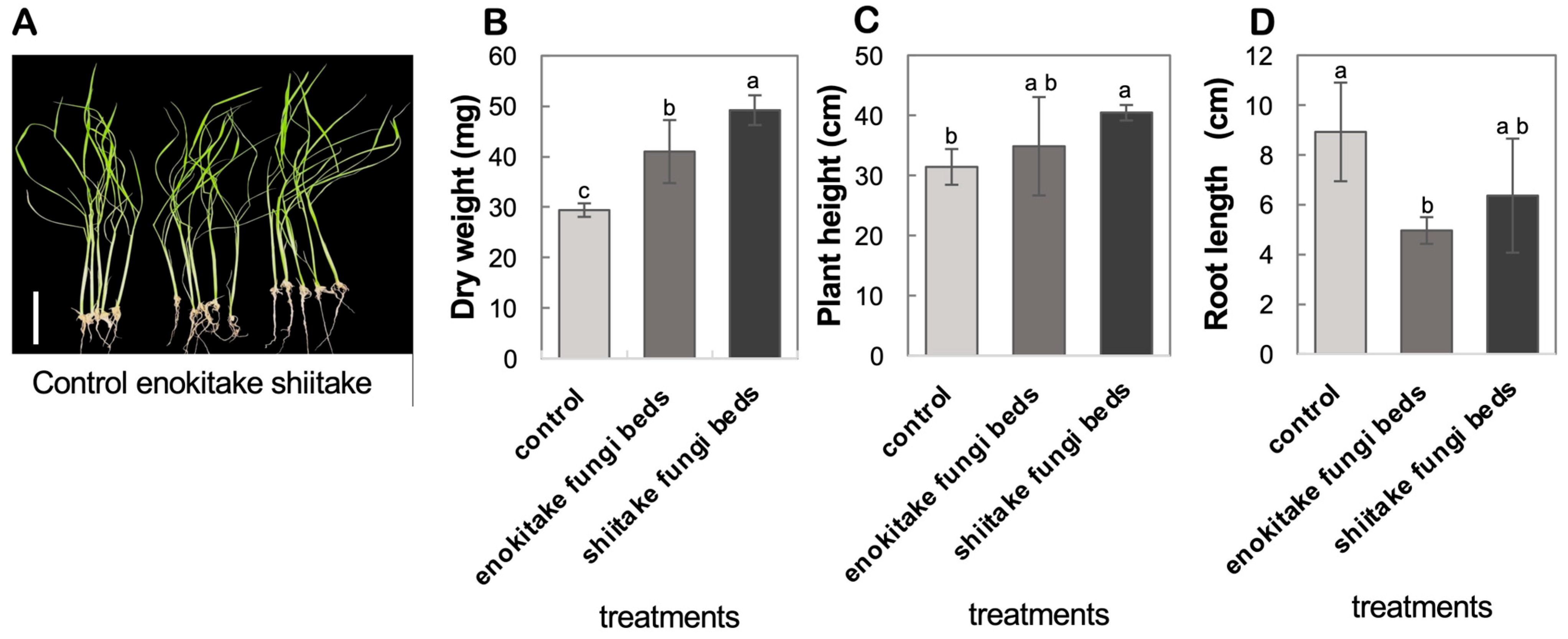
3.1.2. Seedling Cultivation with Enokitake or Shiitake VCs Treatment
- 1.
- Transplant seedlings: Transfer five of the 4-day-old germinated rice seedlings into a Magenta box containing 200 g of sterile Kumiai synthetic culture soil with calcium silicate.
- CRITICAL STEP: Only use germinated seeds of uniform germination stage. Handle seedlings gently to avoid root damage.
- 2.
- For the VCs treatment group (+VCs), place an open Petri dish containing 15 g of FBs in a 3 L plastic container alongside the seedlings that you sowed in the magenta box in step 1, above.
- 3.
- For the control group (−VCs), place seedlings in the filled Magenta box in identical containers without FBs.
- CRITICAL STEP: Ensure no direct contact between the FBs and seedlings to maintain non-contact VCs exposure.
- 4.
- Place containers in a growth chamber for 14 days.
- 5.
- Harvest and measure seedlings: After 14 days, harvest the seedlings and measure key growth parameters:
- Plant height (cm): Measure from the base of the plant to the apex of the uppermost leaf.
- Root length (cm): Measure from the apex of the longest root to the base of the plant.
- Dry weight (mg): Freeze-dry seedlings for 24 h using an EYELA FD-5N freeze dryer and weigh using a Sartorius Entris II BCE124i-1S analytical balance.
- PAUSE STEP: Dried samples can be stored in desiccators for up to 1 week before weighing.
