In Vivo Imaging of Cobalt-Induced Ocular Toxicity in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
- Mice and Experimental Design
- Optical Coherence Tomography (OCT)
- Manganese-Enhanced MRI (MEMRI)
- Histology
- Immunohistochemistry
- TUNEL Assays
- Data Analysis
- Statistical Analysis
3. Results
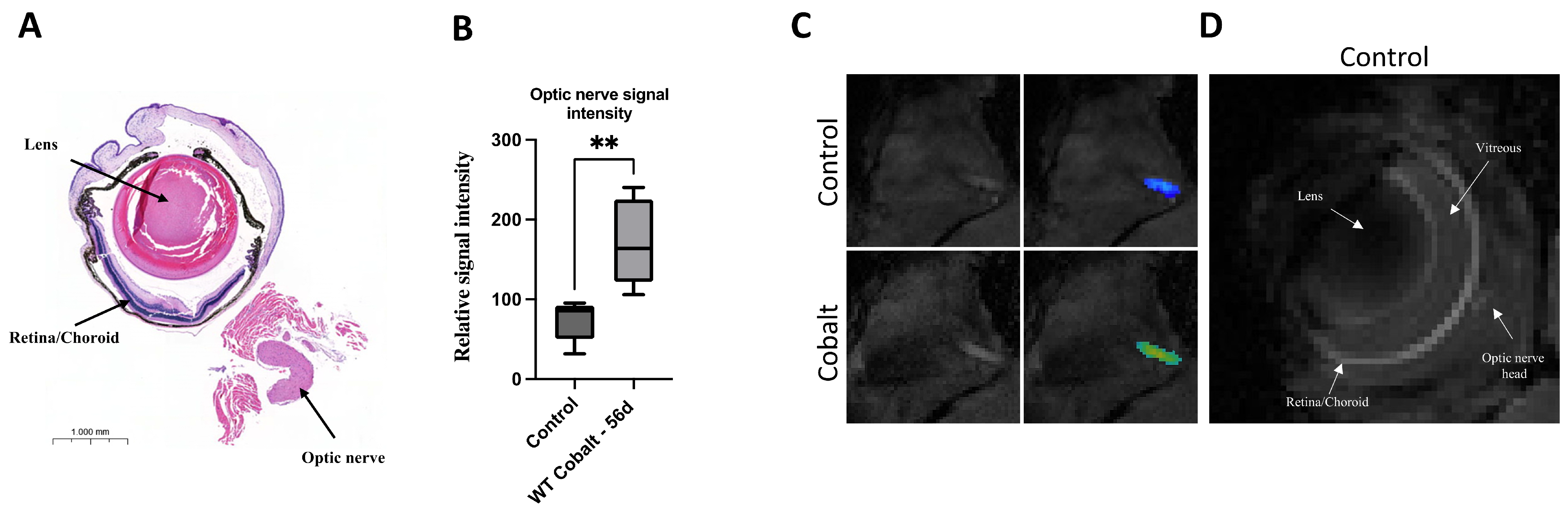
3.1. MRI
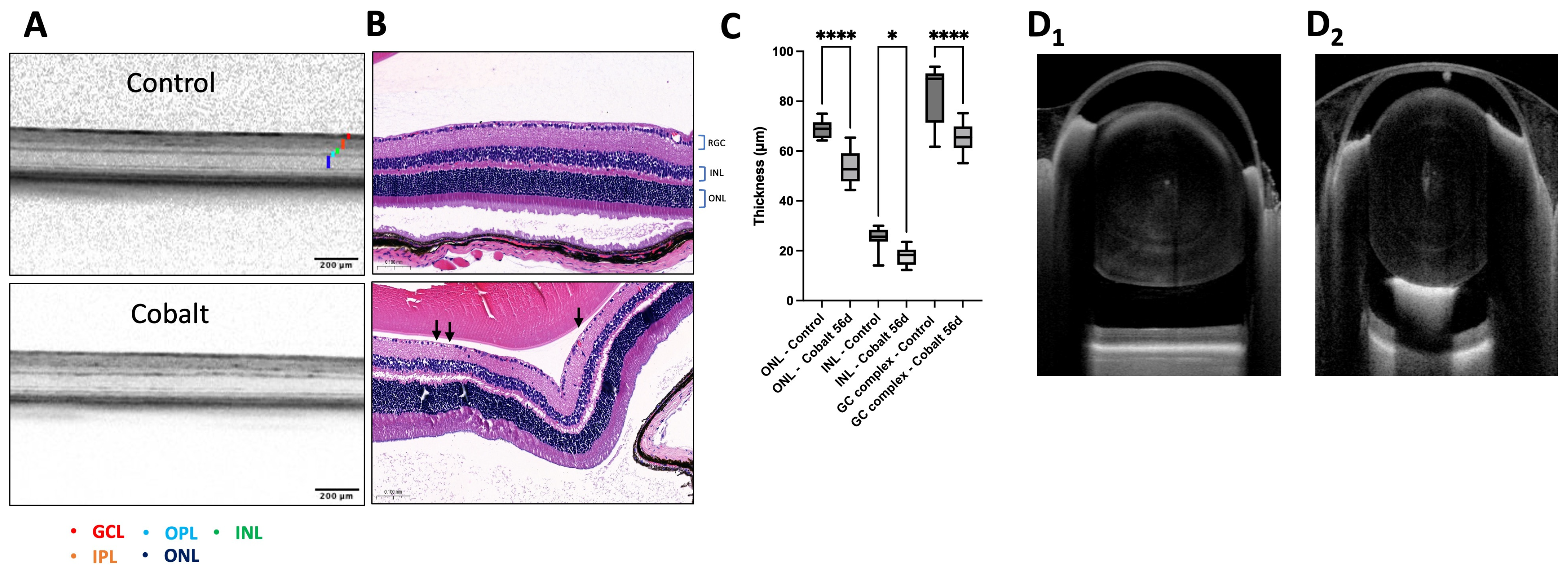
3.2. OCT Imaging
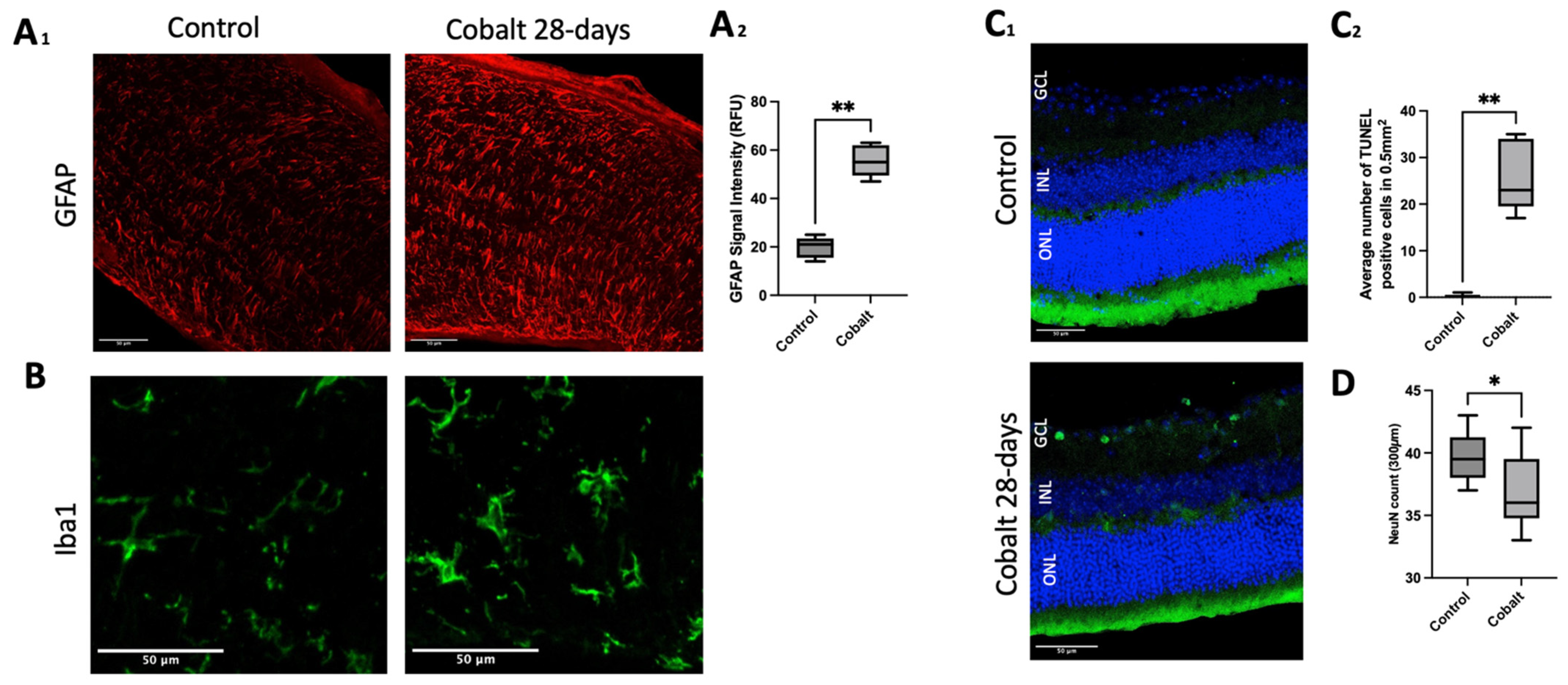
3.3. Histology and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Grillo, L.M.; Nguyen, H.V.; Tsang, S.H.; Hood, D.C.; Odel, J.G. Cobalt-chromium metallosis with normal electroretinogram. J. Neuroophthalmol. 2016, 36, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.R.J.; Meek, R.M.D.; Tate, R.; MacMillan, S.; Grant, M.H.; Currie, S. Cobalt-induced cardiomyopathy—Do circulating cobalt levels matter? Bone Joint Res. 2021, 10, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Castrillo Bustamante, C.; Canteli Álvarez, Á.; Burgos Palacios, V.; Sarralde Aguayo, J.A.; Serrano Lozano, D.; Arana Achaga, X.; Nuñez Rodríguez, Á.; Cobo Belaustegui, M. A case report of cobalt cardiomyopathy leading to electric storm and cardiogenic shock: The importance of the orthopaedic background in patients with heart failure of unknown aetiology. Eur. Heart J. Case Rep. 2021, 5, ytab057. [Google Scholar] [CrossRef] [PubMed]
- Morin, Y.L.; Foley, A.R.; Martineau, G.; Roussel, J. Quebec beer-drinkers’ cardiomyopathy: Forty-eight cases. Can. Med. Assoc. J. 1967, 97, 881–883. [Google Scholar]
- Packer, M. Cobalt cardiomyopathy: A critical reappraisal in light of a recent resurgence. Circ. Heart Fail. 2016, 9, e003604. [Google Scholar] [CrossRef] [PubMed]
- Tower, S.S. Arthroprosthetic cobaltism: Neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: A case report. J. Bone Jt. Surg. Am. 2010, 92, 2847–2851. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.M.; Arac, A.; Shieh, P.B. Hearing and vision loss in an older man. JAMA Neurol. 2018, 75, 1439–1440. [Google Scholar] [CrossRef]
- Catalani, S.; Rizzetti, M.C.; Padovani, A.; Apostoli, P. Neurotoxicity of cobalt. Hum. Exp. Toxicol. 2012, 31, 421–437. [Google Scholar] [CrossRef]
