Wild Birds’ Genetic Resources Bank: Feather Follicle Cell Culture as a Possible Source of Stem Cells
Abstract
1. Introduction
2. Experimental Design
2.1. Materials
- 1.
- Adson serrated tip tweezers
- 2.
- Rubbing ethyl alcohol, 70%
- 3.
- Bonn miniature iris scissors or Noyes c (ABC®, Sydney, Australia; Cat. No. RH636004)
- 4.
- Scalpel (Sigma-Aldrich®; Cat. No. S2896)
- 5.
- Conical centrifuge tube, 15 mL (Perfect, Vancouver, WA, USA; Cat. No. 216090015)
- 6.
- Pipette and pipette tips (Olen-Kasvi®, Olen, Belgium; Cat. No. K31-10000)
- 7.
- Pasteur pipette (Olen-Kasvi®; Cat. No. K30-300S)
- 8.
- Cell culture dish, polystyrene treated, 35 mm × 10 mm (Corning®, Corning, NY, USA; Cat. No. CLS430165)
- 9.
- Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco®, Grand Island, NY, USA; Cat. No. 12100046)
- 10.
- Phosphate-buffered saline (PBS, Boston, MA, USA)
- 11.
- Penicillin/streptomycin (Gibco®; Cat. No. 15140122)
- 12.
- Amphotericin B (Gibco®; Cat. No. 15290026)
- 13.
- Fetal bovine serum (Gibco®; Cat. No. 12657029)
- 14.
- Collagenase type IV (Sigma-Aldrich®; Cat. No. C4221G)
- 15.
- Cell culture plates, 6- and 12-well (Corning®; Cat. No. CLS3548)
2.2. Equipment
- 16.
- Luxeo 4D stereomicroscope (LABOMED®, Los Angeles, CA, USA; Cat. No. 4145000)
- 17.
- Biological culture hood (BIOSEG 12®, Cairo, Egypt; Cat. No. 1EAC3772)
- 18.
- Centrifuge for falcon tubes (Kindly® KC4 model, Kent, UK; Cat. No. KC 4-1)
- 19.
- Cell culture incubator (Thermo Scientific®—3 Water Jacketed CO2 Incubator, Waltham, MA, USA; Cat. No. 4110)
- 20.
- Inverted TCM400 microscope (LABOMED® TCM400; Cat. No. 7125000)
- 21.
- EVOS M5000 microscope (Thermo Scientific®; Cat. No. AMF5000SV)
3. Procedure
3.1. Tissue Collection
3.1.1. Preparation of the Collection Materials
- To guarantee sterile conditions and avoid contamination, clean and disinfect the laminar flow and stereomicroscope hood with 70% ethanol.
- Disinfect and autoclave tools (e.g., scissors and tweezers) before use.
- Prepare the culture medium in advance under a laminar flow hood, under sterile conditions: Dulbecco’s Modified Eagle’s Medium with 15% fetal bovine serum, 1% penicillin/streptomycin and 0.5% amphotericin B. Keep refrigerated and warm to 37 °C before use.
- Prepare the washing solution in advance under a laminar flow hood, under sterile conditions: phosphate-buffered saline with 2% penicillin/streptomycin and 1% amphotericin B.
- Prepare the collagenase type IV in a laminar flow hood under sterile conditions and protected from light: dissolve collagenase type IV in Dulbecco’s Modified Eagle’s Medium at a concentration of 1.5 mg/mL and filter-sterilize using a 0.22 µm pore size filter unit.
- Prepare the conical centrifuge tube with a culture medium.