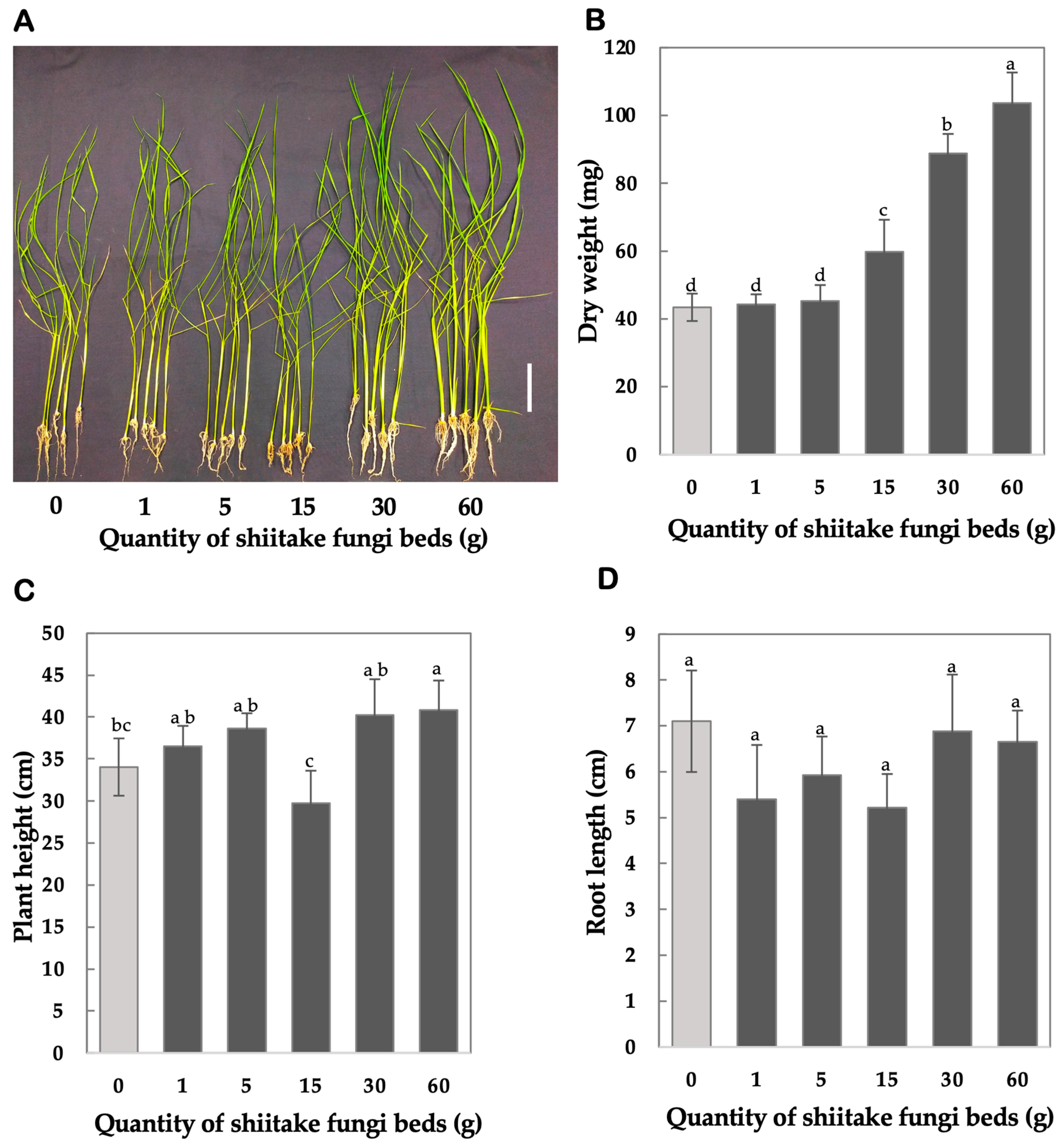
3.1.3. Dose Optimization for Shiitake FBs
- 1.
- Germinate seeds: seed germination was performed as described in Section 3.1.1.
- 2.
- Set up dose treatments: Prepare six treatment groups by placing open Petri dishes containing varying quantities of shiitake FBs (0 g, 1 g, 5 g, 15 g, 30 g, and 60 g) in separate 3 L plastic containers. For the control group (0 g, −VCs), place seedlings in identical containers without FBs. And for the VCs treatment groups (+VCs), place Petri dishes with the specified quantities of FBs alongside the seedlings in each container.
- 3.
- Transplanting seedlings and seedling cultivation, harvest, and measurement were carried out as described in Section 3.1.2.
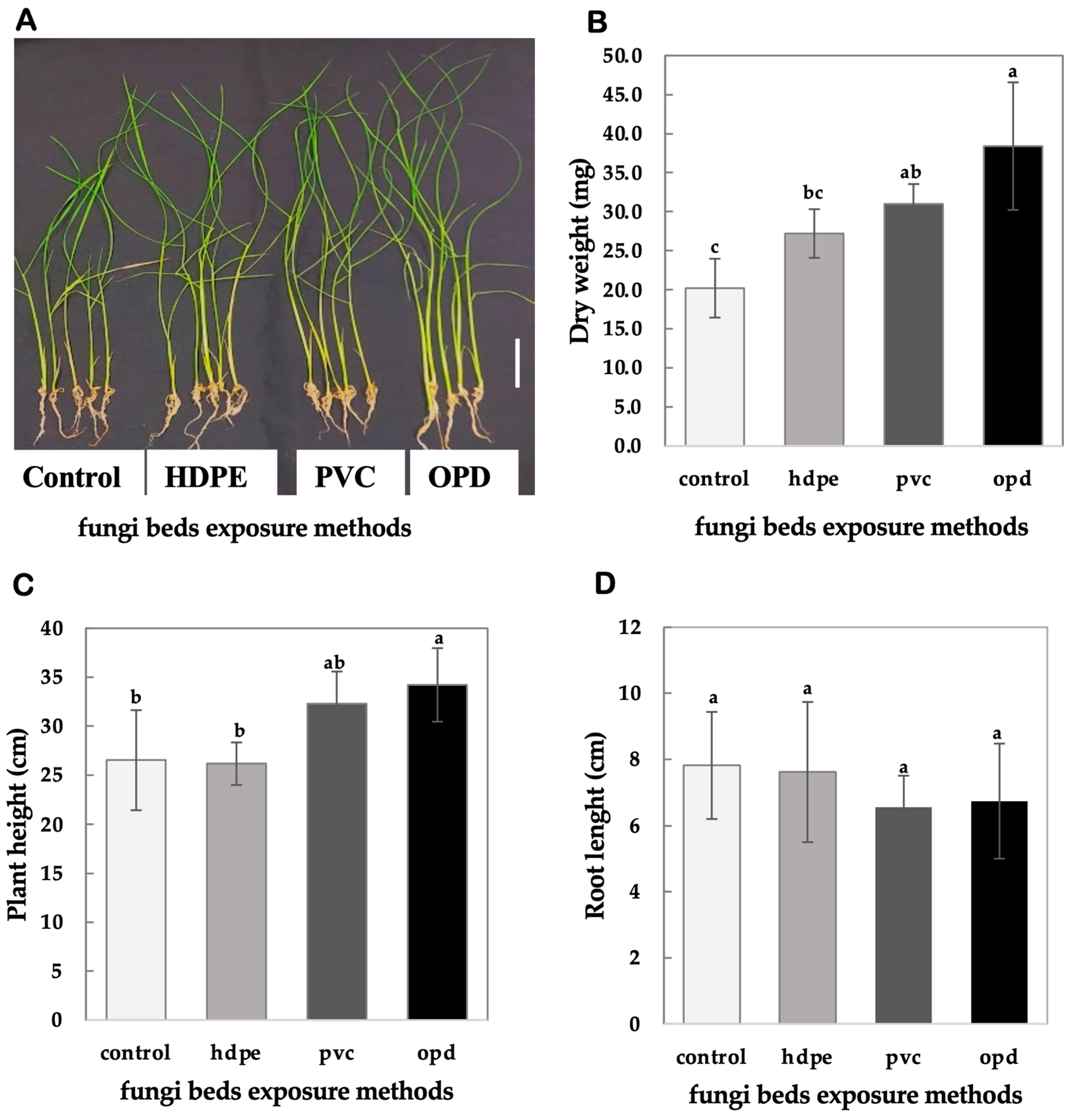
