Assessment of Endocyn on Dental Pulp Stem Cells (DPSCs): A Pilot Study of Endodontic Irrigant Effects
Abstract
1. Introduction
2. Methods
2.1. Study Approval for DPSC Lines
2.2. Experimental Reagents
2.3. Growth Assays
2.4. Viability Assays
2.5. RNA Isolation and cDNA Synthesis
2.6. Real-Time qPCR Screening
| Positive control primer: | Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) |
| GAPDH forward primer | 5′-ATC TTC CAG GAG CGA GAT CC-3′ |
| GAPDH reverse primer | 5′-ACC ACT GAC ACG TTG GCA GT-3′ |
| ISCT (MSC) validation primers: | |
| CD45 forward primer | 5′-CAT ATT TAT TTT GTC CTT CTC CCA-3′ |
| CD45 reverse primer | 5′-GAA AGT TTC CAC GAA CGG-3′ |
| CD73 forward primer | 5′-AGT CCA CTG GAG AGT TCC TGC A-3′ |
| CD73 reverse primer | 5′-TGA GAG GGT CAT AAC TGG GCA C-3′ |
| CD90 forward primer | 5′-ATG AAC CTG GCC ATC AGC A-3′ |
| CD90 reverse primer | 5′-GTG TGC TCA GGC ACC CC-3′ |
| CD105 forward primer | 5′-CCA CTA GCC AGG TCT CGA AG-3′ |
| CD105 reverse primer | 5′-GAT GCA GGA AGA CAC TGC TG-3′ |
| Stem cell biomarker primers: | |
| Nestin forward primer | 5′-CGT TGG AAC AGA GGT TGG AG-3′ |
| Nestin reverse primer | 5′-TCC TGA AAG CTG AGG GAA G-3′ |
| NANOG forward primer | 5′-GCT GAG ATG CCT CAC ACG GAG-3′ |
| NANOG reverse primer | 5′-TCT GTT TCT TGA CTG GGA CCT TGT C-3′ |
| Oct-4 forward primer | 5′-TGG AGA AGG AGA AGC TGG AGC AAA A-3′ |
| Oct-4 reverse primer | 5′-GGC AGA TGG TCG TTT GGC TGA ATA-3′ |
| Sox-2 forward primer | 5′-ATG GGC TCT GTG GTC AAG TC-3′ |
| Sox-2 reverse primer | 5′-CCC TCC CAA TTC CCT TGT AT-3′ |
| Alkaline phosphatase (ALP) | |
| ALP forward primer | 5′-CAC TGC GGA CCA TTC CCA CGT CTT-3′ |
| ALP reverse primer | 5′-GCG CCT GGT AGT TGT TGT GAG CAT-3′ |
| Dentin sialophosphoprotein (DSPP) | |
| DSPP forward primer | 5′-CAA CCA TAG AGA AAG CAA ACG CG-3′ |
| DSPP reverse primer | 5′-TTT CTG TTG CCA CTG CTG GGA C-3′ |
2.7. Statistical Analysis
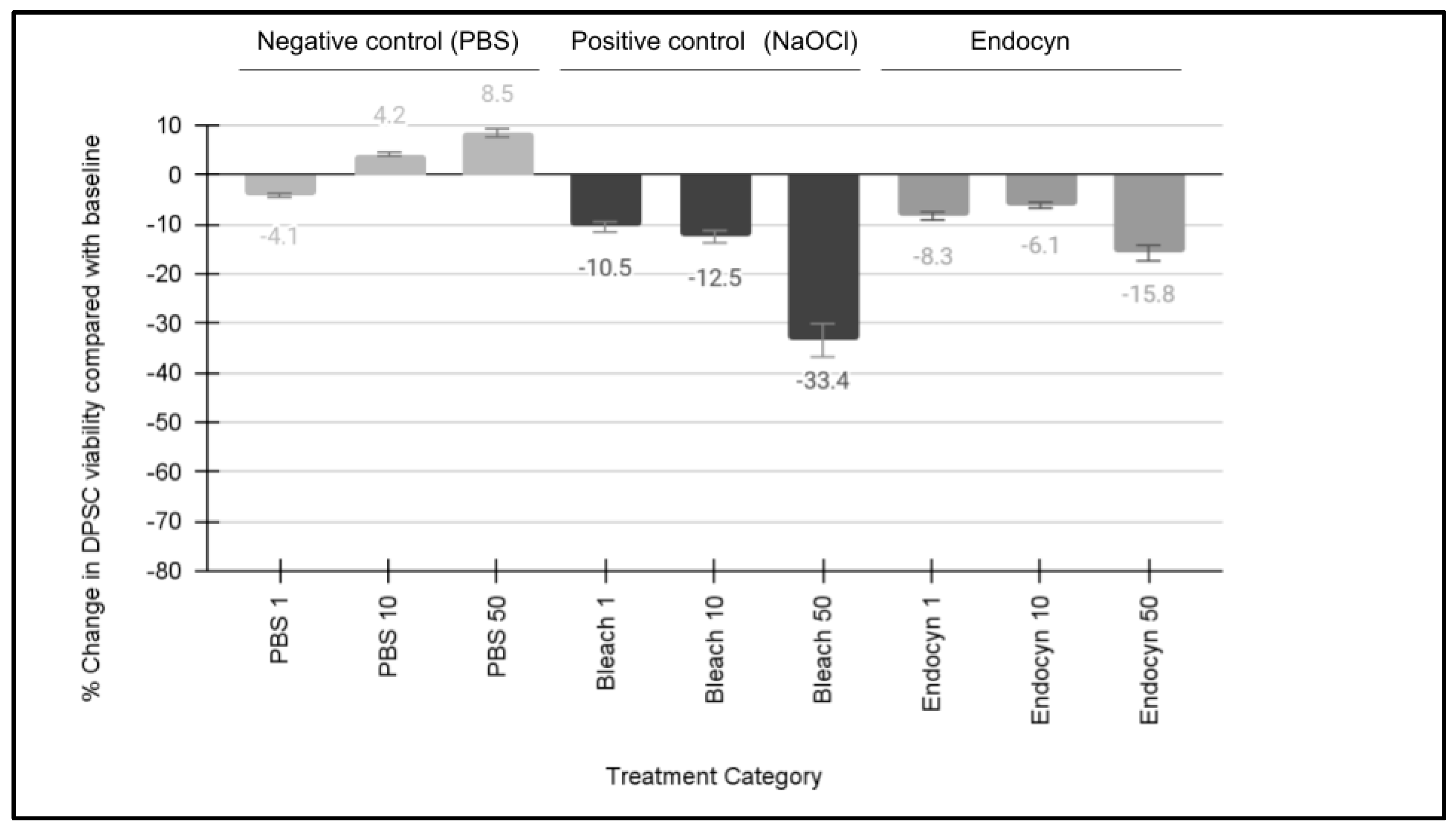
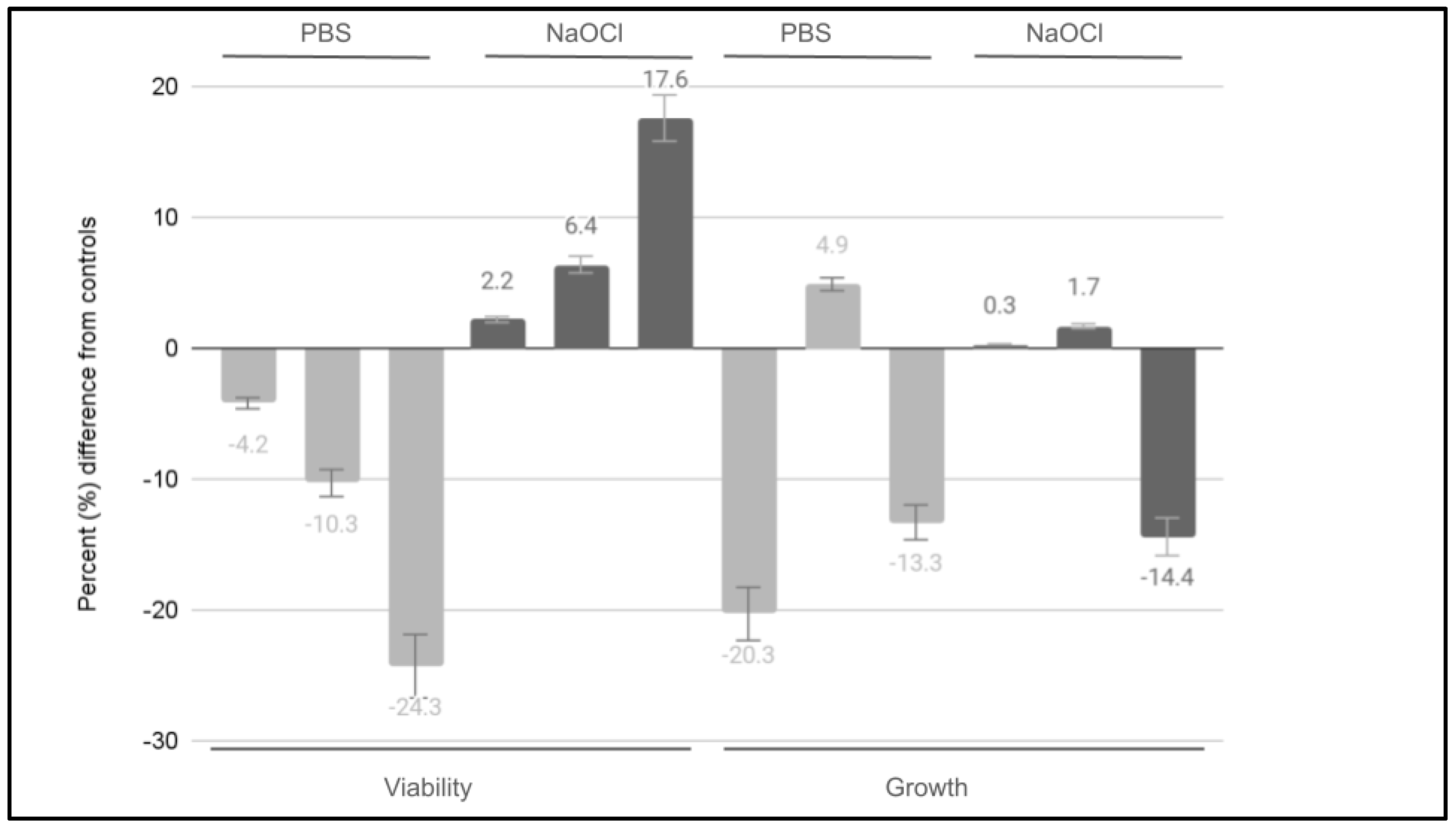
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NaOCl | Sodium hypochlorite |
| DPSC | Dental pulp stem cell |
| UNLV | University of Nevada, Las Vegas |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate-buffered saline |
| CD | Cluster of differentiation |
| ISCT | International Society for Cellular Therapy |
| BGS | Bovine growth serum |
Appendix A
| Experimental Condition Observed Response | Statistical Analysis | |
|---|---|---|
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Viability [1% PBS] −3.7% −3.4% −4.6% −4.3% −4.1% −4.0% −3.2% −4.4% −3.9% −4.2% −4.6% −4.5% | Average: −4.1% Standard deviation: 0.45 Range: −3.2% to −4.6% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Growth [1% PBS] −10.8% −11.4% −8.5% −7.7% −11.4% −9.9% −9.1% −7.5% −8.6% −9.3% −9.1% −9.4% | Average: −9.4% Standard deviation: 1.29 Range: −7.5% to −11.4% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Viability [10% PBS] 3.4% 4.5% 3.9% 4.1% 3.9% 4.5% 4.4% 4.1% 4.6% 4.2% 3.9% 4.4% | Average: 4.2% Standard deviation: 0.35 Range: 3.4% to 4.5% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Growth [10% PBS] −40.2% −32.8% −35.4% −37.2% −41.2% −40.0% −38.4% −32.5% −37.4% −35.6% −31.2% −31.3% −36.2% | Average: −36.1% Standard deviation: 3.54 Range: −31.2% to −41.2% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Viability [50% PBS] 7.7% 10.6% 9.3% 8.7% 8.2% 9.1% 8.9% 7.8% 7.1% 6.8% 8.6% 8.8% | Average: 8.5% Standard deviation: 1.03 Range: 7.7% to 10.6% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Growth [50% PBS] −45.5% −53.1% −48.3% −51.2% −53.3% −46.4% −43.2% −49.8% −53.2% −55.1% −48.2% −49.0% −49.7% | Average: −49.7% Standard deviation: 3.61 Range: −45.5% to −55.1% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Viability [1% NaOCl] −11.4% −12.3% −14.6% −8.8% −9.4% −9.3% −9.4% −10.6% −11.4% −11.7% −8.9% −8.3% | Average: −10.5% Standard deviation: 1.84 Range: −8.3% to −14.6% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Growth [1% NaOCl] −25.5% −28.5% −31.2% −36.7% −33.4% −29.9% −32.4% −27.8% −28.3% −31.6% −28.9% −25.2% −30.0% | Average: −30.0% Standard deviation: 3.31 Range: −25.2% to −33.4% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Viability [10% NaOCl] −13.4% −10.8% −15.6% −11.4% −10.2% −13.3% −11.4% −13.6% −15.1% −11.8% −12.1% −11.8% | Average: −12.5% Standard deviation: 1.67 Range: −10.2% to −15.6% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Growth [10% NaOCl] −35.9% −31.7% −29.8% −28.6% −31.7% −33.5% −35.5% −32.4% −32.5% −29.5% −27.6% −27.9% | Average: −31.4% Standard deviation: 2.77 Range: −27.6% to −35.9% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Viability [50% NaOCl] −39.9% −33.1% −32.6% −37.5% −29.6% −32.7% −33.1% −31.2% −32.4% −29.3% −35.6% −33.5% | Average: −33.4% Standard deviation: 3.05 Range: −29.3% to −39.9% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Growth [50% NaOCl] −55.2% −48.2% −49.7% −48.2% −51.1% −55.3% −44.8% −43.7% −41.7% −47.4% −49.2% −48.1% | Average: −48.6% Standard deviation: 4.09 Range: −41.7% to −55.3% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Viability [1% Endocyn] −10.2% −9.9% −7.4% −7.7% −9.4% −6.9% −9.2% −9.4% −7.8% −7.6% −7.1% −6.8% | Average: −8.3% Standard deviation: 1.24 Range: −6.9% to −10.2% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Growth [1% Endocyn] −33.4% −27.2% −28.6% −33.8% −35.9% −34.5% −26.5% −27.8% −29.3% −31.1% −25.5% −22.4% | Average: −29.7% Standard deviation: 4.11 Range: −22.4% to −35.9% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Viability [10% Endocyn] −5.5% −7.3% −5.5% −5.9% −6.3% −6.6% −5.2% −5.7% −6.2% −7.2% −5.9% −6.2% | Average: −6.1% Standard deviation: 0.66 Range: −5.2% to −7.3% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Growth [10% Endocyn] −32.5% −34.7% −28.7% −31.2% −27.6% −32.4% −33.1% −36.4% −32.4% −28.5% −27.4% −29.5% | Average: −31.2% Standard deviation: 2.88 Range: −27.4% to−36.4% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Viability [50% Endocyn] −11.3% −18.2% −14.3% −15.5% −18.6% −18.4% −17.5% −16.4% −16.9% −11.9% −12.3% −11.2% | Average: −15.8% Standard deviation: 2.89 Range: −11.2% to −18.6% |