3.1.2. Extraction of Developing Feathers
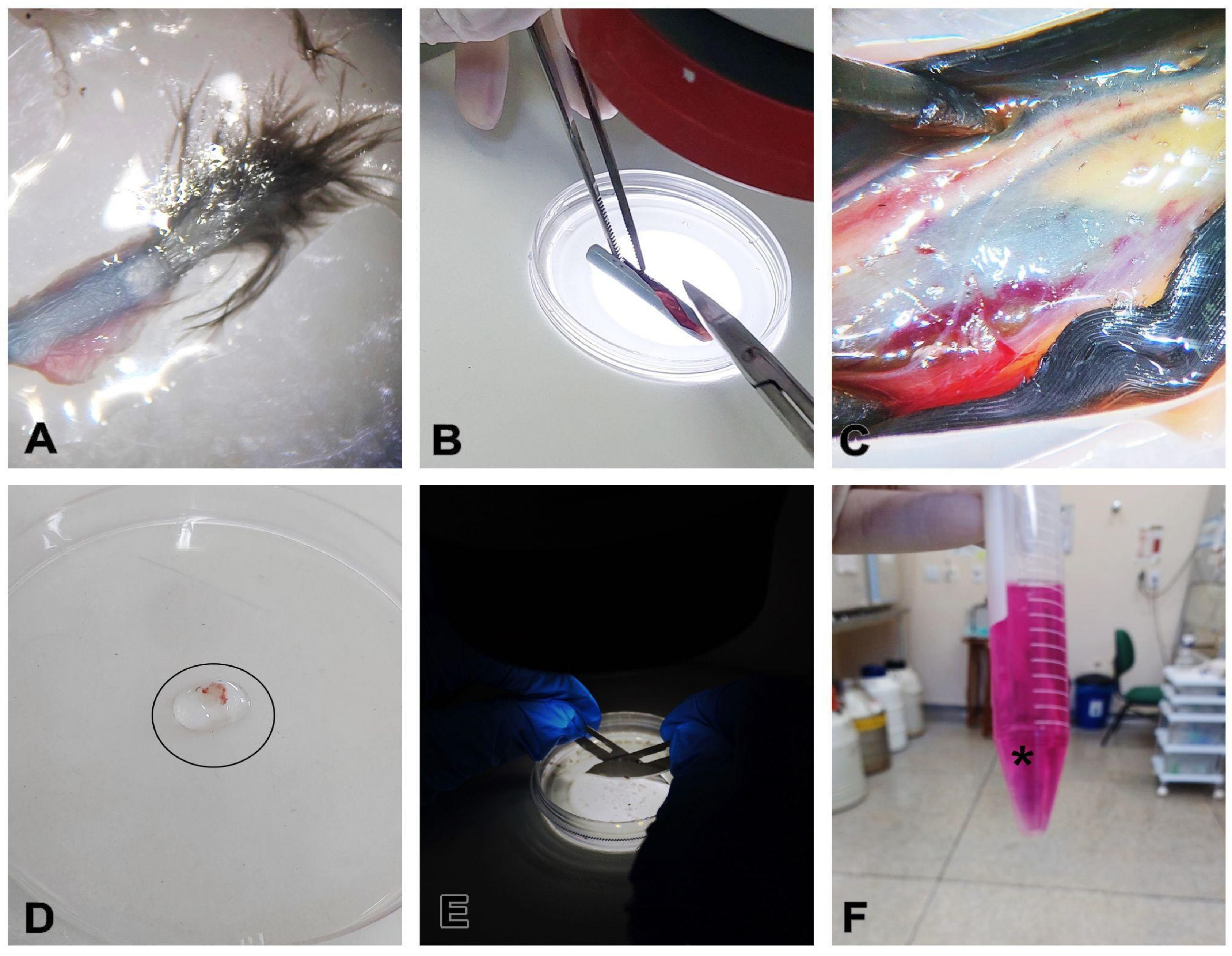
- Select developing feathers which present the feather sheath surrounding them (Figure 2).