3.1.4. Comparison of Fungi Bed Exposure Methods
- Germinate seeds: seed germination was performed as described in Section 3.1.1.
- Test exposure methods: Prepare 60 g of Shiitake FBs and evaluate three treatments:
- HDPE Wrapping: Wrap FBs with 10 μm thick HDPE film (Fukusuke Kogyo Co., Ltd., Ehime, Japan).
- PVC Wrapping: Wrap FBs with 9 μm thick PVC film (Pack Style Co., Ltd., Aichi, Japan).
- Open Petri Dish (OPD): Leave FBs unwrapped in an open Petri dish (OPD).
- 3.
- Transplanting seedlings and seedling cultivation, harvest, and measurement were carried out as described in Section 3.1.2.
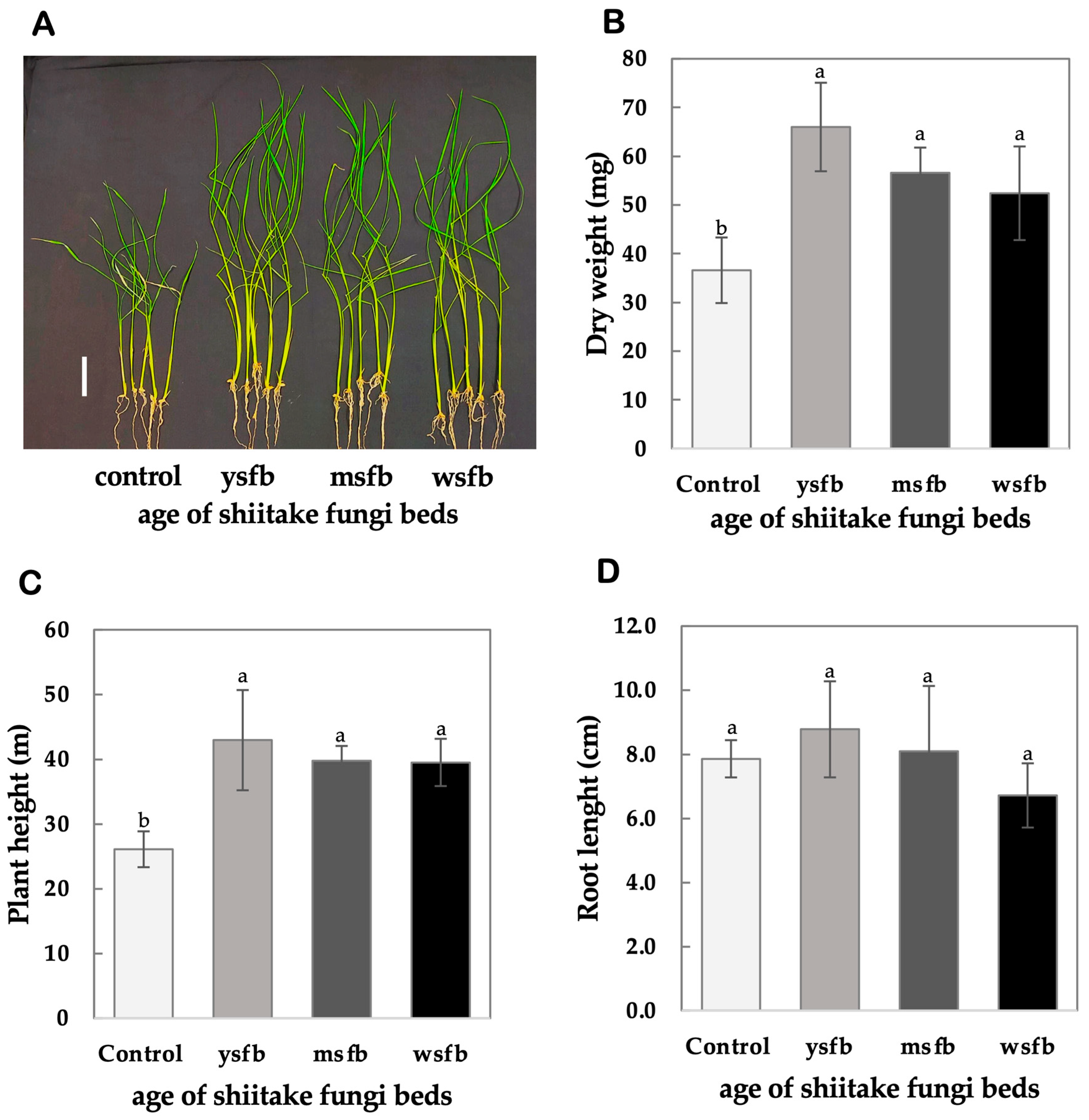
3.1.5. Testing the Effects of Fungi Bed Aging
- Seed germination was performed as described in Section 3.1.1.
