Generating Cytokines and Growth Factors for Functional Activity: Feasibility of Method Using MIF Protein
Abstract
1. Introduction
2. Experimental Design
2.1. Materials
- Complete DMEM media: 500 mL DMEM, high glucose (Gibco, Grand Island, NY, USA, Catalog # 11965092), 50 mL (10%) Heat Inactivated Fetal Bovine Serum (Gibco, Grand Island, NY, USA, Catalog # 10082147), 5 mL (1X) Pen/Strep (Gibco, Grand Island, NY, USA, Catalog # 15140122), and 5 mL (1%) Sodium Pyruvate (Gibco, Grand Island, NY, USA, Catalog # 11360070).
- Fisherbrand™ Surface Treated Sterile Tissue Culture Flasks, Vented Cap (Fisher Healthcare, Houston, TX, USA, Catalog # FB012937).
- Cell counting slides (Bio-Rad, Hercules, CA, USA, Catalog # 1450015).
- Trypsin-EDTA (0.05%), phenol red (Gibco, Grand Island, NY, USA, Catalog # 25300054).
- Opti-MEM™ I Reduced Serum Medium (Gibco, Grand Island, NY, USA, Catalog # 31985070).
- pcDNA3 Mammalian Expression Vector (Invitrogen, Carlsbad, CA, USA, Catalog # V79020).
- FuGENE® HD Transfection Reagent (Promega, Madison, WI, USA, Catalog # E2311).
- Corning™ Costar™ 12-well Clear TC-treated Multiple Well Plates, Individually Wrapped, Sterile (Fisher Healthcare, Houston, TX, USA, Catalog # 07-200-82).
- Geneticin™ Selective Antibiotic (G418 Sulfate) (50 mg/mL) (Gibco, Grand Island, NY, USA, Catalog # 10131035).
- Caco-2 cell line (ATCC, Manassas, VA, USA, Catalog # HTB-37™).
- Amicon Ultra centrifugal filter units 30 kDa (Millipore Sigma, Burlington, MA, USA, Catalog # UFC903024).
- Pierce™ Anti-DYKDDDDK Magnetic Agarose (Thermo Fisher, Waltham, MA, USA, Catalog # A36797).
- Optional: Pierce™ 3x DYKDDDDK Peptide (Thermo Fisher, Waltham, MA, USA, Catalog # A36805).
- Wash buffer (DPBS, no calcium, no magnesium, Thermo Fisher, Waltham, MA, USA, Catalog # 14190144).
- Elution buffer (0.1 M glycine, pH 2.8). Glycine (Millipore Sigma, Burlington, MA, USA, Catalog # G8898).
- Neutralization buffer (1 M Tris-Base, pH 8). Tris-Base (Millipore Sigma, Burlington, MA, USA, Catalog # T6066).
2.2. Equipment
- 37 °C, 5% CO2 incubator (Thermo Scientific, Waltham, MA, USA, Catalog # TH-3110).
- Cell culture microscope (Labomed Inc., Culver City, CA, USA, Catalog # S96062).
- Cell counter (Bio-Rad, Hercules, CA, USA, Catalog # 1450102).
- Centrifuge (VWR, Radnor, PA, USA, Catalog # B30316).
- Magnetic separation rack (Cytiva, Marlborough, MA, USA, Catalog # GE28948964).
- Tube rotator at 4 °C (Fisher Scientific, Waltham, MA, USA, Catalog # 88861049).
- Optional: Pulsing vortex mixer (Fisherbrand, Waltham, MA, USA, Catalog # 02-215-422).
3. Procedure
3.1. Human Cell Line Selection
- 17.
- In the Human Protein Atlas database, Version 23, (https://www.proteinatlas.org/, accessed on 19 June 2023), enter MIF into the search bar. We used MIF for our experiments, but any protein of interest can be selected.
- 18.
- Under MIF, select your cell line. Here, we used the intestinal cell line Caco2 because it expresses MIF and the cell type was relevant for our work. You can choose other cell lines depending on the nature of the study. The American Type Culture Collection (ATCC) has a collection of more than 4000 cell lines that are available for purchase.
3.2. Stable Caco2 Human Cell Line Generation under Antibioitc Selection
- 19.
- Count Caco2 cells using an automated cell counter, and, in a 12-well plate, seed 1 × 105 cells per well in 1 mL Complete DMEM media. Note: the media used will be cell type dependent.
- 20.
- Incubate at 37 °C.
- 21.