- Apostoli, P.; Catalani, S.; Zaghini, A.; Mariotti, A.; Poliani, P.L.; Vielmi, V.; Semeraro, F.; Duse, S.; Porzionato, A.; Macchi, V.; et al. High doses of cobalt induce optic and auditory neuropathy. Exp. Toxicol. Pathol. 2013, 65, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Niwa, M.; Aoki, H.; Kumada, M.; Kunisada, T.; Oyama, T.; Yamamoto, T.; Kozawa, O.; Mori, H. A new model of retinal photoreceptor cell degeneration induced by a chemical hypoxia-mimicking agent, cobalt chloride. Brain Res. 2006, 1109, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Steens, W.; von Foerster, G.; Katzer, A. Severe cobalt poisoning with loss of sight after ceramic-metal pairing in a hip—A case report. Acta Orthop. 2006, 77, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.K.; Ebneter, A.; Gilhotra, J.S. Hip-implant related chorio-retinal cobalt toxicity. Indian J. Ophthalmol. 2013, 61, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Apel, W.; Stark, D.; Stark, A.; O’Hagan, S.; Ling, J. Cobalt-chromium toxic retinopathy case study. Doc. Ophthalmol. 2013, 126, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Rizzetti, M.C.; Liberini, P.; Zarattini, G.; Catalani, S.; Pazzaglia, U.; Apostoli, P.; Padovani, A. Loss of sight and sound. Could it be the hip? Lancet 2009, 373, 1052. [Google Scholar] [CrossRef]
- Garcia, M.D.; Hur, M.; Chen, J.J.; Bhatti, M.T. Cobalt toxic optic neuropathy and retinopathy: Case report and review of the literature. Am. J. Ophthalmol. Case Rep. 2020, 17, 100606. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Fujimoto, J.G. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 2008, 27, 45–88. [Google Scholar] [CrossRef]
- van Velthoven, M.E.; Faber, D.J.; Verbraak, F.D.; van Leeuwen, T.G.; de Smet, M.D. Recent developments in optical coherence tomography for imaging the retina. Prog. Retin. Eye Res. 2007, 26, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.P.; Choo-Smith, L.P.; Flueraru, C.; Mao, Y.; Chang, S.; Disano, J.; Sherif, S.; Sowa, M.G. Optical coherence tomography: Fundamental principles, instrumental designs and biomedical applications. Biophys. Rev. 2011, 3, 155. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Faiq, M.A.; Liu, C.; Adi, V.; Chan, K.C. Applications of manganese-enhanced magnetic resonance imaging in ophthalmology and visual neuroscience. Front. Neural Circuits. 