- Set up treatments: Divide seedlings into control (−VCs) and treatment (+VCs) groups. In the treatment group, expose seedlings to 60 g of shiitake FBs of different ages, categorized as YSFB, MSFB, and WSFB.
- Transplanting seedlings and seedling cultivation, harvest, and measurement were carried out as described in Section 3.1.2.
3.2. Waste Shiitake Mushroom FBs Substractes-Derived VCs
3.2.1. Volatile Compound Identification Via HS-SPME-GC-TOF-MS
- Prepare fungi bed samples: Collect 2.5 g of WSFBs using a sterile knife. Place samples into 20 mL screw-cap vials and seal with silicone septa.
- Extract volatiles: Heat vials in a 60 °C water bath for 10 min to dissipate the VCs. Then, insert SPME fiber assembly and extract volatiles. Incubate vials at 28 °C in a growth chamber for 24 h.
- Analyze samples: Analyze extracted volatiles using a GC-TOF-MS with an Rtx-Wax capillary column. Use the parameters specified in Supplementary Table S1.
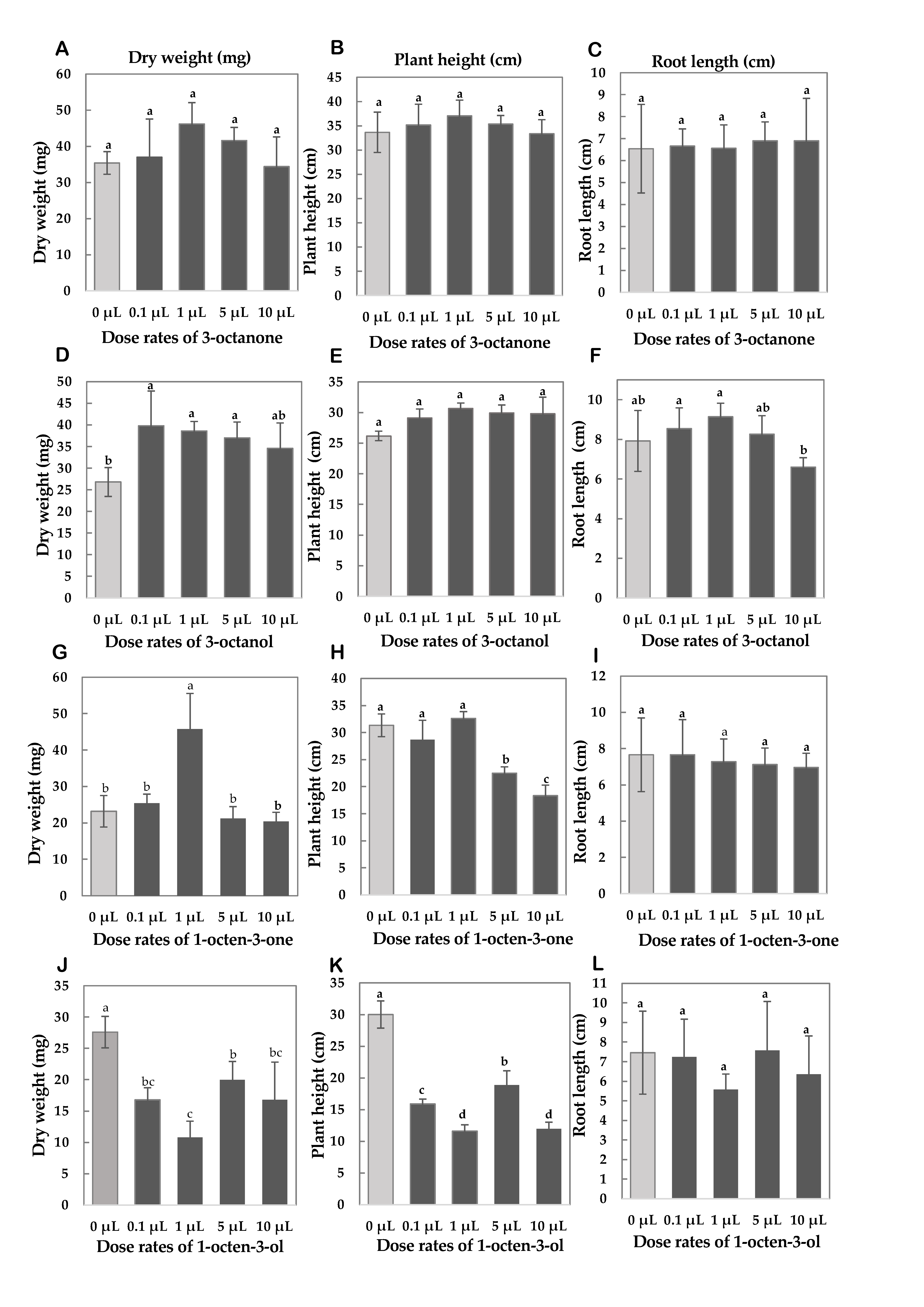
3.2.2. Testing Synthetic Volatile Compounds
- 1.