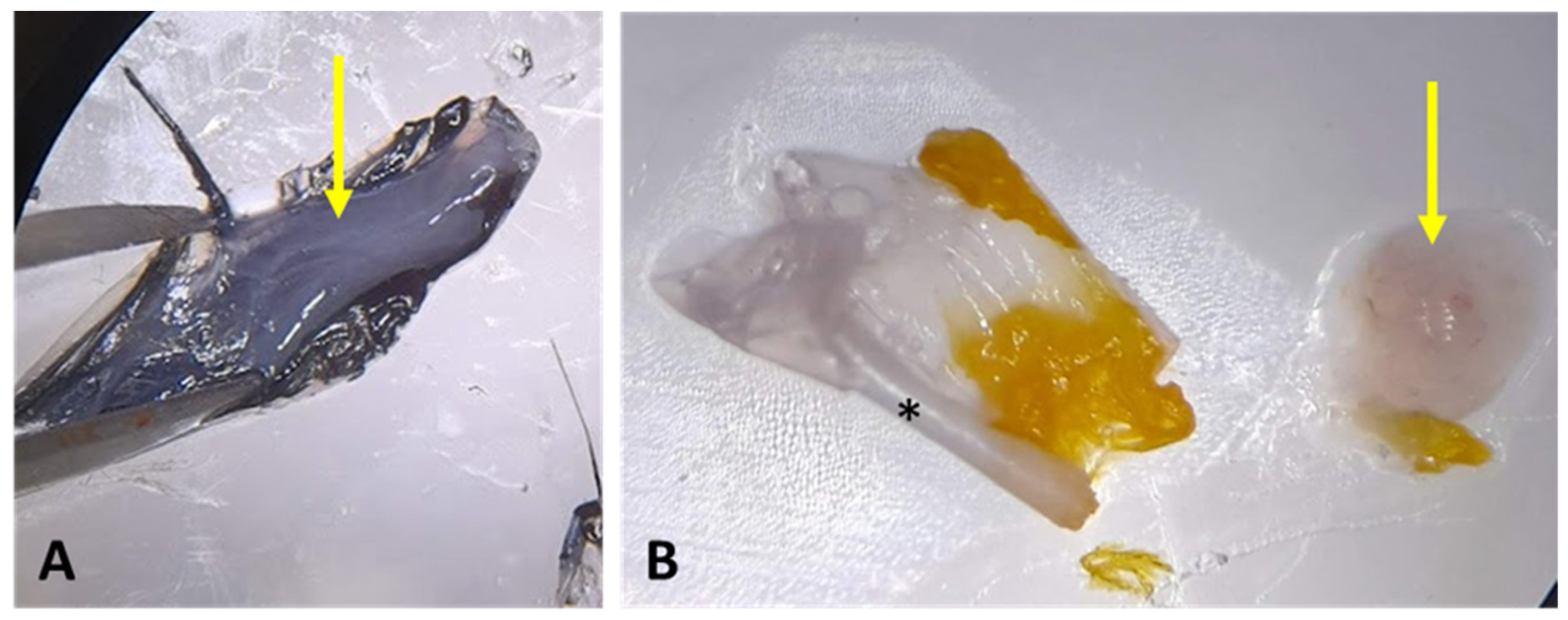
- CRITICAL STEP: Mature feathers present hollow calamus and are not a source of stem cells due to the regression of the feather follicle during the feather development cycle. Make sure to collect developing, immature feathers. The difference between developing and mature feathers is shown in Figure 3A.
- OPTIONAL STEP: Wet the feather area to facilitate the extraction of the feather.
- 2.
- Remove by plucking the developing feathers.
- OPTIONAL STEP: A small incision around the feather may help in the extraction of the feather.
- 3.
- Store plucked feathers at 4 °C in the conical centrifuge tube with medium until use.
3.1.3. Extraction of the Feather Pulp
- In a biological safety cabinet, place the feathers already sectioned in the calamus region in a sterile 60 mm polystyrene Petri dish (Figure 3A).
- Using a stereomicroscope for better visualization, with the aid of small sterile surgical scissors and tweezers, make a longitudinal incision in the calamus of the feather to expose the pulp (Figure 3B,C).
- Scrape the pulp off the calamus and transfer to another Petri dish with PBS (Figure 3C,D).
- Wash the sample 3 times, for 2 min each time, with the washing solution.
- Mechanically dice the content as finely as possible using scalpel blades, then transfer the contents to a falcon or Eppendorf tube and immerse in collagenase IV for enzymatic digestion at a ratio of 1:1 at a concentration of 1.5 mg/mL (Figure 3E,F).
- CRITICAL STEP: Before cutting, remove any blood or fragments from the content.
- 6.
- Leave in the incubator at 39.5 °C from 60 to 90 min. Shake vigorously every 15 min to break the remaining tissue fragments.
- 7.
- Having most of the solid fragments digested, neutralize the collagenase with the same amount of culture medium.
- 8.
- Centrifuge the tubes at room temperature at 1400 rpm for 5 min.
- 9.
- Remove the supernatant and dispose of it.
- 10.
- Add 2 mL of culture medium to the remaining cells in the conical centrifuge tube.
- 11.
- Sow 500 µL of the extracted feather cells in a 6/12-well plate and incubate it in an incubator at 39 °C with 5% CO2.
- PAUSE STEP
- 12.
