Gathering the Evidence on Diet and Depression: A Protocol for an Umbrella Review and Updated Meta-Analyses
Abstract
1. Introduction
- (1)
- To perform an umbrella review on diet and depression. This objective will allow us to synthesize the characteristics, methodology, results, and extent of overlap of existing systematic reviews on diet and depression.
- (2)
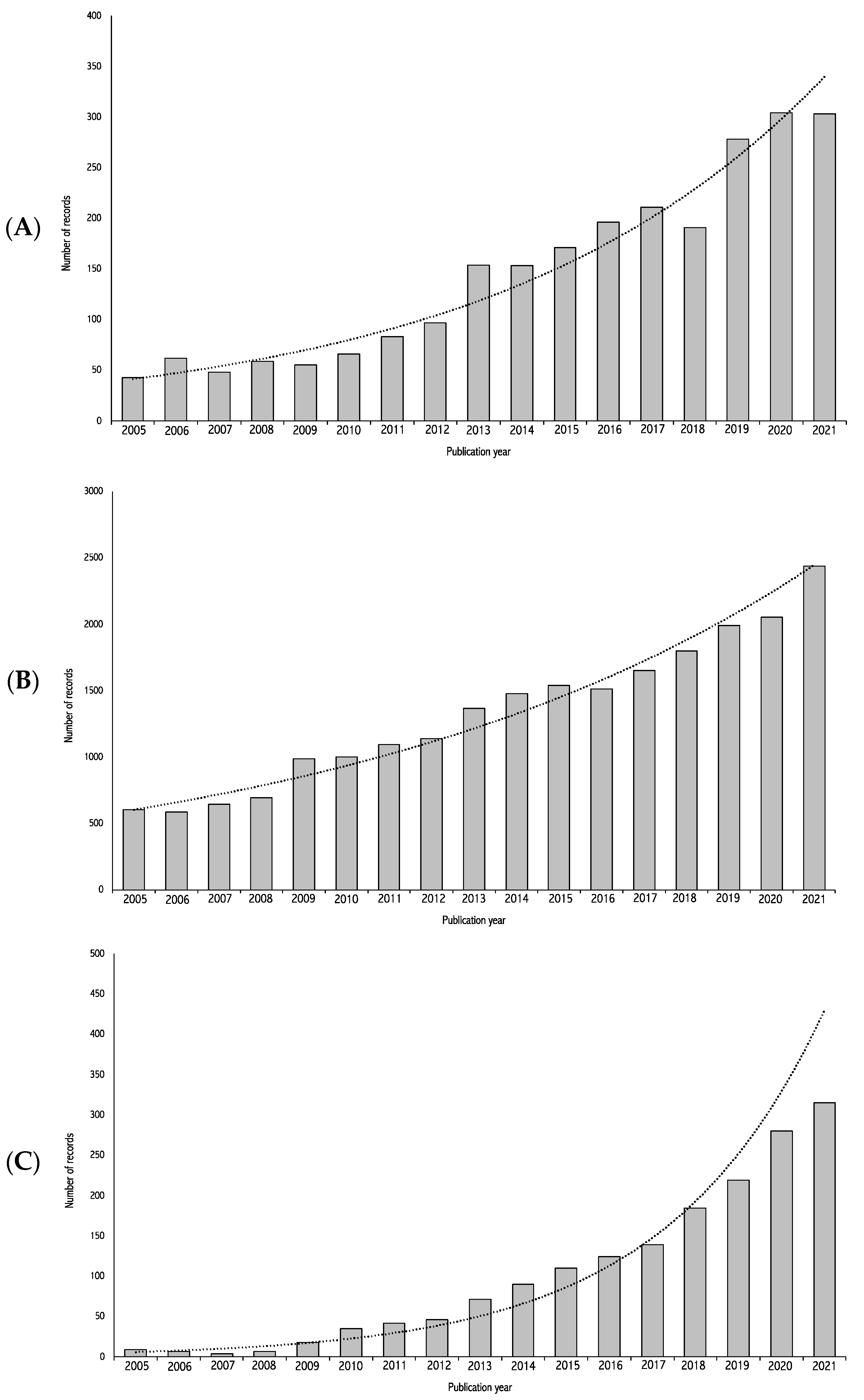
- To perform a systematic review update on dietary patterns and depression. This objective will allow the identification of more recently published primary studies on dietary patterns and depression that were not included in the latest systematic reviews on the topic. The second objective is limited to dietary patterns due to the extensive nature of this work. The decision to limit the dietary variable to dietary patterns specifically is based on the growing interest in this approach and the recommendation to consider diet as-a-whole rather than individual components of the diet [87,88]. This recommendation is reflected in the increasing number of publications on this specific topic (see Figure 1C).
