Protocol for Facile Synthesis of Fmoc-N-Me-AA-OH Using 2-CTC Resin as Temporary and Reusable Protecting Group
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials and Reagents
2.1.1. Resin
- 2-Chlorotrityl chloride resin (2-CTC resin) (loading 1.59 mmol/g)
2.1.2. Fmoc-Amino Acids-OH
- Fmoc-L-βAla-OH
- Fmoc-L-Thr(tBu)-OH
2.1.3. Solvents Synthesis Grade
- N-Methyl-2-pyrrolidine (NMP)
- Methanol (MeOH)
- Anhydrous dichloromethane (anh. DCM)
2.1.4. Reagents to Activate the Resin
- Thionyl chloride
- N-Ethyl diisopropylamine (DIEA)
- Sodium hydroxide (NaOH)
2.1.5. N-Methylation Reagent
- 2-Nitrobenzenesulfonyl chloride (o-NBS-Cl)
- 1,8-Diazabicyclo [5.4.0]undec-7-ene (DBU)
- Dimethyl sulfate
- Methyl iodide
- 2,4,6-Trimethylpyridine (Collidine)
- 2-Mercaptoethanol
- 9-Fluorenylmethyl-succinimidyl carbonate (Fmoc-OSu)
- Piperidine
2.1.6. Cleavage Reagent
- Trifluoroacetic acid (TFA)
- Dichloromethane (DCM)
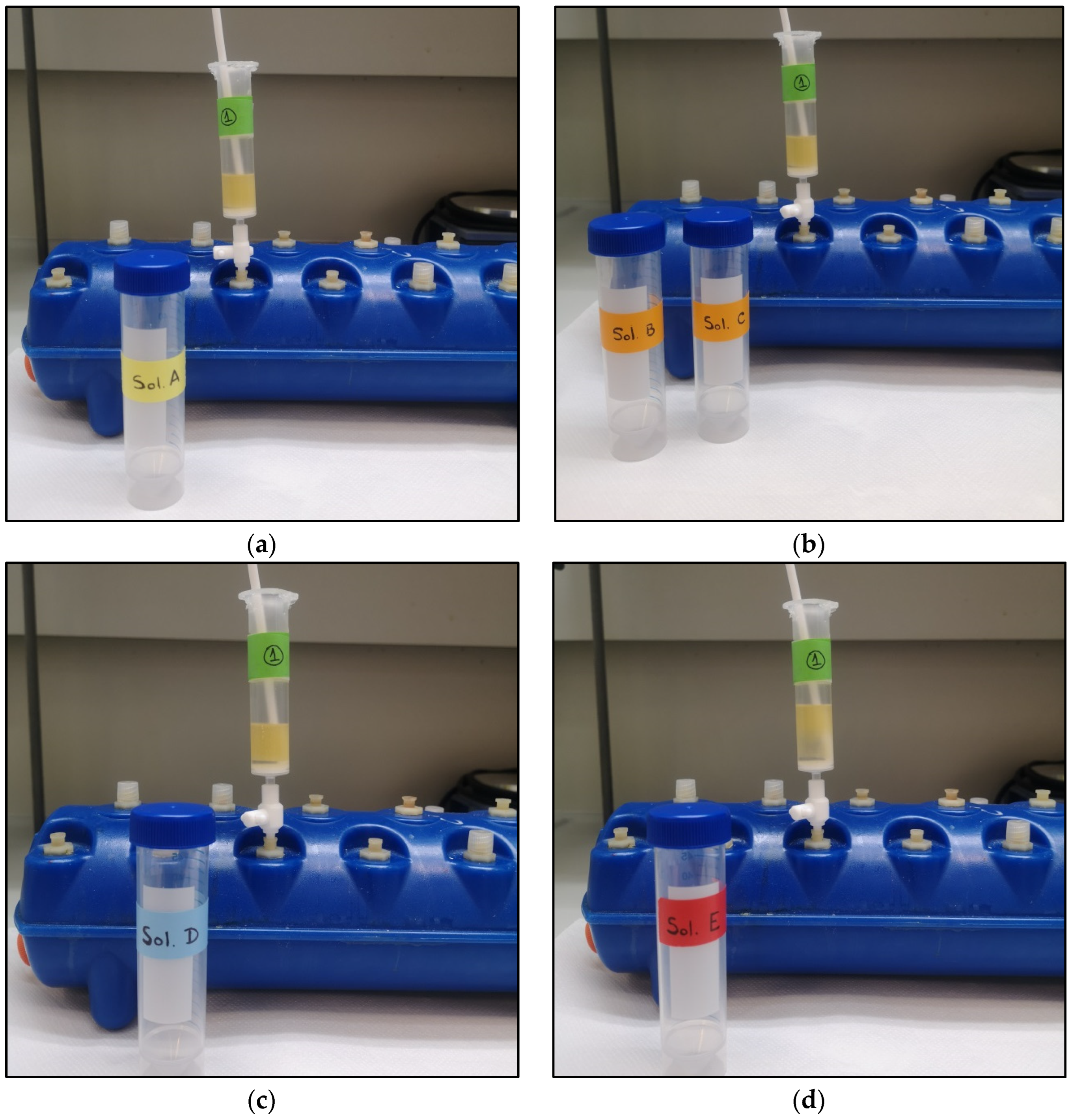
2.2. Preparation of Solutions
2.2.1. o-NBS-Cl (Solution A)
2.2.2. N-Methylation Solution
DBU Solution (Solution B)
Methylating Agent
- Dimethyl sulfate solution (Solution C1)
- Methyl iodide solution (Solution C2)
2.2.3. o-NBS-Cl Deprotection Solution (Solution D)
2.2.4. Fmoc Protection Solution (Solution E)
2.2.5. Cleavage Solution