- Change the medium at 72 h intervals.
3.2. Follicle Cell Culture
3.2.1. Changing the Culture Medium
- Clean and sterilize the laminar flow hood, microscope and tools.
- Prepare the culture medium.
- Remove the plate to be changed from the incubator and observe the follicle cells under the microscope.
- CRITICAL STEP: Before taking the culture plate into the laminar flow hood, it is important to observe the adhesion of the cells to the plate under the microscope. This can be easily visualized by causing a slight trembling of the desk where the microscope is located. If the cells are fully fixed, there will be no movement; in this case, all of the culture medium will be changed. If some cells respond to the movement, only 50% of the medium will be removed from the well to avoid discarding viable cells.
- 4.
- Place the plate in the laminar flow hood and carefully remove the culture medium with a pipette.
- 5.
- Replace the culture medium with the same quantity that was removed from each well.
- 6.
- Return the plate to the incubator.
3.2.2. Passage
- Prepare the solution of EDTA 5 M in a 1:1000 dilution in PBS.
- Place the plate in the laminar flow hood.
- Remove the culture medium with a pipette.
- Add 1–2 mL of the EDTA solution to the wells and return the plate to the incubator for 5 min.
- After 5 min, observe the cell adherence. This can be easily visualized by causing a slight trembling of the desk where the microscope is located.
- CRITICAL STEP: To continue the passage protocol, the cells must no longer be attached to the bottom of the well, meaning that they can be collected and transferred to a conical centrifuge tube for the next step. If the cells are not fully detached, it is recommended to place them back in the incubator for 2 min and gently pipette the medium against the well’s wall.
- 6.
- If all the cells are detached from the bottom of the well, transfer the contents to a 15 mL conical centrifuge tube.
- 7.
- Neutralize with the same amount of culture medium.
- 8.
- Centrifuge the conical centrifuge tube at 1400 rpm for 5 min.
- 9.
- Remove the culture medium from the wells and transfer approximately 2 mL to a conical centrifuge tube.
- 10.
- Sow the cells in a 48-well plate and complete each well with 100–200 µL of culture medium. Incubate the plate at 39.5 °C with 5% CO₂.
- PAUSE STEP
- 11.
- Change the medium at 72 h intervals.
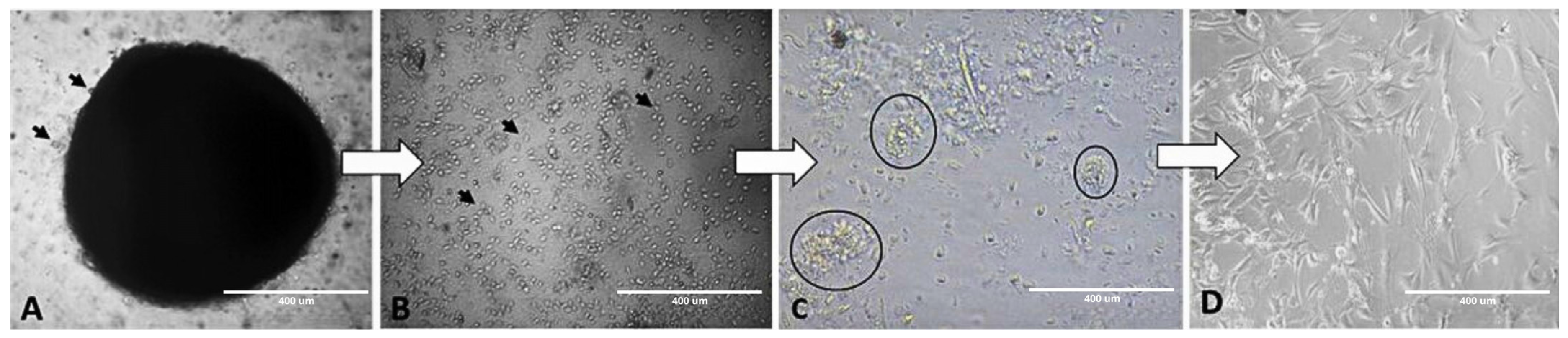
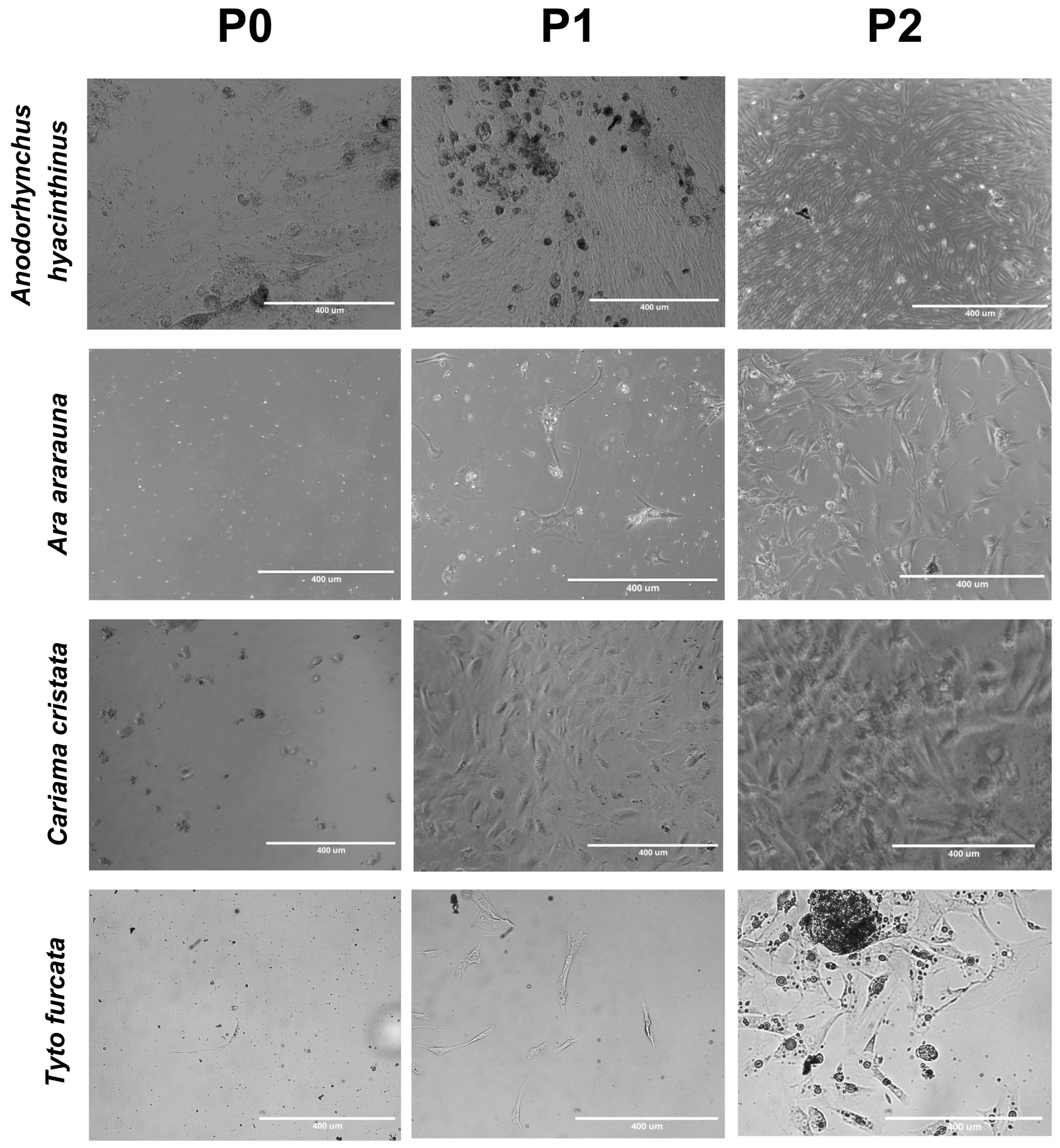
4. Expected Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comizzoli, P.; Wildt, D.E. Cryobanking Biomaterials from Wild Animal Species to Conserve Genes and Biodiversity: Relevance to Human Biobanking and Biomedical Research. In Biobanking of Human Biospecimens; Springer: Cham, Switzerland, 2017; pp. 217–235. [Google Scholar] [CrossRef]