| dpsc-3882 dpsc-3924 dpsc-5653 dpsc-7089 dpsc-8124 dpsc-8604 dpsc-9765 dpsc-9894 dpsc-11418 dpsc-11750 dpsc-11836 dpsc-17322 | Growth [50% Endocyn] −55.8% −64.6% −67.5% −68.1% −61.2% −63.2% −55.5% −65.4% −66.6% −61.2% −67.3% −59.0% | Average: −63.0% Standard deviation: 4.44 Range: −55.5% to −68.1% |
References
- Lin, P.Y.; Chen, H.S.; Wang, Y.H.; Tu, Y.K. Primary molar pulpotomy: A systematic review and network meta-analysis. J. Dent. 2014, 42, 1060–1077. [Google Scholar] [CrossRef] [PubMed]
- Marghalani, A.A.; Omar, S.; Chen, J.W. Clinical and radiographic success of mineral trioxide aggregate compared with formocresol as a pulpotomy treatment in primary molars: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2014, 145, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, A.; Issa, M.A.; Kingsley, K.; Sullivan, V. Analysis of Pediatric Pulpotomy, Pulpectomy, and Extractions in Primary Teeth Revealed No Significant Association with Subsequent Root Canal Therapy and Extractions in Permanent Teeth: A Retrospective Study. Pediatr. Rep. 2024, 16, 438–450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alqaderi, H.; Lee, C.T.; Borzangy, S.; Pagonis, T.C. Coronal pulpotomy for cariously exposed permanent posterior teeth with closed apices: A systematic review and meta-analysis. J. Dent. 2016, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Elhamouly, Y.; Adham, M.M.; Dowidar, K.M.L.; El Backly, R.M. Outcome assessment methods of bioactive and biodegradable materials as pulpotomy agents in primary and permanent teeth: A scoping review. BMC Oral Health 2024, 24, 496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zarabadi, M.S.; Firoozi, P.; Basir Shabestari, S.; Maleki, A.; Nazemi Salman, B. 3Mixtatin versus MTA in pulp therapy of primary teeth: A systematic review and meta-analysis of current randomized controlled trials. Evid. Based Dent. 2024, 25, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Mungekar-Markandey, S.; Mistry, L.; Jawdekar, A. Clinical Success of Iatrogenic Perforation Repair Using Mineral Trioxide Aggregate and Other Materials in Primary Molars: A Systematic Review and Meta-analysis. Int. J. Clin. Pediatr. Dent. 2022, 15, 610–616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ather, A.; Patel, B.; Gelfond, J.A.L.; Ruparel, N.B. Outcome of pulpotomy in permanent teeth with irreversible pulpitis: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 19664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedano, M.S.; Li, X.; Yoshihara, K.; Landuyt, K.V.; Van Meerbeek, B. Cytotoxicity and Bioactivity of Dental Pulp-Capping Agents towards Human Tooth-Pulp Cells: A Systematic Review of In-Vitro Studies and Meta-Analysis of Randomized and Controlled Clinical Trials. Materials 2020, 13, 2670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Chua, P.; Elamin, A.D.; Clarke, M.; El-Karim, I.A. Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Komora, P.; Vámos, O.; Gede, N.; Hegyi, P.; Kelemen, K.; Galvács, A.; Varga, G.; Kerémi, B.; Vág, J. Comparison of bioactive material failure rates in vital pulp treatment of permanent matured teeth—A systematic review and network meta-analysis. Sci. Rep. 2024, 14, 18421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González-Gil, D.; Flores-Fraile, J.; Vera-Rodríguez, V.; Martín-Vacas, A.; López-Marcos, J. Comparative Meta-Analysis of Minimally Invasive and Conventional Approaches for Caries Removal in Permanent Dentition. Medicina 2024, 60, 402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Figundio, N.; Lopes, P.; Tedesco, T.K.; Fernandes, J.C.H.; Fernandes, G.V.O.; Mello-Moura, A.C.V. Deep Carious Lesions Management with Stepwise, Selective, or Non-Selective Removal in Permanent Dentition: A Systematic Review of Randomized Clinical Trials. Healthcare 2023, 11, 2338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kulkarni, P.; Tiwari, S.; Agrawal, N.; Kumar, A.; Umarekar, P.; Bhargava, S. Clinical Outcome of Direct Pulp Therapy in Primary Teeth: A Systematic Review and Meta-analysis. J. Indian Soc. Pedod. Prev. Dent. 2022, 40, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.R.; Bendgude, V.D.; Kakodkar, P. Evaluation of Success Rate of Lesion Sterilization and Tissue Repair Compared to Vitapex in Pulpally Involved Primary Teeth: A Systematic Review. J. Conserv. Dent. 2019, 22, 510–515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garrocho-Rangel, A.; Jalomo-Ávila, C.; Rosales-Berber, M.Á.; Pozos-Guillén, A. Lesion Sterilization Tissue Repair (LSTR) Approach Of Non-Vital Primary Molars With A Chloramphenicol-Tetracycline-ZOE Antibiotic Paste: A Scoping Review. J. Clin. Pediatr. Dent. 2021, 45, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.K.; Brigit, B.; Annapoorna, B.S.; Naik, S.B.; Merwade, S.; Rashmi, K. Effect of triple antibiotic paste and calcium hydroxide on the rate of healing of periapical lesions: A systematic review. J. Conserv. Dent. 2021, 24, 307–313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kharchi, A.S.; Tagiyeva-Milne, N.; Kanagasingam, S. Regenerative Endodontic Procedures, Disinfectants and Outcomes: A Systematic Review. Prim. Dent. J. 2020, 9, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Fedele, G.; Rödig, T. Effectiveness of root canal irrigation and dressing for the treatment of apical periodontitis: A systematic review and meta-analysis of clinical trials. Int. Endod. J. 2023, 56 (Suppl. S3), 422–435. [Google Scholar] [CrossRef] [PubMed]
- Ruksakiet, K.; Hanák, L.; Farkas, N.; Hegyi, P.; Sadaeng, W.; Czumbel, L.M.; Sang-Ngoen, T.; Garami, A.; Mikó, A.; Varga, G.; et al. Antimicrobial Efficacy of Chlorhexidine and Sodium Hypochlorite in Root Canal Disinfection: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2020, 46, 1032–1041.e7. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Herrera, D.R.; Pecorari, V.G.A.; Gomes, B.P.F.A. Endotoxin levels after chemomechanical preparation of root canals with sodium hypochlorite or chlorhexidine: A systematic review of clinical trials and meta-analysis. Int. Endod. J. 2019, 52, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Soltani, M.K.; Shalavi, S. An update on the management of endodontic biofilms using root canal irrigants and medicaments. Iran. Endod. J. 2014, 9, 89–97. [Google Scholar] [PubMed] [PubMed Central]