2019, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.D.; Woodall, R.L.; Kitching, R.E.; Baseler, H.A.; Morland, A.B. Using magnetic resonance imaging to assess visual deficits: A review. Ophthalmic Physiol. Opt. 2016, 36, 240–265. [Google Scholar] [CrossRef]
- Zheng, F.; Li, Y.; Zhang, F.; Sun, Y.; Zheng, C.; Luo, Z.; Wang, Y.L.; Aschner, M.; Zheng, H.; Lin, L.; et al. Cobalt induces neurodegenerative damages through Pin1 inactivation in mice and human neuroglioma cells. J. Hazard. Mater. 2021, 419, 126378. [Google Scholar] [CrossRef]
- Jagodzinska, J.; Sarzi, E.; Cavalier, M.; Seveno, M.; Baecker, V.; Hamel, C.; Péquignot, M.; Delettre, C. Optical coherence tomography: Imaging mouse retinal ganglion cells in vivo. J. Vis. Exp. 2017, 127, 55865. [Google Scholar] [CrossRef]
- Kiernan, D.F.; Mieler, W.F.; Hariprasad, S.M. Spectral-domain optical coherence tomography: A comparison of modern high-resolution retinal imaging systems. Am. J. Ophthalmol. 2010, 149, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.D.; Huber, G.; Beck, S.C.; Tanimoto, N.; Muehlfriedel, R.; Fahl, E.; Grimm, C.; Wenzel, A.; Remé, C.E.; van de Pavert, S.A.; et al. Noninvasive, in vivo assessment of mouse retinal structure using optical coherence tomography. PLoS ONE 2009, 4, e7507. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Wehbe, H.; Jiao, S.; Gregori, G.; Jockovich, M.E.; Hackam, A.; Duan, Y.; Puliafito, C.A. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Fernández-Sánchez, L.; Sauvé, Y.; Segura, F.J.; Martínez-Navarrete, G.; Tamarit, J.M.; Fuentes-Broto, L.; Sanchez-Cano, A.; Pinilla, I. Correlation between SD-OCT, immunocytochemistry and functional findings in an animal model of retinal degeneration. Front. Neuroanat. 2014, 8, 151. [Google Scholar] [CrossRef]
- Anger, E.M.; Unterhuber, A.; Hermann, B.; Sattmann, H.; Schubert, C.; Morgan, J.E.; Cowey, A.; Ahnelt, P.K.; Drexler, W. Ultrahigh resolution optical coherence tomography of the monkey fovea. Identification of retinal sublayers by correlation with semithin histology sections. Exp. Eye Res. 2004, 78, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Puoris’haag, M.; Maguluri, G.N.; Umino, Y.; Cusato, K.; Barlow, R.B.; de Boer, J.F. Monitoring mouse retinal degeneration with high-resolution spectral-domain optical coherence tomography. J. Vis. 2008, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.J.; Wojtkowski, M.; Witkin, A.J.; Duker, J.S.; Ko, T.H.; Carvalho, M.; Schuman, J.S.; Kowalczyk, A.; Fujimoto, J.G. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 2006, 113, 2054–2065. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Kim, U.S. Clinical characteristics depending on magnetic resonance imaging patterns in idiopathic isolated optic neuritis. Sci. Rep. 2023, 13, 2053. [Google Scholar] [CrossRef]
- Pautler, R.G.; Silva, A.C.; Koretsky, A.P. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn. Reson. Med. 1998, 40, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Ai, T.; Goerner, F.; Hu, X.; Runge, V.M.; Tweedle, M. MRI contrast agents: Basic chemistry and safety. J. Magn. Reson. Imaging 2012, 36, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Massaad, C.A.; Pautler, R.G. Manganese-enhanced magnetic resonance imaging (MEMRI). Methods Mol. Biol. 2011, 711, 145–174. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Kidd, G.J.; Mahad, D.; Kiryu-Seo, S.; Avishai, A.; Komuro, H.; Trapp, B.D. Myelination and axonal electrical activity modulate the distribution and motility of mitochondria at CNS nodes of Ranvier. J. Neurosci. 2011, 31, 7249–7258. [Google Scholar] [CrossRef] [PubMed]
- Hervouet, E.; Pecina, P.; Demont, J.; Vojtísková, A.; Simonnet, H.; Houstek, J.; Godinot, C. Inhibition of cytochrome c oxidase subunit 4 precursor processing by the hypoxia mimic cobalt chloride. Biochem. Biophys. Res. Commun. 2006, 344, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Clyne, N.; Hofman-Bang, C.; Haga, Y.; Hatori, N.; Marklund, S.L.; Pehrsson, S.K.; Wibom, R. Chronic cobalt exposure affects antioxidants and ATP production in rat myocardium. Scand. J. Clin. Lab. Investig. 2001, 61, 609–614. [Google Scholar] [CrossRef]
- Millecamps, S.; Julien, J.P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 161–176. [Google Scholar] [CrossRef]