- Cardoso, C.A.; Motta, L.C.B.; Oliveira, V.C.D.; Martins, D.D.S. Somatic Feather Follicle Cell Culture of the Gallus Domesticus Species for Creating a Wild Bird Genetic Resource Bank. Anim. Reprod. 2020, 17, e20200044. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H. The Impact of Mesenchymal Stem Cell Source on Proliferation, Differentiation, Immunomodulation and Therapeutic Efficacy. J. Stem Cell Res. Ther. 2014, 4, 10. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.J.; Ji, H.; Guan, W.; Zhao, Y. Isolation, culture, and characterization of chicken lung-derived mesenchymal stem cells. Can. J. Vet. Res. 2018, 82, 225–235. Available online: https://www.ingentaconnect.com/contentone/cvma/cjvr/2018/00000082/00000003/art00009 (accessed on 16 September 2024). [PubMed]
- Liu, X.; Zhang, S.; Guan, W.; Zheng, D. Isolation and Characterization of Mesenchymal Stem Cells from Chicken Liver. J. Biomater. Tissue Eng. 2020, 10, 8–16. [Google Scholar] [CrossRef]
- Bai, C.; Hou, L.; Ma, Y.; Chen, L.; Zhang, M.; Guan, W. Isolation and Characterization of Mesenchymal Stem Cells from Chicken Bone Marrow. Cell Tissue Bank. 2012, 14, 437–451. [Google Scholar] [CrossRef]
- Bai, C.; Li, X.; Hou, L.; Zhang, M.; Guan, W.; Ma, Y. Biological Characterization of Chicken Mesenchymal Stem/Progenitor Cells from Umbilical Cord Wharton’s Jelly. Mol. Cell. Biochem. 2012, 376, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Pu, Y.; Wang, D.; Hou, L.; Guan, W.; Ma, Y. Isolation and Biological Characterization of Chicken Amnion Epithelial Cells. Eur. J. Histochem. 2012, 56, 33. [Google Scholar] [CrossRef]
- Sasaki, M.; Ikeuchi, T.; Makino, S. A Feather Pulp Culture Technique for Avian Chromosomes, with Notes on the Chromosomes of the Peafowl and the Ostrich. Experientia 1968, 24, 1292–1293. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Nada, Y.; Soh, T.; Fujihara, N.; Hattori, M. Establishment of Feather Follicle Stem Cells as Potential Vehicles for Delivering Exogenous Genes in Birds. J. Reprod. Dev. 2003, 49, 213–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, Y.L.; Sun, J.G.; Wu, F.B.; Xi, Y.M. Investigation of Characteristics of Feather Follicle Stem Cells and Their Regeneration Potential. J. Stem Cell Regen. Med. 2011, 7, 69. [Google Scholar]
- Kim, Y.M.; Park, Y.H.; Lim, J.M.; Jung, H.; Han, J.Y. Technical Note: Induction of Pluripotent Stem Cell-like Cells from Chicken Feather Follicle Cells1. J. Anim. Sci. 2017, 95, 3479–3486. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Plikus, M.V.; Tang, P.C.; Widelitz, R.B.; Chuong, C.M. The modulatable stem cell niche: Tissue interactions during hair and feather follicle regeneration. J. Mol. Biol. 2016, 428, 1423–1440. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yue, Z.; Wu, P.; Wu, D.Y.; Mayer, J.A.; Medina, M.; Widelitz, R.B.; Jiang, T.X.; Chuong, C.M. The biology of feather follicles. Int. J. Dev. Biol. 2004, 48, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.J.G.; Blank, M.H.; Roismann, J. A contribuição das biotecnologias reprodutivas na conservação de espécies selvagens. Rev. Bras. Reprod. Anim. 2024, 48, 46–52. [Google Scholar] [CrossRef]