- (3)
- To perform updated meta-analyses using studies from the previous two objectives This objective will allow the provision of the most up-to-date synthesis of the literature on diet and depression.
2. Methods
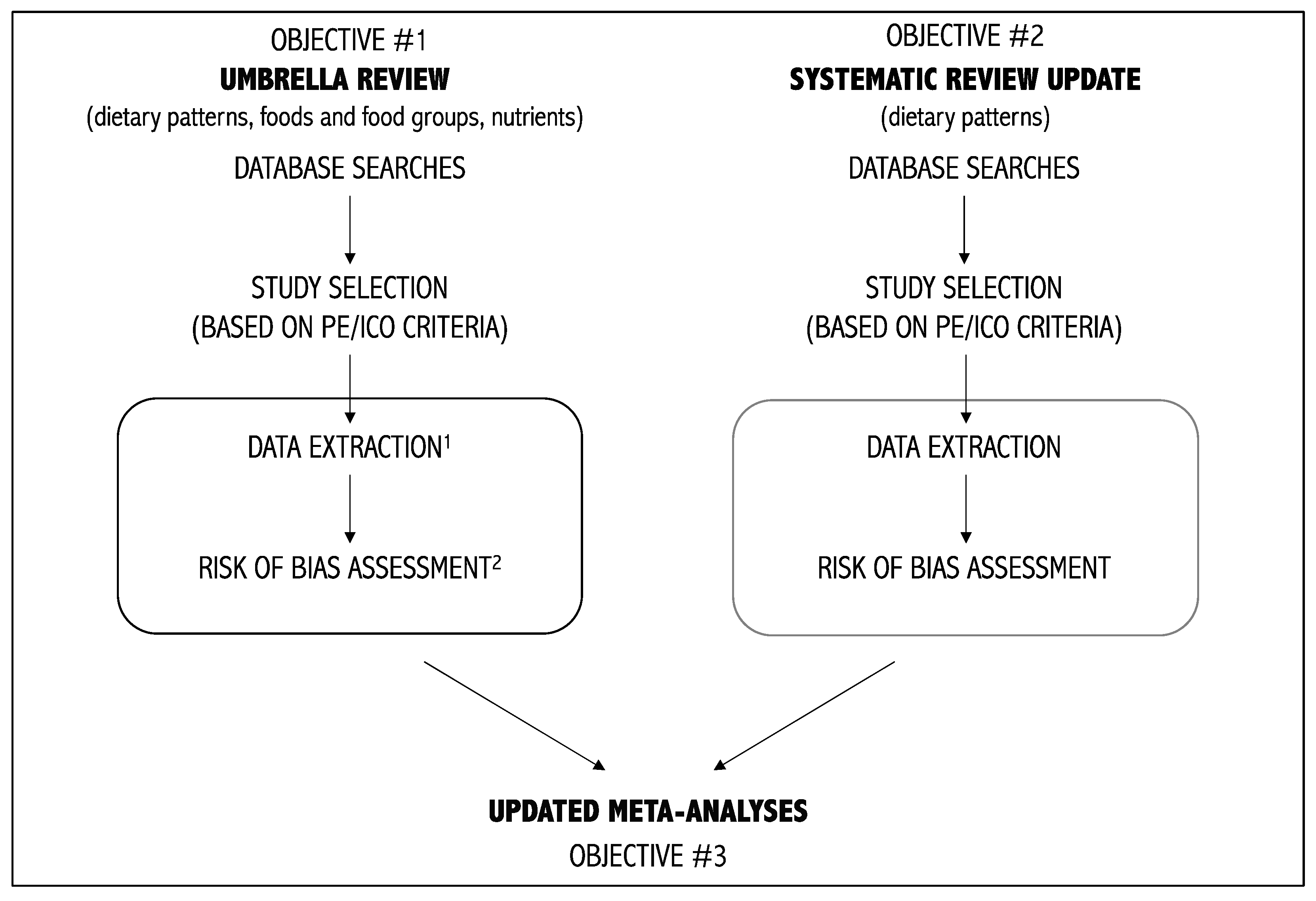
2.1. Objective 1–Umbrella Review on Diet and Depression
2.1.1. Identification of Relevant Studies
2.1.2. Eligibility Criteria
Type of Participants
Type of Exposures and Interventions
Type of Comparators
Type of Outcomes
Type of Study Designs
2.1.3. Data Collection
Selection of Studies
Data Extraction and Management
Assessment of Methodological Quality
2.2. Objective 2–Systematic Review Update on Dietary Patterns and Depression
2.2.1. Identification of Relevant Studies
2.2.2. Eligibility Criteria
Type of Participants
Type of Exposures and Interventions
Type of Comparators
Type of Outcomes
Type of Study Designs
2.2.3. Data Collection
Selection of Studies
Data Extraction and Management
Assessment of the Risk of Bias
- (i).