- When cells reach 60–70% confluency, transfect Caco-2 cells by adding a 50 µL mixture of pcDNA3 expression vector containing c-terminal FLAG-tagged MIF and FuGENE transfection reagent in Opti-MEM media. The plasmid amount should be 1 µg. FuGENE transfection reagent:plasmid DNA volume ratio should be around 3:1. Add 37 °C prewarmed Opti-MEM media to make up the final 50 µL volume.
- 22.
- At 48 h after transfection, aspirate the old media. Add 1 mL of selective media, which is complete DMEM with 400 μg/mL G418, to each well. The pcDNA3 vector contains a neomycin resistance gene that allows for selection with G418. The concentration of G418 may vary depending on the cell type.
- 23.
- For the next week, replace the selective media every 2–3 days.
- 24.
- When the transfected cells have distinct colonies (cells are growing in patches), split the cells into a tissue culture flask.
3.3. FLAG-Tagged MIF Preparation and Protein Purification
- 25.
- Grow four tissue culture flasks of Caco-2 cells expressing FLAG-tagged MIF in 15 mL selective media until cells are 70–80% confluent. No additional tags were present in the MIF protein.
- 26.
- Collect cell culture media from all four flasks into 50 mL centrifuge tubes. You will have a total of 60 mL of media from the four flasks, so you can put 30 mL into two 50 mL tubes.
- 27.
- Centrifuge the media at 3.5 k rpm for 15 min at 4 °C and collect the supernatant with desired secreted protein.
- 28.
- Transfer 15 mL of supernatant into an Amicon centrifugal filter unit. An amount of 15 mL is the capacity for the centrifugal filter unit. Minimize the amount of cell debris you add, because it might clog the centrifugal filter unit.
- 29.
- Centrifuge at 3.5 k rpm for 15 min at 4 °C.
- 30.
- Transfer concentrated media into a 15 mL tube.
- 31.
- Repeat steps 25–27 for remaining media (anticipate 3 mL of concentrated media per 15 mL tube; therefore, if you started with four flasks, you should have 12 mL of concentrated media).
- 32.
- Add 50 μL of anti-DYKDDDDK magnetic agarose to the 15 mL tube.
- 33.
- Incubate the beads overnight while rotating at 4 °C.
- 34.
- Place 15 mL tubes on magnetic separation rack so it binds the magnetic beads and then pour off the media without the beads.
- 35.
- Resuspend magnetic agarose in 1 mL DPBS and transfer it to 1.5 mL tubes.
- 36.
- Wash magnetic agarose with 1 mL wash buffer (DPBS, no calcium, no magnesium). Place tubes on magnetic separation rack and pour off the wash buffer.
- 37.
- Repeat step 36 two times for a total of three washes.
- 38.
- Incubate the beads in 50 μL elution buffer (0.1 M glycine, pH 2.8) for 2 min at room temperature. Neutralize with 5 μL neutralization buffer (1 M Tris-Base, pH 8).
- 39.
- Optional: Vortex for 5 min at 1 k rpm.
- 40.