2.3. Analytical Tests
2.3.1. Ninhydrin Test [24,25]
2.3.2. Chloranil Test [26]
2.3.3. Mini-Cleavage
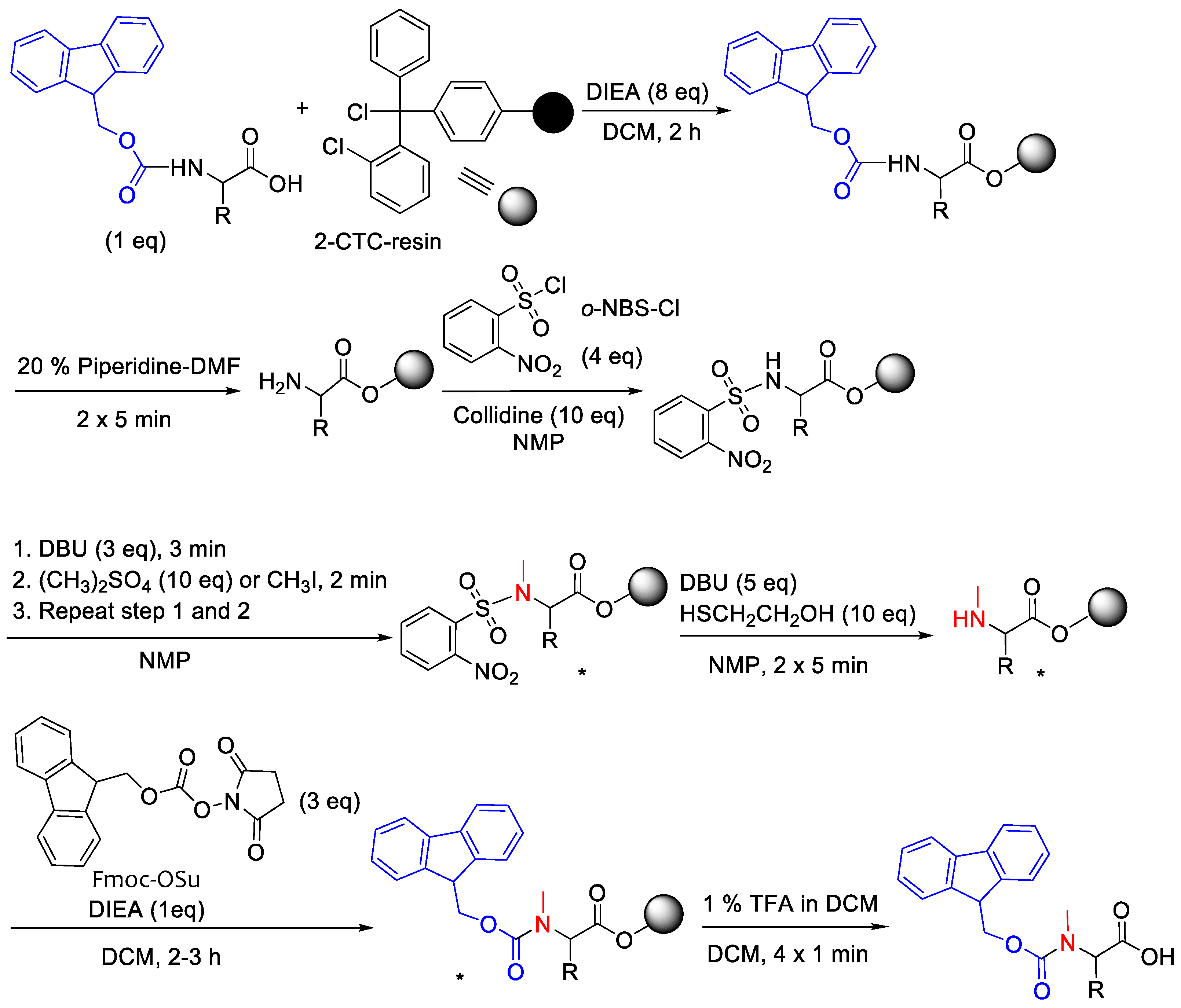
2.4. Synthetic Process
2.4.1. 2-CTC Resin Activation
2.4.2. Fmoc-Amino Acid C-Carboxylic Acid Protection and Fmoc-AA-O-CTC Resin
2.4.3. H-AA-O-CTC Resin and Loading Quantification
2.4.4. Resin Swelling
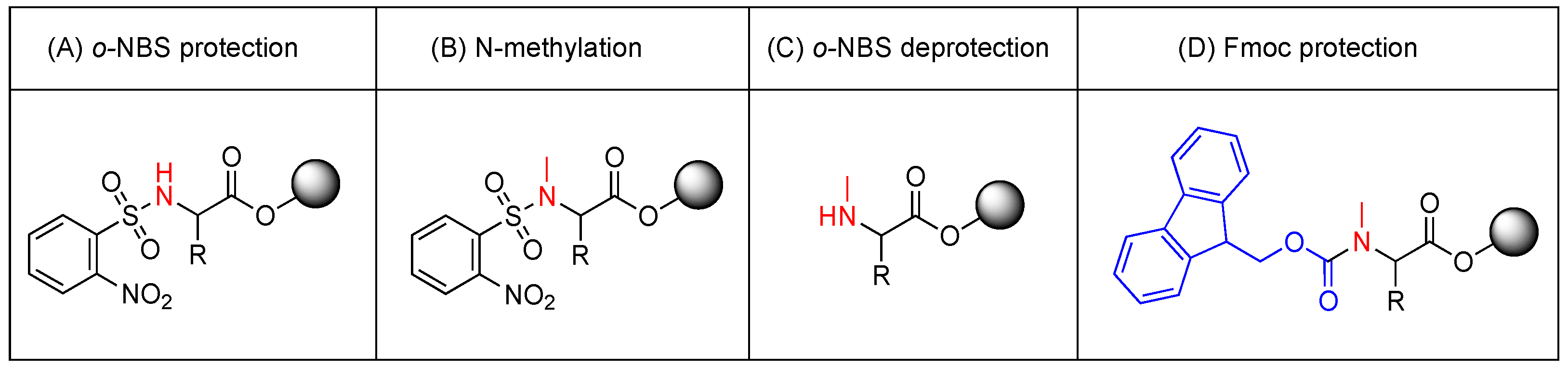
2.4.5. o-NBS-Cl Protection Activation
2.4.6. N-Methylation
2.4.7. o-NBS Removal
2.4.8. Fmoc Protection
2.4.9. Cleavage and Work-Up
2.5. Characterization
2.5.1. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
2.5.2. Liquid Chromatography-Mass Spectrometry (LC-MS)
2.5.3. Electrospray Ionization Time-of-Flight (ESI-TOF) HR-MS
2.5.4. Nuclear Magnetic Resonance (NMR)
2.6. Reuse of 2-CTC Resin
3. Results and Discussion
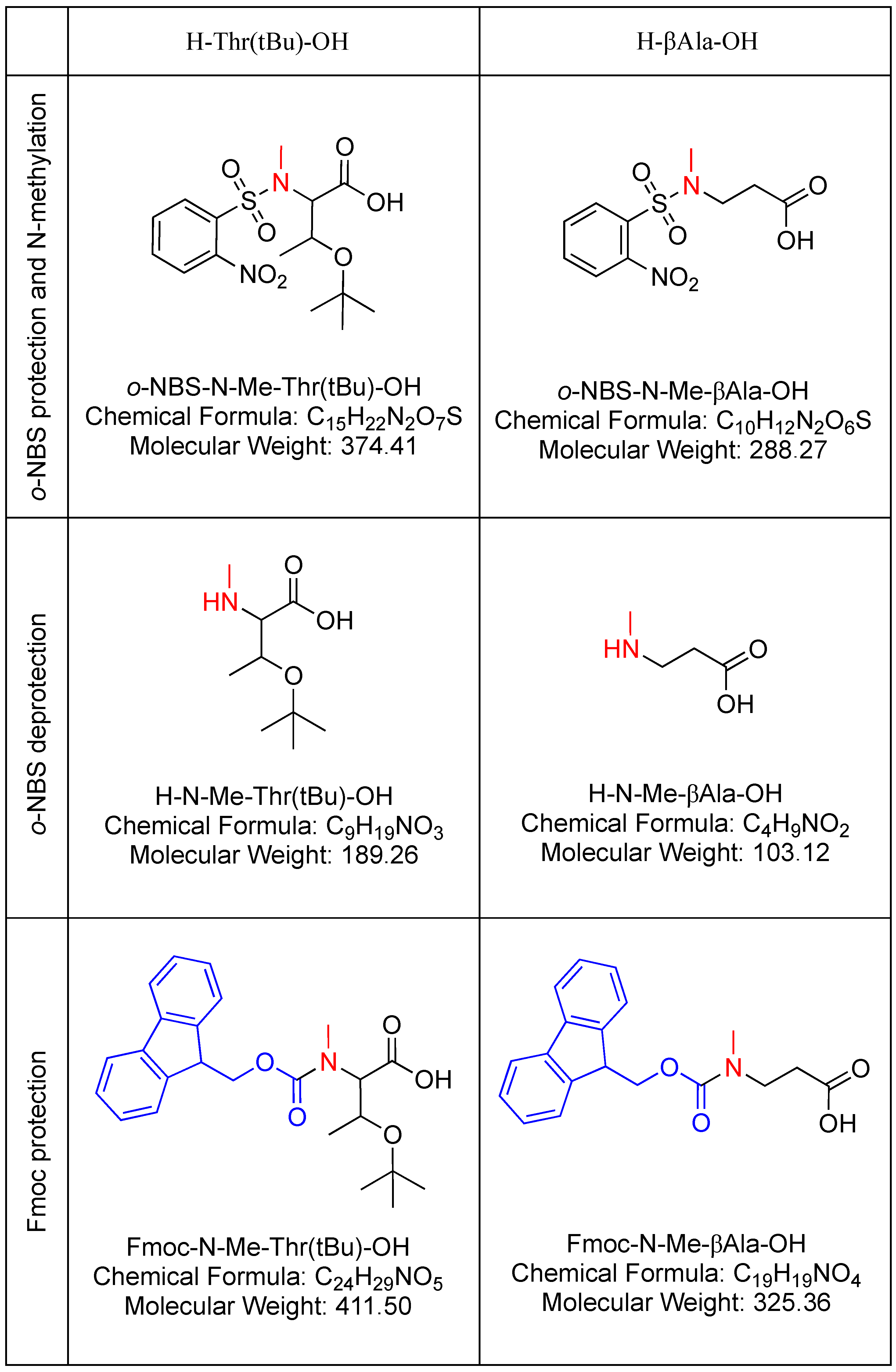
3.1. Yield and Purity
3.2. Characterization
3.3. Recycling and Reagent Usage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.L.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- D’Aloisio, V.; Dognini, P.; Hutcheon, G.A.; Coxon, C.R. PepTherDia: Database and Structural Composition Analysis of Approved Peptide Therapeutics and Diagnostics. Drug Discov. Today 2021, 26, 1409–1419. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2019, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial Host Defence Peptides: Functions and Clinical Potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- Hoang, H.N.; Hill, T.A.; Fairlie, D.P. Connecting Hydrophobic Surfaces in Cyclic Peptides Increases Membrane Permeability. Angew. Chemie 2021, 133, 8466–8471. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Abdel Monaim, S.A.H.; Jad, Y.E.; El-Faham, A.; de la Torre, B.G.; Albericio, F. N-Methylation in Amino Acids and Peptides: Scope and Limitations. Biopolymers 2018, 109, e23110. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Xu, Z. Improvement on Permeability of Cyclic Peptide/Peptidomimetic: Backbone N-Methylation as A Useful Tool. Mar. Drugs 2021, 19, 311. [Google Scholar] [CrossRef]
- Räder, A.F.B.; Reichart, F.; Weinmüller, M.; Kessler, H. Improving Oral Bioavailability of Cyclic Peptides by N-Methylation. Bioorganic Med. Chem. 2018, 26, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, M.L.; Leggio, A.; Malagrinò, F.; Romio, E.; Siciliano, C.; Liguori, A. N-Methylated α-Amino Acids And Peptides: Synthesis And Biological Activity. Mini-Rev. Med. Chem. 2016, 16, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Mo, X.; Liu, X.; Hu, B.; Zhang, Z.; Yu, Z. Arginine Methylation Regulates Self-Assembly of Peptides. Macromol. Rapid Commun. 2023, 18, 2300308. [Google Scholar] [CrossRef] [PubMed]