- Mohammadi, Z.; Jafarzadeh, H.; Shalavi, S.; Kinoshita, J.I. Unusual Root Canal Irrigation Solutions. J. Contemp. Dent. Pract. 2017, 18, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Naladkar, K.; Chandak, M.; Sarangi, S.; Agrawal, P.; Jidewar, N.; Suryawanshi, T.; Hirani, P. Breakthrough in the Development of Endodontic Irrigants. Cureus 2024, 16, e66981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shetty, N.; Mathew, T.; Shetty, A.; Hegde, M.N.; Attavar, S. Ozonated water as an irrigant in disinfecting root canal systems—A systematic review. Evid. Based Dent. 2022. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.N.L.; Rover, G.; Belladonna, F.G.; Herrera, D.R.; De-Deus, G.; da Silva Fidalgo, T.K. Effectiveness of passive ultrasonic irrigation on periapical healing and root canal disinfection: A systematic review. Br. Dent. J. 2019, 227, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.A.; Steier, L.; Vanin, G.N.; Zanella, M.L.; Pizzi, C.M.; Ferreira, E.R.; Dallepiane, F.G.; Piccolo, N.M.; da Silva Koch, J.; Souza, K.R.; et al. Antimicrobial action, cytotoxicity and erosive potential of hypochlorous acid obtained from an electrolytic device compared with sodium hypochlorite. Clin. Oral Investig. 2024, 28, 282. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.A.; Zanella, M.L.; Vanin, G.N.; Dallepiane, F.G.; Pizzi, C.Y.M.; Ferreira, E.R.; Fuhr, M.C.S.; Piccolo, N.M.; Palhano, H.S.; da Silva Koch, J.; et al. Antimicrobial action and cytotoxicity of hypochlorous acid obtained from an innovative electrolytic device—An in vitro study. Arch. Oral Biol. 2024, 163, 105966. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, S.; Menon, K. Comparative Evaluation of Human Pulp Tissue Dissolution by 500-ppm and 200-ppm Hypochlorous Acid and 5.25% Sodium Hypochlorite: An In Vitro Study. J. Contemp. Dent. Pract. 2023, 24, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.; Stewart, S.; Ma, L.; Kingsley, K.; Sullivan, V. Differential Antimicrobial Effects of Endodontic Irrigant Endocyn on Oral Bacteria. Hygiene 2025. in review. [Google Scholar]
- Scott, M.B., 2nd; Zilinski, G.S.; Kirkpatrick, T.C.; Himel, V.T.; Sabey, K.A.; Lallier, T.E. The Effects of Irrigants on the Survival of Human Stem Cells of the Apical Papilla, Including Endocyn. J. Endod. 2018, 44, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Miteva, M.; Mihaylova, Z.; Mitev, V.; Aleksiev, E.; Stanimirov, P.; Praskova, M.; Dimitrova, V.S.; Vasileva, A.; Calenic, B.; Constantinescu, I.; et al. A Review of Stem Cell Attributes Derived from the Oral Cavity. Int. Dent. J. 2024, 74, 1129–1141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, P.; Aghazadeh, M.; Rajasingh, S.; Dixon, D.; Jain, V.; Rajasingh, J. Stem cells in regenerative dentistry: Current understanding and future directions. J. Oral Biosci. 2024, 66, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Mantesso, A.; Nör, J.E. Stem cells in clinical dentistry. J. Am. Dent. Assoc. 