- Harris, A.; Johnson, J.; Mansuripur, P.K.; Limbird, R. Cobalt toxicity after revision to a metal-on-polyethylene total hip arthroplasty for fracture of ceramic acetabular component. Arthroplast. Today 2015, 1, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Wong-Riley, M.T. Energy metabolism of the visual system. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef]
- Goldenberg-Cohen, N.; Guo, Y.; Margolis, F.; Cohen, Y.; Miller, N.R.; Bernstein, S.L. Oligodendrocyte dysfunction after induction experimental anterior optic nerve ischemia. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.L.; Guo, Y.; Kelman, S.E.; Flower, R.W.; Johnson, M.A. Functional and Cellular Responses in a Novel Rodent Model of Anterior Ischemic Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4153–4162. [Google Scholar] [CrossRef]
- Obied, B.; Richard, S.; Zahavi, A.; Fixler, D.; Girshevitz, O.; Goldenberg-Cohen, N. Structure–Function Correlation in Cobalt-Induced Brain Toxicity. Cells 2024, 13, 1765. [Google Scholar] [CrossRef] [PubMed]
- Obied, B.; Richard, S.; Zahavi, A.; Kreizman-Shefer, H.; Bajar, J.; Fixler, D.; Krmpotic, M.; Girshevitz, O.; Goldenberg-Cohen, N. Cobalt Toxicity Induces Retinopathy and Optic Neuropathy in Mice. Investig. Ophthalmol. Vis. Sci. 2024, 65, 59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obied, B.; Saar, G.; Richard, S.; Rotenstreich, Y.; Sher, I.; Zahavi, A.; Goldenberg-Cohen, N. In Vivo Imaging of Cobalt-Induced Ocular Toxicity in a Mouse Model. Methods Protoc. 2025, 8, 1. https://doi.org/10.3390/mps8010001
Obied B, Saar G, Richard S, Rotenstreich Y, Sher I, Zahavi A, Goldenberg-Cohen N. In Vivo Imaging of Cobalt-Induced Ocular Toxicity in a Mouse Model. Methods and Protocols. 2025; 8(1):1. https://doi.org/10.3390/mps8010001
Chicago/Turabian StyleObied, Basel, Galit Saar, Stephen Richard, Ygal Rotenstreich, Ifat Sher, Alon Zahavi, and Nitza Goldenberg-Cohen. 2025. "In Vivo Imaging of Cobalt-Induced Ocular Toxicity in a Mouse Model" Methods and Protocols 8, no. 1: 1. https://doi.org/10.3390/mps8010001
APA StyleObied, B., Saar, G., Richard, S., Rotenstreich, Y., Sher, I., Zahavi, A., & Goldenberg-Cohen, N. (2025). In Vivo Imaging of Cobalt-Induced Ocular Toxicity in a Mouse Model. Methods and Protocols, 8(1), 1. https://doi.org/10.3390/mps8010001