- Germinate seeds: seed germination was performed as described in Section 3.1.1.
- 2.
- Prepare synthetic volatiles: Synthetic VCs exposure experiments were implemented using 3-octanone, 3-octanol, 1-octen-3-ol, and 1-octen-3-one, respectively. All synthetic VCs were solution type and analytical grade and used without further purification.
- 3.
- Mount the compound on filter paper: Test the compound at dose rates of 0.1 μL, 1 μL, 5 μL, and 10 μL. Drop the compounds onto the filter paper, place them on a Petri dish, and immediately place the Petri dish into 3 L containers.
- 4.
- Expose seedlings: Place Petri dishes inside 3 L containers housing rice seedlings in Magenta boxes.
- CRITICAL STEP: Resupply synthetic VCs on day 7 to maintain exposure.
- 5.
- Transplanting seedlings and seedling cultivation, harvest, and measurement were carried out as described in Section 3.1.2.
3.3. Workflow and Experimental Design
- Types of edible mushroom for FBs—enokitake and shiitake FBs (15 g each);
- Dose optimization using different weight quantities of shiitake FBs—1 g, 5 g, 15 g, 30 g, and 60 g;
- Cover FBs with gas-permeable films;
- FBs maturity VCs tests—YSFB, MSFB, and WSFB;
- Synthetic VCs—compounds derived from waste shiitake FBs.
3.4. Statistical Analysis
4. Results
4.1. Application of Method for Short-Term Seedling Cultivation with VCs from Edible Mushroom FBs Resulted in Significant Biomass Increase
4.2. Results from Dose Optimization for Shiitake FBs
4.3. Comparison of Fungi Bed Exposure Methods
4.4. Testing the Effects of Fungi Bed Aging
4.5. Results for Volatile Compound Identification Via HS-SPME-GC-TOF-MS
4.6. Testing Synthetic Volatile Compounds
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fincheira, P.; Quiroz, A.; Tortella, G.; Diez, M.C.; Rubilar, O. Current advances in plant-microbe communication via volatile organic compounds as an innovative strategy to improve plant growth. Microbiol. Res. 2021, 247, 126726. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal volatile organic compounds: More than just a funky smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Jiang, L.; Lee, M.H.; Kim, C.Y.; Kim, S.W.; Kim, P.I.; Min, S.R.; Lee, J. Plant growth promotion by two volatile organic compounds emitted from the fungus Cladosporium halotolerans NGPF1. Front. Plant Sci. 2021, 12, 794349. [Google Scholar] [CrossRef]
- Rao, Y.; Zeng, L.; Jiang, H.; Mei, L.; Wang, Y. Trichoderma atroviride LZ42 Releases volatile organic compounds promoting plant growth and suppressing Fusarium wilt disease in tomato seedlings. BMC Microbiol. 2022, 22, 88. [Google Scholar] [CrossRef]
- You, J.; Li, G.; Li, C.; Zhu, L.; Yang, H.; Song, R.; Gu, W. Biological control and plant growth promotion by volatile organic compounds of Trichoderma koningiopsis T-51. J. Fungi 2022, 8, 131. [Google Scholar] [CrossRef]
- Fiers, M.; Lognay, G.; Fauconnier, M.-L.; Jijakli, M.H. Volatile compound-mediated interactions between barley and pathogenic fungi in the soil. PLoS ONE 2013, 8, e66805. [Google Scholar] [CrossRef]
- Fiddaman, P.J.; Rossall, S. The production of antifungal volatiles by Bacillus subtilis. J. Appl. Bacteriol. 1993, 74, 119–126. [Google Scholar] [CrossRef]
- Mackie, A.E.; Wheatley, R.E. Effects and Incidence of volatile organic compound interactions between soil bacterial and fungal isolates. Soil Biol. Biochem. 1999, 31, 375–385. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Alborn, H.T.; Tumlinson, J.H. In vivo volatile emissions from peanut plants induced by simultaneous fungal infection and insect damage. J. Chem. Ecol. 2002, 28, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Humphris, S.N.; Bruce, A.; Buultjens, E.; Wheatley, R.E. The effects of volatile microbial secondary metabolites on protein synthesis in Serpula lacrymans. FEMS Microbiol. Lett. 2002, 210, 215–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stinson, M.; Ezra, D.; Hess, W.M.; Sears, J.; Strobel, G. An endophytic Gliocladium Sp. of Eucryphia cordifolia producing selective volatile antimicrobial compounds. Plant Sci. 2003, 165, 913–922. [Google Scholar] [CrossRef]