- Biron, E.; Kessler, H. Convenient Synthesis of N -Methylamino Acids Compatible with Fmoc Solid-Phase Peptide Synthesis. J. Org. Chem. 2005, 70, 5183–5189. [Google Scholar] [CrossRef]
- Biron, E.; Chatterjee, J.; Kessler, H. Optimized SelectiveN-Methylation of Peptides on Solid Support. J. Pept. Sci. 2006, 12, 213–219. [Google Scholar] [CrossRef]
- Chatterjee, J.; Gilon, C.; Hoffman, A.; Kessler, H. N-Methylation of Peptides: A New Perspective in Medicinal Chemistry. Acc. Chem. Res. 2008, 41, 1331–1342. [Google Scholar] [CrossRef]
- Chatterjee, J.; Laufer, B.; Kessler, H. Synthesis of N-Methylated Cyclic Peptides. Nat. Protoc. 2012, 7, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, T.; Jow, C.-K.; Cheung, M. 2- and 4-Nitrobenzenesulfonamides: Exceptionally Versatile Means for Preparation of Secondary Amines and Protection of Amines. Tetrahedron Lett. 1995, 36, 6373–6374. [Google Scholar] [CrossRef]
- Miller, S.C.; Scanlan, T.S. Site-Selective N-Methylation of Peptides on Solid Support. J. Am. Chem. Soc. 1997, 119, 2301–2302. [Google Scholar] [CrossRef]
- Chen, Y. Recent Advances in Methylation: A Guide for Selecting Methylation Reagents. Chem.–Eur. J. 2019, 25, 3405–3439. [Google Scholar] [CrossRef]