- Blank, M.H.; Silva, V.C.; Rui, B.R.; Novaes, G.N.; Castiglione, V.C.; Pereira, R.J.G. Beneficial influence of fetal serum on in vitro cryosurvival of chicken spermatozoa. Cryobiology 2020, 95, 103–109. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, D.H.; Noh, K.H.; Jung, C.; Kang, H.M. The Proliferative and Multipotent Epidermal Progenitor Cells for Human Skin Reconstruction in Vitro and in Vivo. Cell Prolif. 2022, 55, e13284. [Google Scholar] [CrossRef]
- Song, S.-H.; Kumar, B.M.; Kang, E.-J.; Lee, Y.-M.; Kim, T.-H.; Ock, S.-A.; Lee, S.-L.; Jeon, B.-G.; Rho, G.-J. Characterization of Porcine Multipotent Stem/Stromal Cells Derived from Skin, Adipose, and Ovarian Tissues and Their Differentiation In Vitro into Putative Oocyte-like Cells. Stem Cells Dev. 2011, 20, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Cheng, S.-F.; Dyce, P.W.; De Felici, M.; Shen, W. Skin-Derived Stem Cells as a Source of Primordial Germ Cell- and Oocyte-like Cells. Cell Death Dis. 2016, 7, e2471. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Fukuda, T.; Kaneko, T.; Nakagawa, Y.; Tajima, A.; Naito, M.; Ohmaki, H.; Endo, D.; Asano, M.; Nagamine, T.; et al. Induced Pluripotent Stem Cells of Endangered Avian Species. Commun. Biol. 2022, 5, 1049. [Google Scholar] [CrossRef]
- Roe, M.; McDonald, N.; Durrant, B.; Jensen, T. Xenogeneic Transfer of Adult Quail (Coturnix coturnix) Spermatogonial Stem Cells to Embryonic Chicken (Gallus gallus) Hosts: A Model for Avian Conservation1. Biol. Reprod. 2013, 88, 129. [Google Scholar] [CrossRef] [PubMed]


| Order | Family | Scientific Name | Representative Picture | Conservation Status | Collection Date |
|---|---|---|---|---|---|
| Accipitriformes | Accipitridae | Geranospiza caerulescens |  https://ebird.org/species/crahaw (accessed on 28 November 2024). |  https://www.iucnredlist.org/species/22695729/168785689 (accessed on 28 November 2024) | 21 November 2024 |
| Apodiformes | Trochilidae | Eupetomena macroura |  https://ebird.org/species/swthum1 (accessed on 28 November 2024) |  https://www.iucnredlist.org/species/22687094/263628596 (accessed on 28 November 2024) | 6 April 2022 |
| Caprimulgiformes | Caprimulgidae | Nyctidromus albicollis |  https://ebird.org/species/compau (accessed on 28 November 2024) |  https://www.iucnredlist.org/species/22689731/168860360 (accessed on 28 November 2024) | 1 November 2024 |