- Bruce, A.; Stewart, D.; Verrall, S.; Wheatley, R.E. Effect of volatiles from bacteria and yeast on the growth and pigmentation of sapstain fungi. Int. Biodeterior. Biodegrad. 2003, 51, 101–108. [Google Scholar] [CrossRef]
- Chaurasia, B.; Pandey, A.; Palni, L.M.S.; Trivedi, P.; Kumar, B.; Colvin, N. Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol. Res. 2005, 160, 75–81. [Google Scholar] [CrossRef]
- Dandurishvili, N.; Toklikishvili, N.; Ovadis, M.; Eliashvili, P.; Giorgobiani, N.; Keshelava, R.; Tediashvili, M.; Vainstein, A.; Khmel, I.; Szegedi, E.; et al. Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumours on tomato plants. J. Appl. Microbiol. 2011, 110, 341–352. [Google Scholar] [CrossRef]
- Atmosukarto, I.; Castillo, U.; Hess, W.M.; Sears, J.; Strobel, G. Isolation and characterization of Muscodor albus I-41.3s, a volatile antibiotic producing fungus. Plant Sci. 2005, 169, 854–861. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, S.; Pandey, S.; Bhandari, M.S.; Pandey, A.; Giri, K. Biocontrol potential of Pseudomonas azotoformans, Serratia marcescens and Trichoderma virens against fusarium wilt of Dalbergia sissoo. For. Pathol. 2020, 50, e12581. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Fialho, M.B.; Toffano, L.; Pedroso, M.P.; Augusto, F.; Pascholati, S.F. Volatile organic compounds produced by Saccharomyces cerevisiae inhibit the in vitro development of Guignardia citricarpa, the causal agent of citrus black spot. World J. Microbiol. Biotechnol. 2010, 26, 925–932. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Cai, J.; Liu, X.; Huang, G. Antifungal activity of volatile compounds generated by endophytic fungi Sarocladium brachiariae HND5 against Fusarium oxysporum f. Sp. Cubense. PLoS ONE 2021, 16, e0260747. [Google Scholar] [CrossRef]
- Inayati, A.; Sulistyowati, L.; Aini, L.Q.; Yusnawan, E. Antifungal activity of volatile organic compounds from Trichoderma virens. In Proceedings of the International Conference on Biology and Applied Science (ICOBAS), Malang, Indonesia, 13–14 March 2019. [Google Scholar]
- Gutiérrez-Luna, F.M.; López-Bucio, J.; Altamirano-Hernández, J.; Valencia-Cantero, E.; De La Cruz, H.R.; Macías-Rodríguez, L. Plant growth-promoting Rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 2010, 51, 75–83. [Google Scholar] [CrossRef]
- Blom, D.; Fabbri, C.; Eberl, L.; Weisskopf, L. Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl. Environ. Microbiol. 2011, 77, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hung, R.; Yap, M.; Bennett, J.W. Age Matters: The effects of volatile organic compounds emitted by Trichoderma atroviride on plant growth. Arch. Microbiol. 2015, 197, 723–727. [Google Scholar] [CrossRef]
- Duc, N.H.; Vo, H.T.N.; van Doan, C.; Hamow, K.Á.; Le, K.H.; Posta, K. Volatile organic compounds shape belowground plant–fungi interactions. Front. Plant Sci. 2022, 13, 1046685. [Google Scholar] [CrossRef]
- Şesan, T.E.; Oancea, A.O.; Ştefan, L.M.; Mănoiu, V.S.; Ghiurea, M.; Răut, I.; Constantinescu-Aruxandei, D.; Toma, A.; Savin, S.; Bira, A.F.; et al. Effects of foliar treatment with a Trichoderma plant biostimulant consortium on Passiflora caerulea L. yield and quality. Microorganisms 2020, 8, 123. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O.; Santoyo, G.; Perazzolli, M. The Potential Role of microbial biostimulants in the amelioration of climate change-associated abiotic stresses on crops. Front. Microbiol. 2022, 12, 829099. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.-L.; Sassi, K.; Nasraoui, B.; Jijakli, M.-H. Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef]
- Razo-Belmán, R.; Ángeles-López, Y.I.; García-Ortega, L.F.; León-Ramírez, C.G.; Ortiz-Castellanos, L.; Yu, H.; Martínez-Soto, D. Fungal volatile organic compounds: Mechanisms involved in their sensing and dynamic communication with plants. Front. Plant Sci. 2023, 14, 1257098. [Google Scholar] [CrossRef]
- Fujita, R.; Yokono, M.; Ube, N.; Okuda, Y.; Ushijima, S.; Fukushima-Sakuno, E.; Ueno, K.; Osaki-Oka, K.; Ishihara, A. Suppression of Alternaria brassicicola infection by volatile compounds fromspent mushroom substrates. J. Biosci. Bioeng. 2021, 132, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Fukushima-Sakuno, E.; Ishihara, A.; Osaki-Oka, K. Antimicrobial activity of octan-3-one released from spent mushroom substrate of shiitake (Lentinula edodes) and its inhibitory effects on plant diseases. J. Gen. Plant Pathol. 2023, 89, 122–131. [Google Scholar] [CrossRef]