- Yang, L.; Chiu, K. Solid Phase Synthesis of Fmoc N-Methyl Amino Acids: Application of the Fukuyama Amine Synthesis. Tetrahedron Lett. 1997, 38, 7307–7310. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Leggio, A.; Liguori, A.; Perri, F. Solid-Phase Synthesis of N-Nosyl- and N-Fmoc-N-Methyl-α-Amino Acids. J. Org. Chem. 2007, 72, 3723–3728. [Google Scholar] [CrossRef]
- García-Martín, F.; Bayó-Puxan, N.; Cruz, L.J.; Bohling, J.C.; Albericio, F. Chlorotrityl Chloride (CTC) Resin as a Reusable Carboxyl Protecting Group. QSAR Comb. Sci. 2007, 26, 1027–1035. [Google Scholar] [CrossRef]
- Sarin, V.K.; Kent, S.B.H.; Tam, J.P.; Merrifield, R.B. Quantitative Monitoring of Solid-Phase Peptide Synthesis by the Ninhydrin Reaction. Anal. Biochem. 1981, 117, 147–157. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color Test for Detection of Free Terminal Amino Groups in the Solid-Phase Synthesis of Peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Vojkovsky, T. Detection of Secondary Amines on Solid Phase. Pept. Res. 1995, 8, 236–237. [Google Scholar] [PubMed]
- Luna, O.; Gomez, J.; Cárdenas, C.; Albericio, F.; Marshall, S.; Guzmán, F. Deprotection Reagents in Fmoc Solid Phase Peptide Synthesis: Moving away from Piperidine? Molecules 2016, 21, 1542. [Google Scholar] [CrossRef]