2023, 154, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bae, A.; Kim, J.; Kingsley, K. Differential Effects of Extracellular Matrix Glycoproteins Fibronectin and Laminin-5 on Dental Pulp Stem Cell Phenotypes and Responsiveness. J. Funct. Biomater. 2023, 14, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bae, S.; Kang, B.; Lee, H.; Luu, H.; Mullins, E.; Kingsley, K. Characterization of Dental Pulp Stem Cell Responses to Functional Biomaterials Including Mineralized Trioxide Aggregates. J. Funct. Biomater. 2021, 12, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bassett, C.; Triplett, H.; Lott, K.; Howard, K.M.; Kingsley, K. Differential Expression of MicroRNA (MiR-27, MiR-145) among Dental Pulp Stem Cells (DPSCs) Following Neurogenic Differentiation Stimuli. Biomedicines 2023, 11, 3003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lott, K.; Collier, P.; Ringor, M.; Howard, K.M.; Kingsley, K. Administration of Epidermal Growth Factor (EGF) and Basic Fibroblast Growth Factor (bFGF) to Induce Neural Differentiation of Dental Pulp Stem Cells (DPSC) Isolates. Biomedicines 2023, 11, 255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, A.; Raik, S.; Sharma, P.; Rattan, V.; Bhattacharyya, S. Primary Culture of Dental Pulp Stem Cells. J. Vis. Exp. 2023, 195, e65223. [Google Scholar] [CrossRef] [PubMed]
- Hollands, P.; Aboyeji, D.; Orcharton, M. Dental pulp stem cells in regenerative medicine. Br. Dent. J. 2018, 224, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Koosha, F.; Cymerman, J.; Manders, T.; Simon, M.; Walker, S.; Rafailovich, M. Non-cytotoxic Root Canal Dressing with Improved Antimicrobial Efficacy. J. Endod. 2023, 49, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Chen, Y.L.; Huang, J.S.; Huang, G.T.; Chuang, S.F. Effects of Restorative Materials on Dental Pulp Stem Cell Properties. J. Endod. 2019, 45, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; D’Amico, C.; Meto, A.; Mehta, V.; Lo Giudice, G.; Cervino, G. Sodium Hypochlorite Accidents in Endodontic Practice: Clinical Evidence and State of the Art. J. Craniofac. Surg. 2024, 35, e636–e645. [Google Scholar] [CrossRef] [PubMed]
- Parchami, K.; Dastorani, M.; Barati, M. What is the impact of Endodontic Irrigant Solutions on the Viability of Stem Cells from Apical Papilla in an in-vitro setting: A Systematic Review. Saudi Dent. J. 2024, 36, 1170–1178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Susila, A.V.; Sai, S.; Sharma, N.; Balasubramaniam, A.; Veronica, A.K.; Nivedhitha, S. Can natural irrigants replace sodium hypochlorite? A systematic review. Clin. Oral Investig. 2023, 27, 1831–1849. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Delgado, M.; Camps-Font, O.; Luz, L.; Sanz, D.; Mercade, M. Update on citric acid use in endodontic treatment: A systematic review. Odontology 2023, 111, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mrinalini, M.; Gupta, A.; Abraham, D.; Duraisamy, A.K.; Sharma, R. A Systematic Review of the Comparative Efficacy of Lactobacillus Probiotics and Sodium Hypochlorite as Intracanal Irrigants Against Enterococcus faecalis. Cureus 2024, 