| Cariamiformes | Cariamidae | Cariama cristata |  https://ebird.org/species/relser1 (accessed on 28 November 2024) |  https://www.iucnredlist.org/species/22692205/263629033 (accessed on 28 November 2024) | 20 March 2023 |
| Cathartiformes | Cathartidae | Coragyps atratus |  https://ebird.org/species/blkvul (accessed on 28 November 2024) |  https://www.iucnredlist.org/species/22697624/93624950 (accessed on 28 November 2024) | 1 November 2022 |
| Cuculiformes | Cuculidae | Guira guira |  https://ebird.org/species/guicuc1 (accessed on 28 November 2024) |  https://www.iucnredlist.org/species/22684441/263682441 (accessed on 28 November 2024) | 1 November 2024 |
| Galliformes | Cracidae | Pauxi mitu |  https://ebird.org/species/alacur1 (accessed on 28 November 2024) |  https://www.iucnredlist.org/species/22678486/132315266 (accessed on 28 November 2024) | 3 May 2024 |
| Pipile cujubi |  https://ebird.org/species/rtpgua1 (accessed on 28 November 2024) |  https://www.iucnredlist.org/species/22678422/194242016 (accessed on 28 November 2024) | 8 April 2022 | ||
| Phasianidae | Gallus gallus |  https://ebird.org/species/redjun (accessed on 28 November 2024) |  https://www.iucnredlist.org/species/22679199/263732457 (accessed on 28 November 2024) | 6 July 2022 | |
| Lophura edwardsi |  https://commons.wikimedia.org/wiki/File:Ba%C5%BEant_Edwards%C5%AFv.jpg (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/45354985/249120158 (accessed on 29 November 2024) | 8 April 2022 | ||
| Meleagris gallopavo |  https://ebird.org/species/wiltur (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/22679525/132051953 (accessed on 29 November 2024) | 22 August 2023 | ||
| Passeriformes | Icteridae | Cacicus cela |  https://ebird.org/species/yercac1 (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/103792683/138350097 (accessed on 29 November 2024) | 21 April 2022 |
| Psittaciformes | Psittacidae | Amazona festiva |  https://ebird.org/species/fespar1 (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/22727626/209890756 (accessed on 29 November 2024) | 19 July 2022 |
| Anodorhynchus hyacinthinus |  https://ebird.org/species/hyamac1 (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/22685516/93077457 (accessed on 29 November 2024) | 3 May 2024 | ||
| Ara Ararauna |  https://ebird.org/species/baymac (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/22685539/131917270 (accessed on 29 November 2024) | 5 August 2022 | ||
| Ara rubrogenys |  https://ebird.org/species/refmac1 (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/22685572/196567391 (accessed on 29 November 2024) | 18 April 2022 | ||
| Pyrrhura frontalis |  https://ebird.org/species/mabpar (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/22685793/93088076 (accessed on 29 November 2024) | 8 April 2022 | ||