| o-NBS Deprotection Reagents | Fmoc-N-Me-[AA]-OH | Resin (mg) | Loading (mEq/g) | MW (g/mol) | Theoretical Yield (mg) | Experimental Yield (mg) | % Yield | Retention Time (tr) | % Purity |
|---|---|---|---|---|---|---|---|---|---|
| First activation of 2-CTC resin | |||||||||
| Dimethyl sulfate | 1. [βAla] | 160.2 | 1.76 | 325.4 | 91.9 | 85.3 | 92.8 | 5.253 | 98.59 |
| 3. [Thr(tBu)] | 152.5 | 1.54 | 411.5 | 97.0 | 71.1 | 73.3 | 6.007 | 90.83 | |
| Methyl iodide | 2. [βAla] | 155.5 | 1.76 | 325.4 | 89.2 | 81.5 | 91.3 | 5.257 | 94.07 |
| 4. [Thr(tBu)] | 155.5 | 1.54 | 411.5 | 98.9 | 83.7 | 84.6 | 6.050 | 93.29 | |
| REUSE: Second activation of 2-CTC resin | |||||||||
| Dimethyl sulfate | R1. [βAla] | 150.8 | 1.773 | 325.4 | 87.1 | 74.0 | 84.9 | 5.220 | 98.38 |
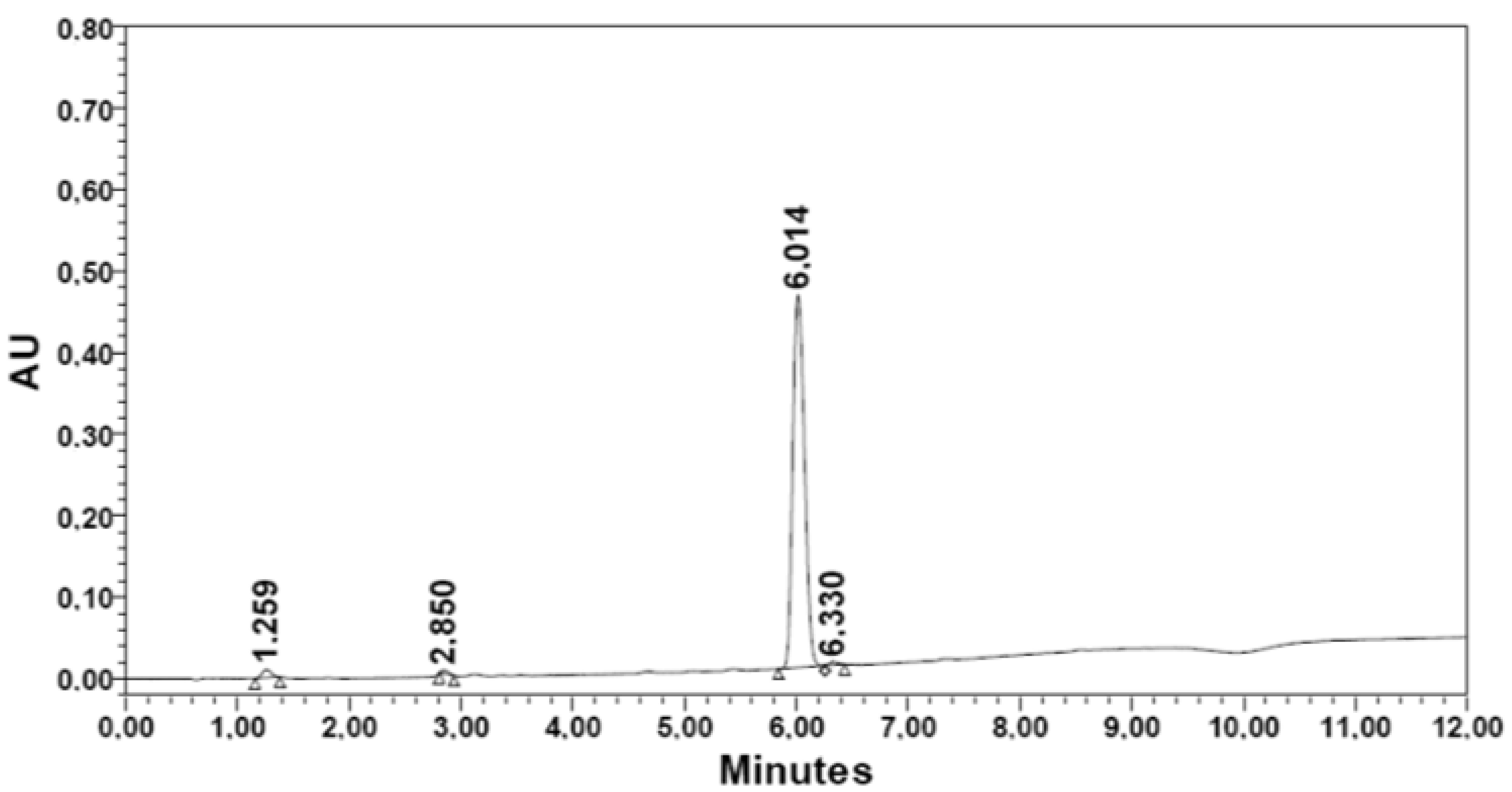
| R3. [Thr(tBu)] | 111.0 | 1.36 | 411.5 | 62.3 | 50.5 | 81.0 | 6.014 | 97.15 | |
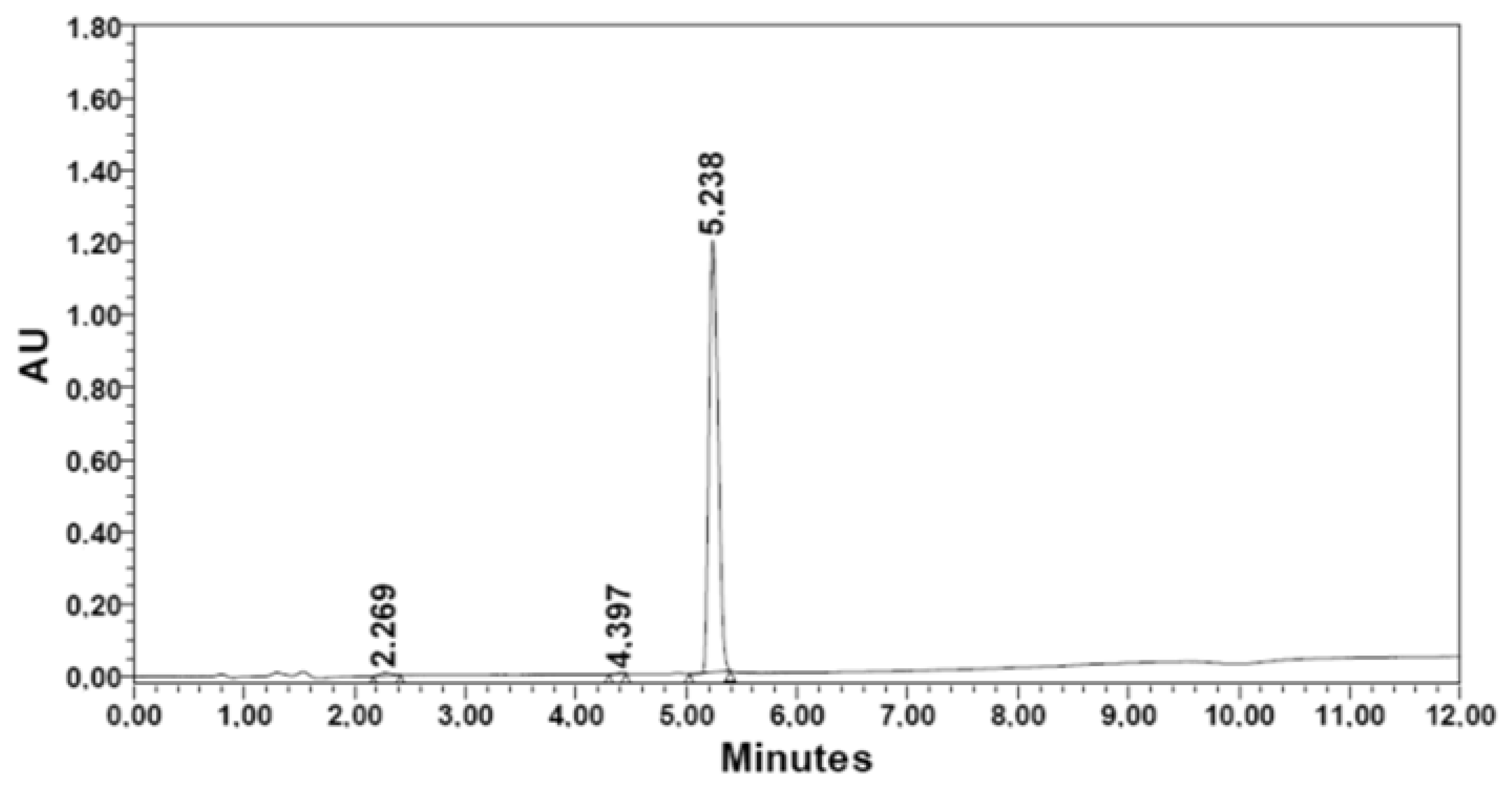
| Methyl iodide | R2. [βAla] | 141.3 | 1.816 | 325.4 | 83.5 | 67.7 | 81.1 | 5.238 | 99.2 |
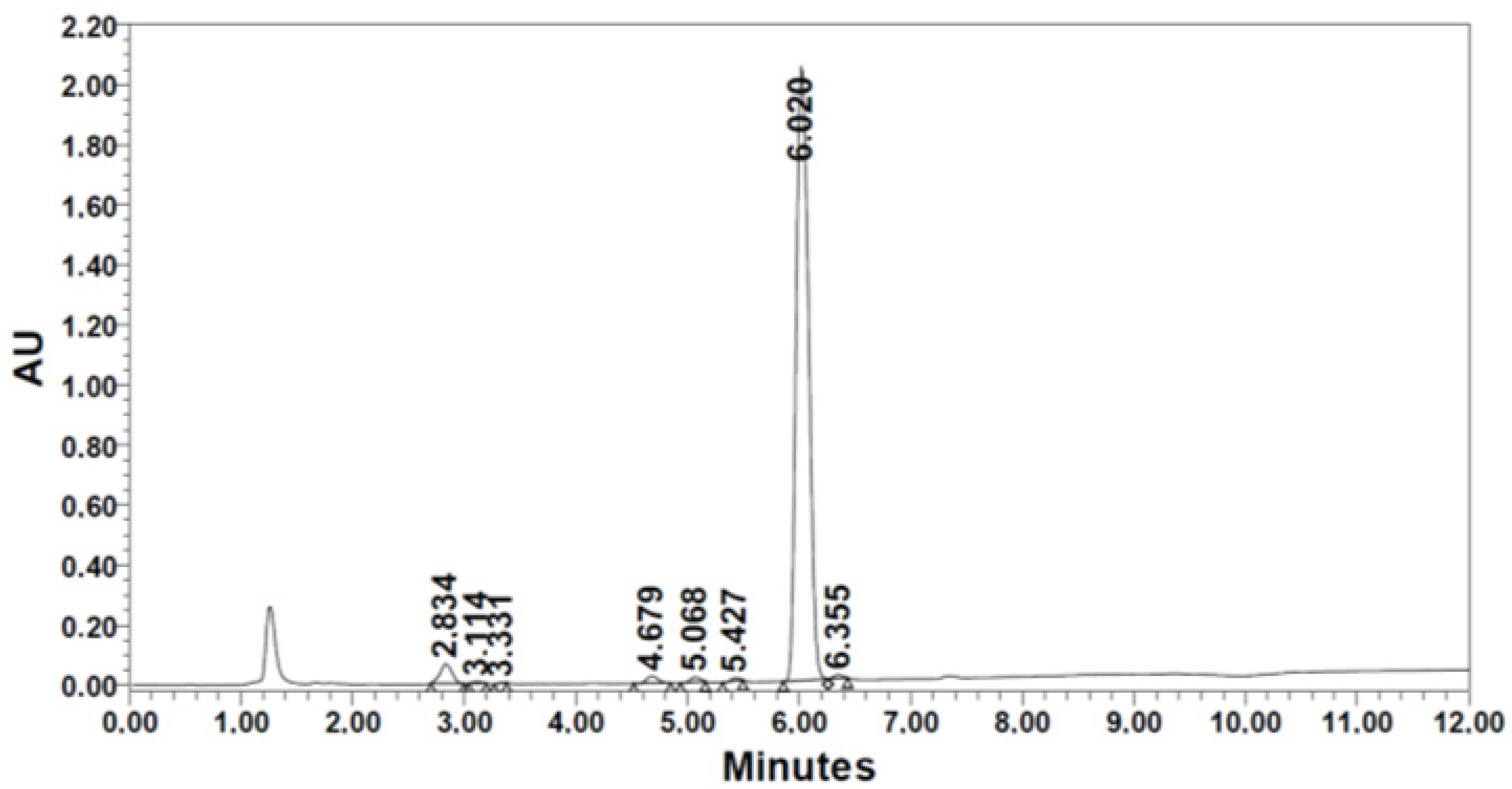
| R4. [Thr(tBu)] | 124.5 | 1.42 | 411.5 | 73.0 | 59.9 | 82.0 | 6.020 | 94.63 | |
| Fmoc-N-Me-βAla-OH | Fmoc-N-Me-Thr(tBu)-OH | ||
|---|---|---|---|
| Synthesis with first activation of 2-CTC-resin | Dimethyl sulfate | 1 | 3 |