- Place 1.5 mL tubes on magnetic separation rack and collect the elute containing the FLAG-tagged MIF in separate 1.5 mL tubes.
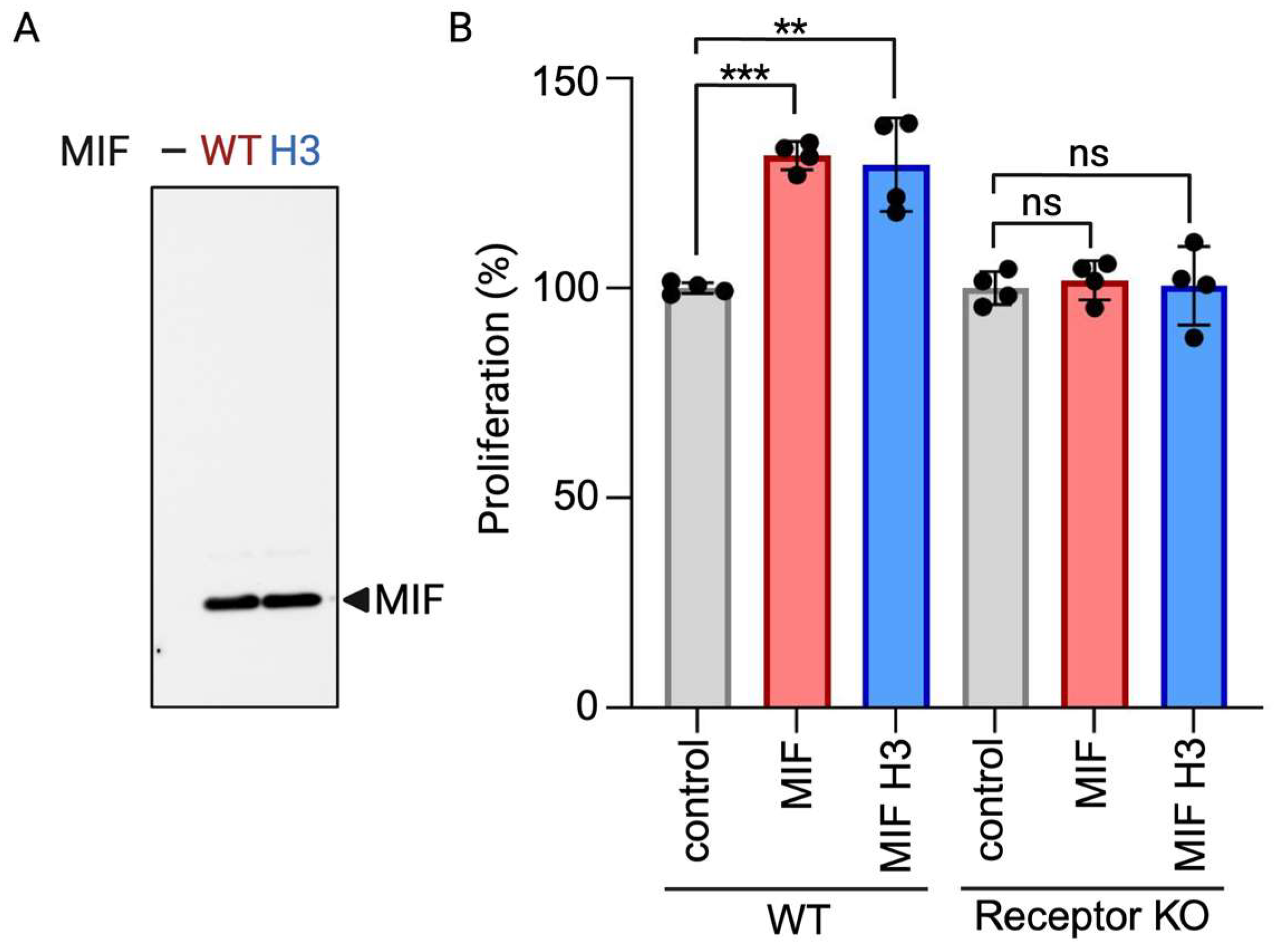
4. Expected Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bohnacker, S.; Hildenbrand, K.; Aschenbrenner, I.; Müller, S.I.; Esser-von Bieren, J.; Feige, M.J. Influence of glycosylation on IL-12 family cytokine biogenesis and function. Mol. Immunol. 2020, 126, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based human protein atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, C.; Zwahlen, M.; Von Feilitzen, K.; Karlsson, M.; Shi, M.; Yuan, M.; Song, X.Y.; Li, X.Y.; Yang, H.; et al. Systematic transcriptional analysis of human cell lines for gene expression landscape and tumor representation. Nat. Commun. 2023, 14, 5417. [Google Scholar] [CrossRef]
- Farr, L.; Ghosh, S.; Moonah, S. Role of MIF cytokine/CD74 receptor pathway in protecting against injury and promoting repair. Front. Immunol. 2020, 11, 1273. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Qian, X.; Wang, Y.; Lee, J.-H.; Xia, Y.; Hawake, D.H.; Zhang, G.; Lyu, J.X.; Lu, Z.M. Secreted and O-GlcNAcylated MIF binds to the human EGF receptor and inhibits its activation. Nat. Cell Biol. 2015, 17, 1348–1355. [Google Scholar] [CrossRef]
- Schindler, L.; Dickerhof, N.; Hampton, M.B.; Bernhagen, J. Post-translational regulation of macrophage migration inhibitory factor: Basis for functional fine-tuning. Redox. Biol. 2018, 15, 135–142. [Google Scholar] [CrossRef]
- Tornel, W.; Sharma, I.; Osmani, H.; Moonah, S. Prosurvival Pathway Protects From Clostridioides difficile Toxin-Mediated Cell Death. J. Infect. Dis. 2024, 229, 1519–1522. [Google Scholar] [CrossRef]
- Cao, R.; Bråkenhielm, E.; Pawliuk, R.; Wariaro, D.; Post, M.J.; Wahlberg, E.; Leboulch, P.; Cao, Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 2003, 9, 604–613. [Google Scholar] [CrossRef]
- Klooster, J.P.t.; Bol-Schoenmakers, M.; van Summeren, K.; van Vliet, A.L.; de Haan, C.A.; van Kuppeveld, F.J.; Verkoeijen, S.; Pieters, R. Enterocytes, fibroblasts and myeloid cells synergize in anti-bacterial and anti-viral pathways with IL22 as the central cytokine. Commun. Biol. 2021, 4, 631. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Harrison, S.T.; Tai, S.L. Advances in bioreactor systems for the production of biologicals in mammalian cells. ChemBioEng. Rev. 2022, 9, 42–62. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.H.; Ha, T.K.; Samoudi, M.; Lewis, N.E.; Palsson, B.O.; Kildegaard, H.F.; Lee, G.M. Revealing key determinants of clonal variation in transgene expression in recombinant CHO cells using targeted genome editing. ACS Synth. Biol. 2018, 7, 2867–2878. [Google Scholar] [CrossRef] [PubMed]
- Kausar, H.; Gull, S.; Ijaz, B.; Ahmad, W.; Sarwar, M.T.; Iqbal, Z.; Nawaz, Z.; Riazuddin, S.; Hassan, S. Huh-7 cell line as an alternative cultural model for the production of human like erythropoietin (EPO). J. Transl. Med. 2011, 9, 1–8. [Google Scholar] [CrossRef]