- being at low risk of bias when all domains are rated as such,
- (ii).
- raising some concerns when at least one domain is rated as such, but no domain is rated as being at high risk of bias, and as
- (iii).
- being at high risk of bias when at least one domain is rated as such, or when multiple domains are rated as raising some concerns.
2.3. Objective 3–Updated Meta-Analyses
2.3.1. Data Analysis and Synthesis
Outcome Measures
Synthesis of Results
Qualitative Synthesis of Results
Quantitative Synthesis of Result
- Observational Studies
- Experimental Studies
- Subgroup and Sensitivity Analyses
- Assessment of Heterogeneity
- Assessment of Publication Bias
- Certainty of Evidence
- (i).
- High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
- (ii).
- Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
- (iii).
- Low certainty: We have little confidence in the effect estimate. The true effect may be substantially different from the estimate of the effect.
- (iv).
- Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uher, R.; Payne, J.L.; Pavlova, B.; Perlis, R.H. Major Depressive Disorder in DSM-5: Implications for Clinical Practice and Research of Changes from DSM-IV. Depress. Anxiety 2014, 31, 459–471. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Mental Disorders Collaborators Global, Regional, and National Burden of 12 Men al Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [CrossRef] [PubMed]
- Sohn, J.H.; Ahn, S.H.; Seong, S.J.; Ryu, J.M.; Cho, M.J. Prevalence, Work-Loss Days and Quality of Life of Community Dwelling Subje ts with Depressive Symptoms. J. Korean Med. Sci. 2013, 28, 280–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alonso, J.; Petukhova, M.; Vilagut, G.; Chatterji, S.; Heeringa, S.; Üstün, T.B.; Alhamzawi, A.O.; Viana, M.C.; Angermeyer, M.; Bromet, E.; et al. Days out of Role Due to Common Physical and Mental Conditions: Results from the WHO World Mental Health Surveys. Mol. Psychiatry 2010, 16, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Lerner, D.; Adler, D.A.; Chang, H.; Lapitsky, L.; Hood, M.Y.; Perissinotto, C.; Reed, J.; McLaughlin, T.J.; Berndt, E.R.; Rogers, W.H. Unemployment, Job Retention, and Productivity Loss among Employees with Depression. Psychiatr. Serv. 2004, 55, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Crain, L.A.; Solberg, L.I.; Unützer, J.; Maciosek, M.V.; Whitebird, R.R.; Rossom, R.C. Does Severity of Depression Predict Magnitude of Productivity Loss? Am. J. Manag. Care 2014, 20, e294. [Google Scholar] [PubMed]
- Hofmann, S.G.; Curtiss, J.; Carpenter, J.K.; Kind, S. Effect of Treatments for Depression on Quality of Life: A Meta-Analysis. Cogn. Behav. Ther. 2017, 46, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Seabury, S.A.; Axeen, S.; Pauley, G.; Tysinger, B.; Schlosser, D.; Hernandez, J.B.; Heun-Johnson, H.; Zhao, H.; Goldman, D.P. Measuring the Lifetime Costs of Serious Mental Illness and the Mitigating Effects of Educational Attainment. Behav. Health Care 2019, 38, 652–659. [Google Scholar] [CrossRef]
- Insel, T.R. Assessing the Economic Costs of Serious Mental Illness. Am. J. Psychiatry 2008, 165, 663–665. [Google Scholar] [CrossRef]
- Rotella, F.; Mannucci, E. Depression as a Risk Factor for Diabetes: A Meta-Analysis of Longitudinal Studies. J. Clin. Psychiatry 2013, 74, 4231. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Fahey, P.; Cochrane, B.; Smith, S. Bidirectional Associations Between Clinically Relevant Depression or Anxiety and COPD: A Systematic Review and Meta-Analysis. Chest 2013, 144, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Koutsimani, P.; Montgomery, A.; Georganta, K. The Relationship between Burnout, Depression, and Anxiety: A Systematic Review and Meta-Analysis. Front. Psychol. 2019, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, N.C.; Newman, M.G. Anxiety and Depression as Bidirectional Risk Factors for One Another: A Meta-Analysis of Longitudinal Studies. Psychol. Bull. 2017, 143, 1155–1200. [Google Scholar] [CrossRef] [PubMed]