- Bitas, V.; Kim, H.-S.; Bennett, J.W.; Kang, S. Sniffing on microbes: Diverse roles of microbial volatile organic compounds in plant health. Mol. Plant Microbe. Interact. 2013, 26, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef]
- Naznin, H.A.; Kimura, M.; Miyazawa, M.; Hyakumachi, M. Analysis of volatile organic compounds emitted by plant growth-promoting fungus Phoma Sp. GS8-3 for growth promotion effects on Tobacco. Microb. Environ. 2013, 28, 42–49. [Google Scholar] [CrossRef]
- Song, G.C.; Jeon, J.-S.; Sim, H.-J.; Lee, S.; Jung, J.; Kim, S.-G.; Moon, S.Y.; Ryu, C.-M. Dual functionality of natural mixtures of Bacterial volatile compounds on plant growth. J. Exp. Bot. 2022, 73, 571–583. [Google Scholar] [CrossRef]
- Wood, M.J.; Kortsinoglou, A.M.; Khoja, S.; Kouvelis, V.N.; Myrta, A.; Midthassel, A.; Loveridge, E.J.; Butt, T.M. Metarhizium brunneum (Hypocreales: Clavicipitaceae) and its derived volatile organic compounds as biostimulants of commercially valuable Angiosperms and Gymnosperms. J. Fungi 2022, 8, 1052. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Zhao, Y.; Kakumyan, P. Impact of cultivation substrate and microbial community on improving mushroom productivity: A Review. Biology 2022, 11, 569. [Google Scholar] [CrossRef]
- Kumar, P.; Eid, E.M.; Taher, M.A.; El-Morsy, M.H.E.; Osman, H.E.M.; Al-Bakre, D.A.; Adelodun, B.; Abou Fayssal, S.; Goala, M.; Mioč, B.; et al. Biotransforming the spent substrate of shiitake mushroom (Lentinula edodes berk.): A synergistic approach to biogas production and tomato (Solanum lycopersicum L.) Fertilization. Acta Hortic. 2022, 8, 479. [Google Scholar] [CrossRef]
- Lee, P.; Lin, X.; Khan, F.; Bennett, A.E.; Winter, J.O. Translating controlled release systems from biomedicine to Agriculture. Front. Biomater. Sci. 2022, 1, 1011877. [Google Scholar] [CrossRef]
- Pavithran, R.K.; Reddy, S.G.; Kumar, B.S.; Kugabalasooriar, S. Enhancing sustainability in agriculture: Natural polymer-based controlled release systems for effective pest management and environmental protection. ESFAF 2024, 18, 1276. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.-M. Formulation and agricultural application of bacterial volatile compounds. In Bacterial Volatile Compounds as Mediators of Airborne Interactions; Ryu, C.-M., Weisskopf, L., Piechulla, B., Eds.; Springer: Singapore, 2020; pp. 317–336. ISBN 978-981-15-7293-7. [Google Scholar]
- Aisala, H.; Sola, J.; Hopia, A.; Linderborg, K.M.; Sandell, M. Odor-contributing volatile compounds of wild edible nordic mushrooms analyzed with HS–SPME–GC–MS and HS–SPME–GC–O/FID. Food Chem. 2019, 283, 566–578. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.; Tohamy, A.; Hussein, K. Plant protection properties of the plant growth promoting fungi (PGPF): Mechanisms and potentiality. CREAM 2021, 11, 391–415. [Google Scholar] [CrossRef]
- Zhao, M.; Su, H.; Huang, Y.; Abdugheni, R.; Ma, J.; Gao, J.; Guo, F.; Li, L. Plant growth-promoting properties and anti-fungal activity of endophytic Bacterial strains isolated from Thymus altaicus and Salvia deserta in Arid Lands. J. Arid Land 2023, 15, 1405–1420. [Google Scholar] [CrossRef]
- Cordovez, V.; Mommer, L.; Moisan, K.; Lucas-Barbosa, D.; Pierik, R.; Mumm, R.; Carrion, V.J.; Raaijmakers, J.M. Plant phenotypic and transcriptional changes induced by volatiles from the fungal root pathogen Rhizoctonia solani. Front. Plant Sci. 2017, 8, 1262. [Google Scholar] [CrossRef]
- Estrada-Rivera, M.; Rebolledo-Prudencio, O.G.; Pérez-Robles, D.A.; Rocha-Medina, M.D.C.; González-López, M.D.C.; Casas-Flores, S. Trichoderma Histone Deacetylase HDA-2 modulates multiple responses in arabidopsis. Plant Physiol. 2019, 179, 1343–1361. [Google Scholar] [CrossRef]
- Bitas, V. Volatile Compounds from Soilborne Pathogenic Fungi Affect Plant Growth and Enhance Plant Biotic and Abiotic Stress Resistance. Master’s Thesis, Pennsylvania State University, University Park, PA, USA, 2014. Available online: https://etda.libraries.psu.edu/files/final_submissions/10158 (accessed on 3 March 2025).