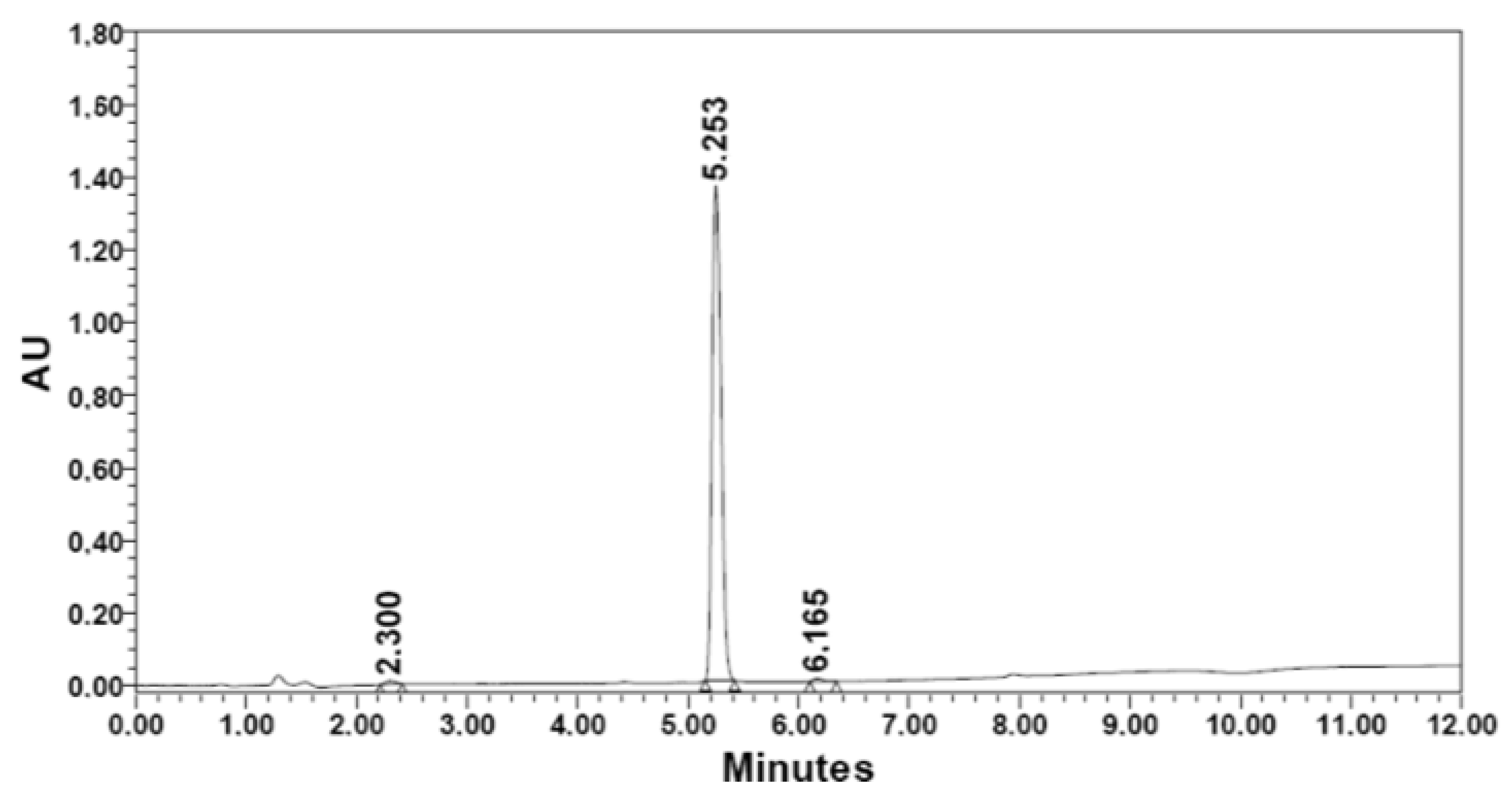 | 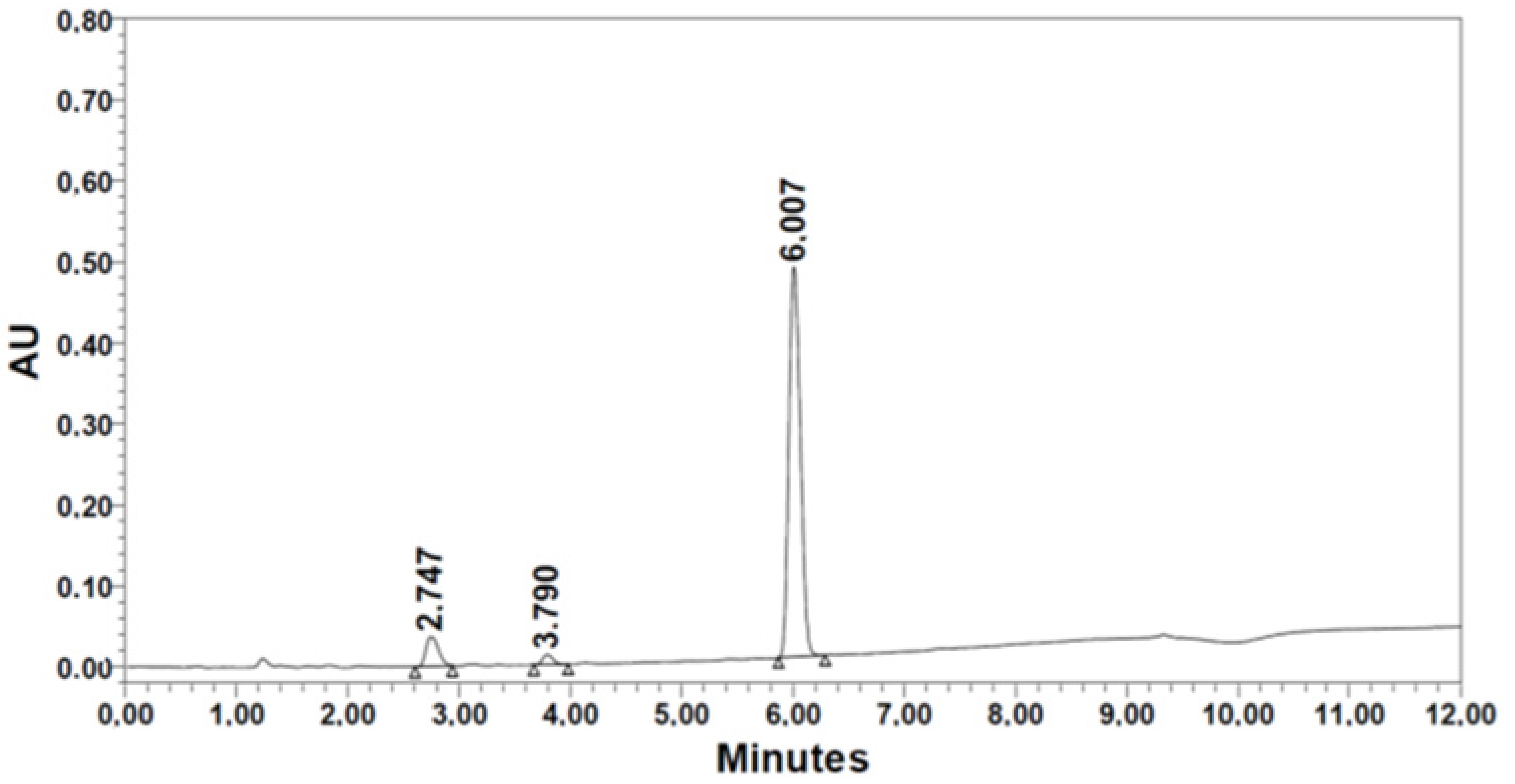 | ||
| Methyl iodide | 2 | 4 | |
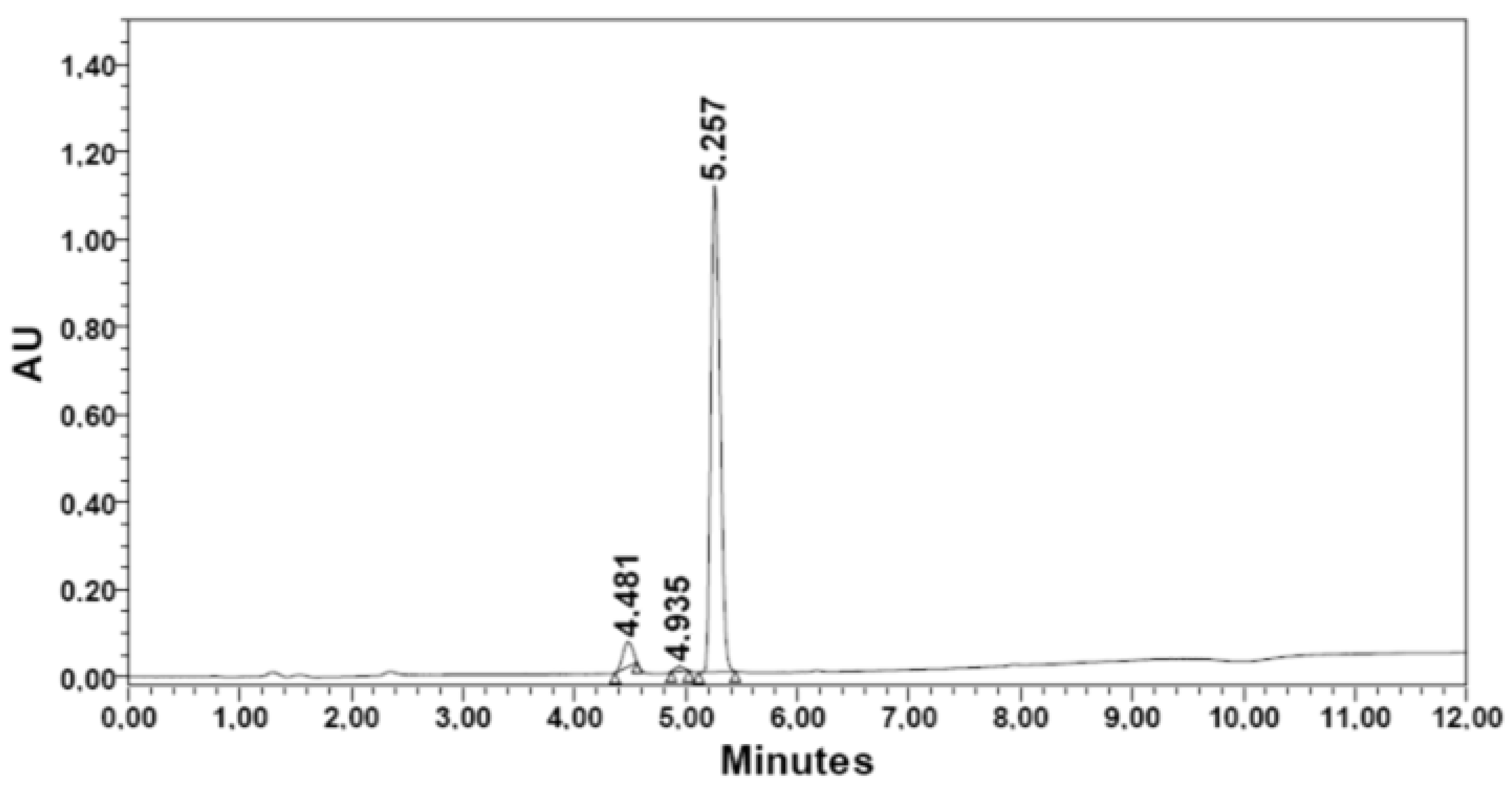 | 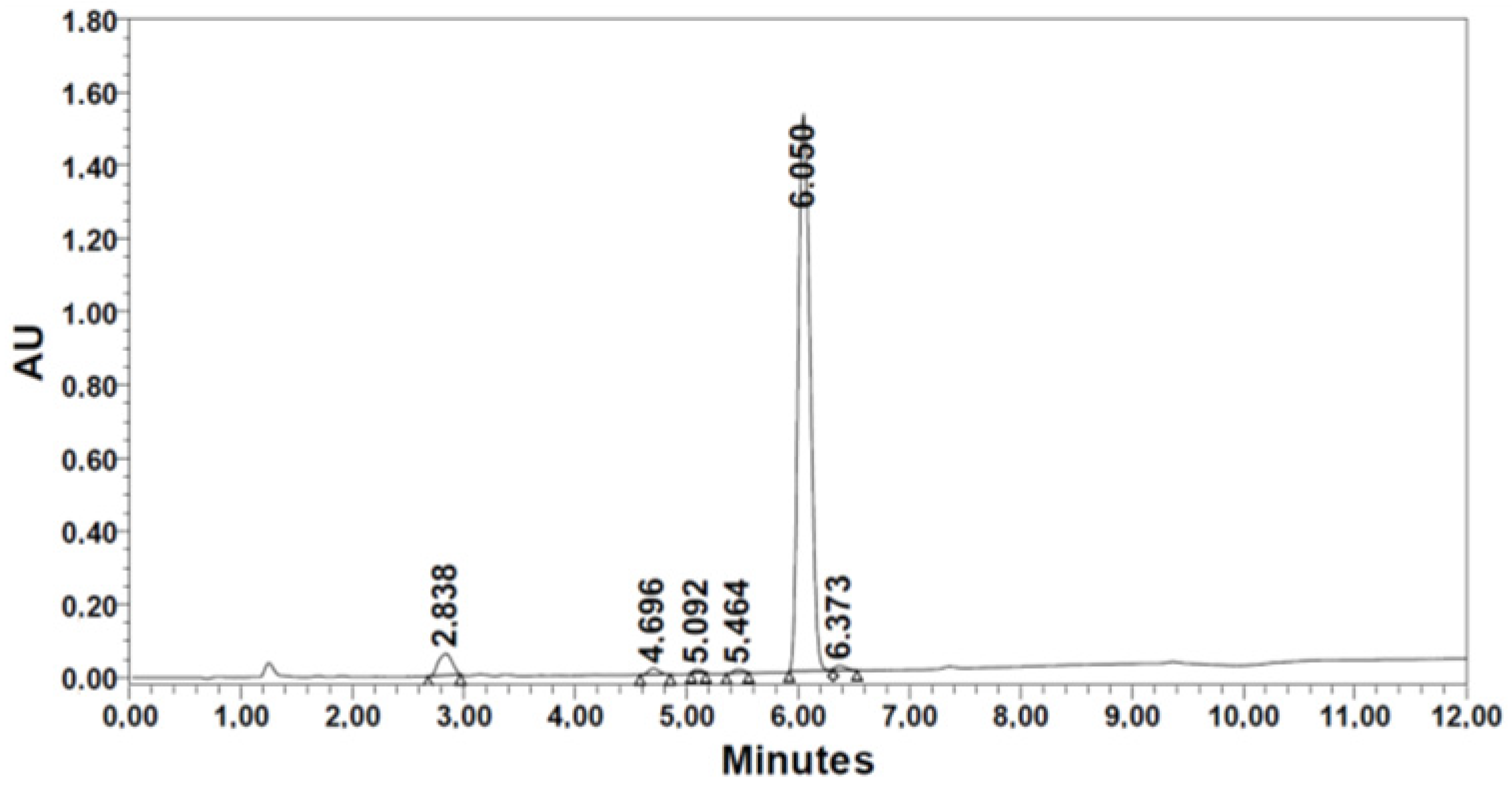 | ||
| REUSE: Synthesis with second activation of 2-CTC-resin | Dimethyl sulfate | R1 | R3 |
 |  | ||
| Methyl iodide | R2 | R4 | |
 |  | ||
| Reagents to Remove o-NBS | Methylation Steps | Sequence | MW (g/mol) | Ion M1+ | ||
|---|---|---|---|---|---|---|
| Theoretical | Experimental (First Activation) | Experimental (Second Activation) | ||||
| Dimethyl sulfate | o-NBS protection and N-methylation | o-NBS-N-Me-βAla-OH | 288.27 | 289.27 | * | 289.3 |
| o-NBS-N-Me-Thr(tBu)-OH | 374.41 | 375.41 | * | 375.4 | ||
| o-NBS deprotection | H-N-Me-βAla-OH | 103.12 | 104.12 | 104.2 | 104.2 | |
| H-N-Me-Thr(tBu)-OH | 189.26 | 190.26 | 190.3 | 190.4 | ||
| Fmoc protection | Fmoc-N-Me-βAla-OH | 325.36 | 326.36 | 326.5 | 326.5 | |
| Fmoc-N-Me-Thr(tBu)-OH | 411.5 | 412.5 | 412.5 | 412.4 | ||
| Methyl iodide | o-NBS protection and N-methylation | o-NBS-N-Me-βAla-OH | 288.27 | 289.27 | * | 289.3 |
| o-NBS-N-Me-Thr(tBu)-OH | 374.41 | 375.41 | * | 375.4 | ||
| o-NBS deprotection | H-N-Me-βAla-OH | 103.12 | 104.12 | 104.2 | 104.2 | |
| H-N-Me-Thr(tBu)-OH | 189.26 | 190.26 | 190.3 | 190.4 | ||