- Zanoletti, L.; Valdata, A.; Nehlsen, K.; Faris, P.; Casali, C.; Cacciatore, R.; Sbarsi, I.; Carriero, F.; Arfini, D.; Baarle, L.V.; et al. Cytological, molecular, cytogenetic, and physiological characterization of a novel immortalized human enteric glial cell line. Front. Cell. Neurosci. 2023, 17, 11703–11709. [Google Scholar] [CrossRef]
- Nukpook, T.; Ekalaksananan, T.; Kiyono, T.; Kasemsiri, P.; Teeramatwanich, W.; Vatanasapt, P.; Chaiwiriyakul, S.; Ungarreevittaya, P.; Kampan, J.; Muisuk, K.; et al. Establishment and genetic characterization of cell lines derived from proliferating nasal polyps and sinonasal inverted papillomas. Sci. Rep. 2021, 11, 17100. [Google Scholar] [CrossRef]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef]
- Tani, T.; Mathsyaraja, H.; Campisi, M.; Li, Z.H.; Haratani, K.; Fahey, C.G.; Ota, K.; Mahadevan, N.R.; Shi, Y.X.; Saito, S.; et al. TREX1 inactivation unleashes cancer cell STING–interferon signaling and promotes antitumor immunity. Cancer Discov. 2024, 14, 752–765. [Google Scholar] [CrossRef]
- Norrman, K.; Fischer, Y.; Bonnamy, B.; Sand, W.F.; Ravassard, P.; Semb, H. Quantitative comparison of constitutive promoters in human ES cells. PLoS ONE 2010, 5, 12413. [Google Scholar] [CrossRef]
- Gossen, M.; Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. 1992, 89, 5547–5551. [Google Scholar] [CrossRef]
- Cheng, J.; Novati, G.; Pan, J.; Bycroft, C.; Žemgulytė, A.; Applebaum, T.; Pritzel, A.; Wong, L.H.; Zielinski, M.; Sargeant, T.; et al. Accurate proteome-wide missense variant effect prediction with AlphaMissense. Science 2023, 381, 7492. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.M.; Rehm, H.L. Will variants of uncertain significance still exist in 2030? Am. J. Hum. Genet. 2024, 111, 5–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmani, H.; Sharma, I.; Moonah, S. Generating Cytokines and Growth Factors for Functional Activity: Feasibility of Method Using MIF Protein. Methods Protoc. 2024, 7, 72. https://doi.org/10.3390/mps7050072
Osmani H, Sharma I, Moonah S. Generating Cytokines and Growth Factors for Functional Activity: Feasibility of Method Using MIF Protein. Methods and Protocols. 2024; 7(5):72. https://doi.org/10.3390/mps7050072
Chicago/Turabian StyleOsmani, Hiba, Ishrya Sharma, and Shannon Moonah. 2024. "Generating Cytokines and Growth Factors for Functional Activity: Feasibility of Method Using MIF Protein" Methods and Protocols 7, no. 5: 72. https://doi.org/10.3390/mps7050072
APA StyleOsmani, H., Sharma, I., & Moonah, S. (2024). Generating Cytokines and Growth Factors for Functional Activity: Feasibility of Method Using MIF Protein. Methods and Protocols, 7(5), 72. https://doi.org/10.3390/mps7050072