| Strigiformes | Tytonidae | Tyto furcata (old Tyto alba) |  https://ebird.org/species/brnowl (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/22688504/155542941 (accessed on 29 November 2024) | 31 August 2022 |
| Struthioniformes | Tinamidae | Crypturellus noctivagus |  https://ebird.org/species/yeltin1 (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/22678217/221182790 (accessed on 29 November 2024) | 21 April 2022 |
| Crypturellus obsoletus |  https://ebird.org/species/brotin1 (accessed on 29 November 2024) |  https://www.iucnredlist.org/species/22678176/234006619 (accessed on 29 November 2024) | 16 April 2022 |
| Species | Collection Timepoint | Scattering from the Fragment | Cell Aggregate | Scattered Cells | Fibroblast-like Morphology (days) | Presence of Rounded Cells with No Apparent Nucleus | Adhesion to Plate (days) |
|---|---|---|---|---|---|---|---|
| Pipile cujubi | After euthanasia | + | + | + | + | + | 14 |
| Lophura edwards | After euthanasia | + | + | + | − | + | 4 |
| Gallus gallus | 8 h after death | − | − | − | − | − | absent |
| Amazona aestiva | 5 h after death | + | + | + | − | + | absent |
| Ara arauna | 2 h after death | + | + | + | 5 | + | 5 |
| Eupetomena macroura | 24 h after death | + | − | + | absent | + | 3 |
| Tyto furcata | 6 h after death | + | + | + | 22 | + | 16 |
| Cariama cristata | 6 h after death | + | + | + | 3 | + | 3 |
| Meleagris gallopavo | 24 h after death | + | + | + | 3 | + | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, Y.G.d.; Guerra, M.E.P.; Coutinho, M.d.P.; Santos, S.I.P.; Mota, B.D.; Munhoz, L.L.d.S.; Rossetti, D.P.; Martins, D.d.S. Wild Birds’ Genetic Resources Bank: Feather Follicle Cell Culture as a Possible Source of Stem Cells. Methods Protoc. 2025, 8, 17. https://doi.org/10.3390/mps8010017
Reis YGd, Guerra MEP, Coutinho MdP, Santos SIP, Mota BD, Munhoz LLdS, Rossetti DP, Martins DdS. Wild Birds’ Genetic Resources Bank: Feather Follicle Cell Culture as a Possible Source of Stem Cells. Methods and Protocols. 2025; 8(1):17. https://doi.org/10.3390/mps8010017
Chicago/Turabian StyleReis, Yasmin Godoi dos, Maria Eduarda Pralon Guerra, Meline de Paula Coutinho, Sarah Ingrid Pinto Santos, Bruna Dias Mota, Lauriene Luiza de Souza Munhoz, Diogo Pascoal Rossetti, and Daniele dos Santos Martins. 2025. "Wild Birds’ Genetic Resources Bank: Feather Follicle Cell Culture as a Possible Source of Stem Cells" Methods and Protocols 8, no. 1: 17. https://doi.org/10.3390/mps8010017
APA StyleReis, Y. G. d., Guerra, M. E. P., Coutinho, M. d. P., Santos, S. I. P., Mota, B. D., Munhoz, L. L. d. S., Rossetti, D. P., & Martins, D. d. S. (2025). Wild Birds’ Genetic Resources Bank: Feather Follicle Cell Culture as a Possible Source of Stem Cells. Methods and Protocols, 8(1), 17. https://doi.org/10.3390/mps8010017