- Esparza-Reynoso, S.; Pelagio-Flores, R.; López-Bucio, J. Role of volatile organic compounds in establishment of the Trichoderma–plant interaction. In Plant Rel; Scott, B., Mesarich, C., Eds.; The Mycota; Springer International Publishing: Cham, Switzerland, 2023; Volume 5, pp. 239–252. ISBN 978-3-031-16502-3. [Google Scholar]
- Sheikh, T.M.M.; Zhou, D.; Haider, M.S.; Hussain, S.; Wang, N.; Chen, S.; Zhao, Y.; Wen, X.; Feng, H.; Wang, X.; et al. Volatile organic compounds from Pythium oligandrum play a role in its parasitism on plant-pathogenic Pythium myriotylum. Appl. Environ. Microbiol. 2023, 89, e0203622. [Google Scholar] [CrossRef]
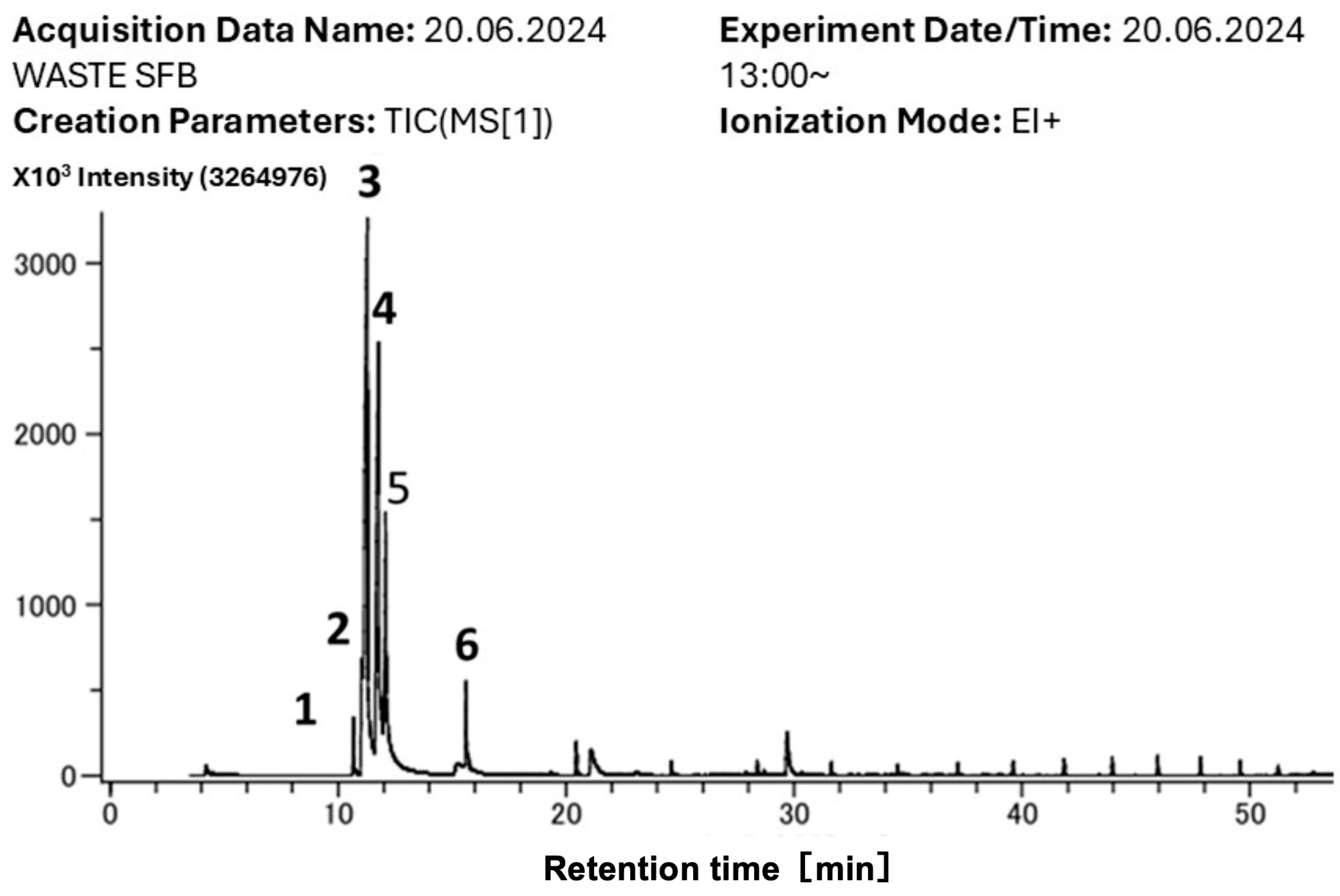
| Peak | RT (min) | Compound Name | Molecular Formula | Molecular Weight (g·mol−1) | Family |
|---|---|---|---|---|---|
| *1 | 10.2 | Octamethylcyclotetrasiloxane | [(CH3)2SiO]4 | 296.62 | Cyclomethicones |
| 2 | 10.3 | 1-octen-3-one | C8H14O | 126.20 | Ketone |
| 3 | 11.8 | 3-octanone | C8H16O | 128.21 | Ketone |
| 4 | 12 | 1-octen-3-ol | C8H16O | 128.21 | Alcohol |
| 5 | 12.1 | 3-octanol | C8H18O | 130.23 | Alcohol |
| *6 | 14.9 | Decamethylcyclopentasiloxane | [(CH3)2SiO]5 | 370.77 | Cyclomethicones |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanga, C.N.; Okisaka, Y.; Hanamata, S.; Ueda, D.; Sato, T.; Mitsui, T.; Itoh, K. Development of an Application Method for Volatile Compounds Derived from Mushroom Fungi Beds as Plant Growth-Promoting Biostimulants. Methods Protoc. 2025, 8, 29. https://doi.org/10.3390/mps8020029
Kanga CN, Okisaka Y, Hanamata S, Ueda D, Sato T, Mitsui T, Itoh K. Development of an Application Method for Volatile Compounds Derived from Mushroom Fungi Beds as Plant Growth-Promoting Biostimulants. Methods and Protocols. 2025; 8(2):29. https://doi.org/10.3390/mps8020029
Chicago/Turabian StyleKanga, Clever N., Yui Okisaka, Shigeru Hanamata, Daijiro Ueda, Tsutomu Sato, Toshiaki Mitsui, and Kimiko Itoh. 2025. "Development of an Application Method for Volatile Compounds Derived from Mushroom Fungi Beds as Plant Growth-Promoting Biostimulants" Methods and Protocols 8, no. 2: 29. https://doi.org/10.3390/mps8020029
APA StyleKanga, C. N., Okisaka, Y., Hanamata, S., Ueda, D., Sato, T., Mitsui, T., & Itoh, K. (2025). Development of an Application Method for Volatile Compounds Derived from Mushroom Fungi Beds as Plant Growth-Promoting Biostimulants. Methods and Protocols, 8(2), 29. https://doi.org/10.3390/mps8020029