16, e70926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anbalagan, K.; Jena, A.; Mohanty, S.; Mallick, R.; Shashirekha, G.; Sarangi, P. Smear layer removal and antimicrobial efficacy of chitosan as a root canal irrigant: A systematic review of in-vitro studies. Odontology 2024, 113, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Elfarraj, H.; Lizzi, F.; Bitter, K.; Zaslansky, P. Effects of endodontic root canal irrigants on tooth dentin revealed by infrared spectroscopy: A systematic literature review. Dent. Mater. 2024, 40, 1138–1163. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.A.; Falacho, R.I.; Santos, J.M.; Ramos, J.C.; Palma, P.J. Effects of endodontic irrigation solutions on structural, chemical, and mechanical properties of coronal dentin: A scoping review. J. Esthet. Restor. Dent. 2024, 36, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Aksel, H.; Albanyan, H.; Bosaid, F.; Azim, A.A. Dentin Conditioning Protocol for Regenerative Endodontic Procedures. J. Endod. 2020, 46, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; D’Souza, R.N.; Federlin, M.; Cavender, A.C.; Hartgerink, J.D.; Hecker, S.; Schmalz, G. Dentin conditioning codetermines cell fate in regenerative endodontics. J. Endod. 2011, 37, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Alghilan, M.A.; Windsor, L.J.; Palasuk, J.; Yassen, G.H. Attachment and proliferation of dental pulp stem cells on dentine treated with different regenerative endodontic protocols. Int. Endod. J. 2017, 50, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.C.; Murray, P.E.; Namerow, K.N.; Kuttler, S.; Garcia-Godoy, F. The comparison of the effect of endodontic irrigation on cell adherence to root canal dentin. J. Endod. 2008, 34, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Dinh, Y.; Yesares Rubi, P.; Gibbs, J.L.; Michot, B. Effects of aqueous and ethanolic extracts of Chinese propolis on dental pulp stem cell viability, migration and cytokine expression. PeerJ 2024, 12, e18742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zawadzka-Knefel, A.; Rusak, A.; Mrozowska, M.; Machałowski, T.; Żak, A.; Haczkiewicz-Leśniak, K.; Kulus, M.; Kuropka, P.; Podhorska-Okołów, M.; Skośkiewicz-Malinowska, K. Chitin scaffolds derived from the marine demosponge Aplysina fistularis stimulate the differentiation of dental pulp stem cells. Front. Bioeng. Biotechnol. 2023, 11, 1254506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padmawar, N.; Pawar, N.; Tripathi, V.; Banerjee, S.; Tyagi, G.; Joshi, S.R. Comparative analysis of rotary versus manual instrumentation in paediatric pulpectomy procedures: A systematic review and meta-analysis. Aust. Endod. J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Tysiąc-Miśta, M.; Tanasiewicz, M.; Amini, S.; Najary, S.; Baghani, M.T.; Eftekhar Ashtiani, R.; Shidfar, S.; Nasiri, M.J. Traumatic Dental Injuries’ Prevalence across Diverse Healthcare Settings: A Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2024, 13, e11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Namjoynik, A.; Islam, M.A.; Islam, M. Evaluating the efficacy of human dental pulp stem cells and scaffold combination for bone regeneration in animal models: A systematic review and meta-analysis. Stem Cell Res. Ther. 2023, 14, 132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandes, T.L.; Cortez de SantAnna, J.P.; Frisene, I.; Gazarini, J.P.; Gomes Pinheiro, C.C.