- Panagioti, M.; Gooding, P.A.; Tarrier, N. A Meta-Analysis of the Association between Posttraumatic Stress Disorder and Suicidality: The Role of Comorbid Depression. Compr. Psychiatry 2012, 53, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H. Tools and Strategies for Ongoing Assessment of Depression: A Measurement-Based Approach to Remission. J. Clin. Psychiatry 2009, 70, 21046. [Google Scholar] [CrossRef] [PubMed]
- McCormack, J.; Korownyk, C. Effectiveness of Antidepressants. BMJ 2018, 360, k1073. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Focus 2018, 16, 420–429. [Google Scholar] [CrossRef]
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; Norquist, G.; Howland, R.H.; Lebowitz, B.; McGrath, P.J.; et al. Evaluation of Outcomes with Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice. Am. J. Psychiatry 2006, 163, 28–40. [Google Scholar] [CrossRef]
- Jansen, L.; van Schijndel, M.; van Waarde, J.; van Busschbach, J. Health-Economic Outcomes in Hospital Patients with Medical-Psychiatric Comorbidity: A Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0194029. [Google Scholar] [CrossRef]
- Ismail, K.; Moulton, C.D.; Winkley, K.; Pickup, J.C.; Thomas, S.M.; Sherwood, R.A.; Stahl, D.; Amiel, S.A. The Association of Depressive Symptoms and Diabetes Distress with Glycaemic Control and Diabetes Complications over 2 Years in Newly Diagnosed Type 2 Diabetes: A Prospective Cohort Study. Diabetologia 2017, 60, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Reimer, A.; Hermanns, N.; Kulzer, B.; Ehrmann, D.; Krichbaum, M.; Huber, J.; Haak, T. Depression Is Linked to Hyperglycaemia via Suboptimal Diabetes Self-Management: A Cross-Sectional Mediation Analysis. J. Psychosom. Res. 2017, 94, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Adan, R.A.H.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional Psychiatry: Towards Improving Mental Health by What You Eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Teasdale, S.B.; Ward, P.B.; Veronese, N.; Shivappa, N.; Hebert, J.R.; Berk, M.; Yung, A.R.; Sarris, J. Diet as a Hot Topic in Psychiatry: A Population-scale Study of Nutritional Intake and Inflammatory Potential in Severe Mental Illness. World Psychiatry 2018, 17, 365. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Paul Amminger, G.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. International Society for Nutritional Psychiatry Research Consensus Position Statement: Nutritional Medicine in Modern Psychiatry. World Psychiatry 2015, 14, 370. [Google Scholar] [CrossRef] [PubMed]
- Levinson, D.F. The Genetics of Depression: A Review. Biol. Psychiatry 2006, 60, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Dunn, E.C.; Brown, R.C.; Dai, Y.; Rosand, J.; Nugent, N.R.; Amstadter, A.B.; Smoller, J.W. Genetic Determinants of Depression: Recent Findings and Future Directions. Harv. Rev. Psychiatry 2015, 23, 1. [Google Scholar] [CrossRef]
- Nelson, J.; Klumparendt, A.; Doebler, P.; Ehring, T. Childhood Maltreatment and Characteristics of Adult Depression: Meta-Analysis. Br. J. Psychiatry 2017, 210, 96–104. [Google Scholar] [CrossRef]
- Gardner, M.J.; Thomas, H.J.; Erskine, H.E. The Association between Five Forms of Child Maltreatment and Depressive and Anxiety Disorders: A Systematic Review and Meta-Analysis. Child. Abus. Negl. 2019, 96, 104082. [Google Scholar] [CrossRef]
- Normann, C.; Buttenschøn, H.N. Gene–Environment Interactions between HPA-Axis Genes and Stressful Life Events in Depression: A Systematic Review. Acta Neuropsychiatr. 2019, 31, 186–192. [Google Scholar] [CrossRef]
- Kraaij, V.; Arensman, E.; Spinhoven, P. Negative Life Events and Depression in Elderly PersonsA Meta-Analysis. J. Gerontol. Ser. B 2002, 57, P87–P94. [Google Scholar] [CrossRef] [PubMed]