| Fmoc protection | Fmoc-N-Me-βAla-OH | 325.36 | 326.36 | 326.5 | 326.4 | |
| Fmoc-N-Me-Thr(tBu)-OH | 411.5 | 412.5 | 412.5 | 412.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, T.; Acosta, G.; Cárdenas, C.; de la Torre, B.G.; Guzmán, F.; Albericio, F. Protocol for Facile Synthesis of Fmoc-N-Me-AA-OH Using 2-CTC Resin as Temporary and Reusable Protecting Group. Methods Protoc. 2023, 6, 110. https://doi.org/10.3390/mps6060110
Román T, Acosta G, Cárdenas C, de la Torre BG, Guzmán F, Albericio F. Protocol for Facile Synthesis of Fmoc-N-Me-AA-OH Using 2-CTC Resin as Temporary and Reusable Protecting Group. Methods and Protocols. 2023; 6(6):110. https://doi.org/10.3390/mps6060110
Chicago/Turabian StyleRomán, Tanya, Gerardo Acosta, Constanza Cárdenas, Beatriz G. de la Torre, Fanny Guzmán, and Fernando Albericio. 2023. "Protocol for Facile Synthesis of Fmoc-N-Me-AA-OH Using 2-CTC Resin as Temporary and Reusable Protecting Group" Methods and Protocols 6, no. 6: 110. https://doi.org/10.3390/mps6060110
APA StyleRomán, T., Acosta, G., Cárdenas, C., de la Torre, B. G., Guzmán, F., & Albericio, F. (2023). Protocol for Facile Synthesis of Fmoc-N-Me-AA-OH Using 2-CTC Resin as Temporary and Reusable Protecting Group. Methods and Protocols, 6(6), 110. https://doi.org/10.3390/mps6060110