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Franco Bueno, D. Systematic Review of Human Dental Pulp Stem Cells for Cartilage Regeneration. Tissue Eng. Part B Rev. 2020, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Amghar-Maach, S.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Regeneration of periodontal bone defects with dental pulp stem cells grafting: Systematic Review. J. Clin. Exp. Dent. 2019, 11, e373–e381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conde, M.C.; Chisini, L.A.; Grazioli, G.; Francia, A.; Carvalho, R.V.; Alcázar, J.C.; Tarquinio, S.B.; Demarco, F.F. Does Cryopreservation Affect the Biological Properties of Stem Cells from Dental Tissues? A Systematic Review. Braz. Dent. J. 2016, 27, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A. Editorial: Rejuvenation of aging adult stem cells to improve their regenerative potential. Front. Cell Dev. Biol. 2023, 11, 1232970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwack, K.H.; Lee, H.W. Clinical Potential of Dental Pulp Stem Cells in Pulp Regeneration: Current Endodontic Progress and Future Perspectives. Front. Cell Dev. Biol. 2022, 10, 857066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

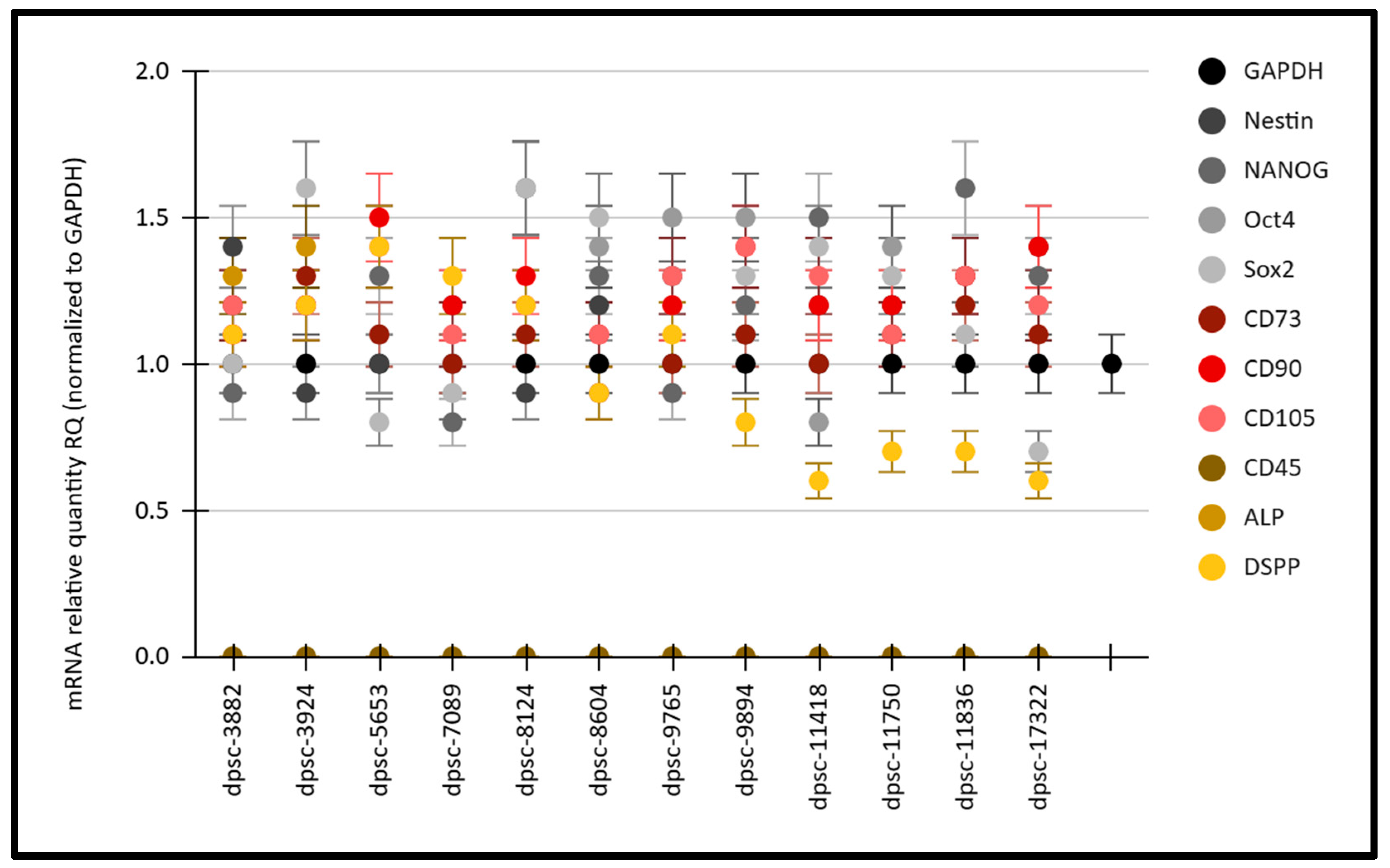
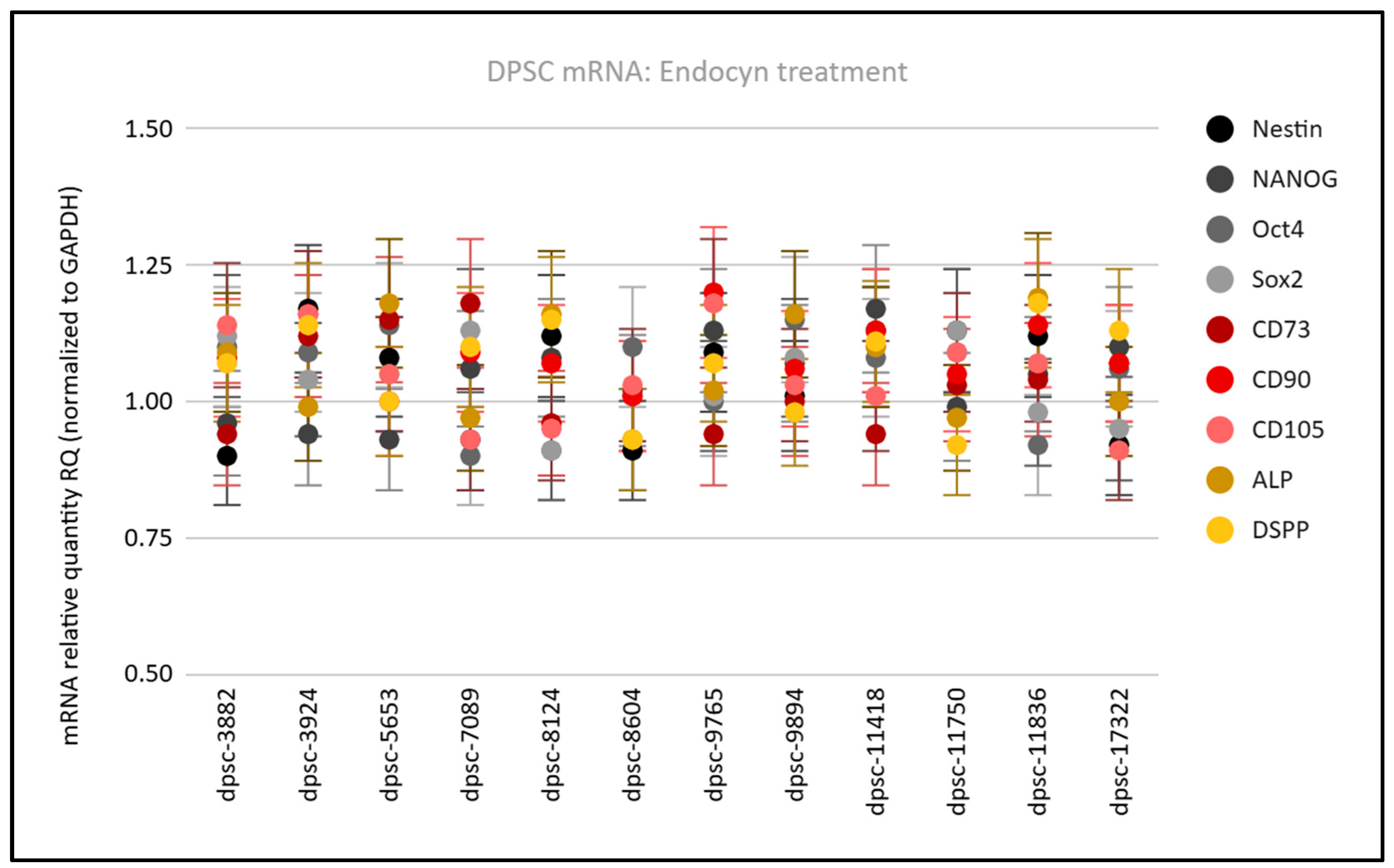
| Cell Line | Doubling Time (Growth Rate) | Viability (Following Thaw) |
|---|---|---|
| dpsc-3882 | 1.8 days | 88% |
| dpsc-3924 | 2.2 days | 86% |
| dpsc-5653 | 1.9 days | 87% |
| dpsc-7089 | 1.7 days | 82% |
| dpsc-8124 | 4.4 days | 73% |
| dpsc-8604 | 4.1 days | 71% |
| dpsc-9765 | 2.1 days | 79% |
| dpsc-9894 | 5.2 days | 76% |
| dpsc-11418 | 8.4 days | 63% |
| dpsc-11750 | 9.9 days | 62% |
| dpsc-11836 | 11.2 days | 67% |
| dpsc-17322 | 10.6 days | 66% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truman, B.; Ma, L.; Stewart, S.; Kingsley, K.; Sullivan, V. Assessment of Endocyn on Dental Pulp Stem Cells (DPSCs): A Pilot Study of Endodontic Irrigant Effects. Methods Protoc. 2025, 8, 18. https://doi.org/10.3390/mps8010018
Truman B, Ma L, Stewart S, Kingsley K, Sullivan V. Assessment of Endocyn on Dental Pulp Stem Cells (DPSCs): A Pilot Study of Endodontic Irrigant Effects. Methods and Protocols. 2025; 8(1):18. https://doi.org/10.3390/mps8010018
Chicago/Turabian StyleTruman, Brennan, Linda Ma, Samuel Stewart, Karl Kingsley, and Victoria Sullivan. 2025. "Assessment of Endocyn on Dental Pulp Stem Cells (DPSCs): A Pilot Study of Endodontic Irrigant Effects" Methods and Protocols 8, no. 1: 18. https://doi.org/10.3390/mps8010018
APA StyleTruman, B., Ma, L., Stewart, S., Kingsley, K., & Sullivan, V. (2025). Assessment of Endocyn on Dental Pulp Stem Cells (DPSCs): A Pilot Study of Endodontic Irrigant Effects. Methods and Protocols, 8(1), 18. https://doi.org/10.3390/mps8010018