- Leigh-Hunt, N.; Bagguley, D.; Bash, K.; Turner, V.; Turnbull, S.; Valtorta, N.; Caan, W. An Overview of Systematic Reviews on the Public Health Consequences of Social Isolation and Loneliness. Public Health 2017, 152, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Gariépy, G.; Honkaniemi, H.; Quesnel-Vallée, A. Social Support and Protection from Depression: Systematic Review of Current Findings in Western Countries. Br. J. Psychiatry 2016, 209, 284–293. [Google Scholar] [CrossRef]
- Everson, S.A.; Maty, S.C.; Lynch, J.W.; Kaplan, G.A. Epidemiologic Evidence for the Relation between Socioeconomic Status and Depression, Obesity, and Diabetes. J. Psychosom. Res. 2002, 53, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Muntaner, C.; Eaton, W.W.; Miech, R.; O’Campo, P. Socioeconomic Position and Major Mental Disorders. Epidemiol. Rev. 2004, 26, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.H.; Ho, F.Y.Y.; Shi, N.K.; Sarris, J.; Chung, K.F.; Yeung, W.F. Lifestyle Medicine for Depression: A Meta-Analysis of Randomized Controlled Trials. J. Affect. Disord. 2021, 284, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. A Review of Lifestyle Factors That Contribute to Important Pathways Associated with Major Depression: Diet, Sleep and Exercise. J. Affect. Disord. 2013, 148, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and Depression: Exploring the Biological Mechanisms of Action. Mol. Psychiatry 2020, 26, 134–150. [Google Scholar] [CrossRef]
- Mulinari, S. Monoamine Theories of Depression: Historical Impact on Biomedical Research. J. Hist. Neurosci. 2012, 21, 366–392. [Google Scholar] [CrossRef]
- Elhwuegi, A.S. Central Monoamines and Their Role in Major Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 435–451. [Google Scholar] [CrossRef]
- Lin, P.Y.; Tseng, P.T. Decreased Glial Cell Line-Derived Neurotrophic Factor Levels in Patients with Depression: A Meta-Analytic Study. J. Psychiatr. Res. 2015, 63, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Duman, R.; Sanacora, G. Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biol. Psychiatry 2008, 64, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, G.E. Depression and Hypothalamic-Pituitary-Adrenal Activation: A Quantitative Summary of Four Decades of Research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- Lopez-Duran, N.L.; Kovacs, M.; George, C.J. Hypothalamic–Pituitary–Adrenal Axis Dysregulation in Depressed Children and Adolescents: A Meta-Analysis. Psychoneuroendocrinology 2009, 34, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is Depression Associated with Increased Oxidative Stress? A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and Oxidative Stress: Results from a Meta-Analysis of Observational Studies. Psychosom. Med. 2014, 76, 12. [Google Scholar] [CrossRef]
- Jimenez-Fernandez, S.; Gurpegui, M.; Diaz-Atienza, F.; Perez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative Stress and Antioxidant Parameters in Patients with Major Depressive Disorder Compared to Healthy Controls Before and After Antidepressant Treatment: Results from a Meta-Analysis. J. Clin. Psychiatry 2015, 76, 13705. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of Central Inflammation in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Studies Examining Cerebrospinal Fluid, Positron Emission Tomography and Post-Mortem Brain Tissue. Brain Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons between Schizophrenia, Bipolar Disorder and Depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative Meta-Analysis of Interleukins 6 and 1β, Tumour Necrosis Factor α and C-Reactive Protein in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Bayes, J.; Schloss, J.; Sibbritt, D. Effects of Polyphenols in a Mediterranean Diet on Symptoms of Depression: A Systematic Literature Review. Adv. Nutr. 2020, 11, 602–615. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Rahimi, R.; Nikfar, S.; Abdollahi, M. Effect of Resveratrol on Cognitive and Memory Performance and Mood: A Meta-Analysis of 225 Patients. Pharmacol. Res. 2018, 128, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Wilder, H.; Bhagwagar, Z.; Geddes, J. Inositol for Depressive Disorders. Cochrane Database Syst. Rev. 2004, CD004049. [Google Scholar] [CrossRef] [PubMed]
- Phelan, D.; Molero, P.; Martínez-González, M.A.; Molendijk, M. Magnesium and Mood Disorders: Systematic Review and Meta-Analysis. BJPsych Open 2018, 4, 167–179. [Google Scholar] [CrossRef]
- Lomagno, K.A.; Hu, F.; Riddell, L.J.; Booth, A.O.; Szymlek-Gay, E.A.; Nowson, C.A.; Byrne, L.K. Increasing Iron and Zinc in Pre-Menopausal Women and Its Effects on Mood and Cognition: A Systematic Review. Nutrients 2014, 6, 5117–5141. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Moxey, A.; Nowak, G.; Vashum, K.; Bailey, K.; McEvoy, M. The Efficacy of Zinc Supplementation in Depression: Systematic Review of Randomised Controlled Trials. J. Affect. Disord. 2012, 136, e31–e39. [Google Scholar] [CrossRef]
- Williams, A.L.; Cotter, A.; Sabina, A.; Girard, C.; Goodman, J.; Katz, D.L. The Role for Vitamin B-6 as Treatment for Depression: A Systematic Review. Fam. Pract. 2005, 22, 532–537. [Google Scholar] [CrossRef]
- Taylor, M.J.; Carney, S.M.; Goodwin, G.M.; Geddes, J.R. Folate for Depressive Disorders: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Psychopharmacol. 2004, 18, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, H.; Amirani, E.; Milajerdi, A.; Kolahdooz, F.; Mirzaei, H.; Zaroudi, M.; Ghaderi, A.; Asemi, Z. The Effects of Vitamin D Supplementation on Mental Health, and Biomarkers of Inflammation and Oxidative Stress in Patients with Psychiatric Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 94, 109651. [Google Scholar] [CrossRef]
- Appleton, K.M.; Sallis, H.M.; Perry, R.; Ness, A.R.; Churchill, R. ω-3 Fatty Acids for Major Depressive Disorder in Adults: An Abridged Cochrane Review. BMJ Open 2016, 6, e010172. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.H.O.; Jimoh, O.F.; Biswas, P.; O’Brien, A.; Hanson, S.; Abdelhamid, A.S.; Fox, C.; Hooper, L. Omega-3 and Polyunsaturated Fat for Prevention of Depression and Anxiety Symptoms: Systematic Review and Meta-Analysis of Randomised Trials. Br. J. Psychiatry 2021, 218, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hausenblas, H.A.; Saha, D.; Dubyak, P.J.; Anton, S.D. Saffron (Crocus sativus L.) and Major Depressive Disorder: A Meta-Analysis of Randomized Clinical Trials. J. Integr. Med. 2013, 11, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for Depression: A Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2019, 60, 2643–2653. [Google Scholar] [CrossRef]
- Hu, D.; Cheng, L.; Jiang, W. Sugar-Sweetened Beverages Consumption and the Risk of Depression: A Meta-Analysis of Observational Studies. J. Affect. Disord. 2019, 245, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Castellano, S.; Pajak, A.; Galvano, F. Coffee, Tea, Caffeine and Risk of Depression: A Systematic Review and Dose–Response Meta-Analysis of Observational Studies. Mol. Nutr. Food Res. 2016, 60, 223–234. [Google Scholar] [CrossRef]
- Dobersek, U.; Wy, G.; Adkins, J.; Altmeyer, S.; Krout, K.; Lavie, C.J.; Archer, E. Meat and Mental Health: A Systematic Review of Meat Abstention and Depression, Anxiety, and Related Phenomena. Crit. Rev. Food Sci. Nutr. 2021, 61, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, Y.; Je, Y. Fish Consumption and Risk of Depression: Epidemiological Evidence from Prospective Studies. Asia-Pac. Psychiatry 2018, 10, e12335. [Google Scholar] [CrossRef]
- Hockey, M.; McGuinness, A.J.; Marx, W.; Rocks, T.; Jacka, F.N.; Ruusunen, A. Is Dairy Consumption Associated with Depressive Symptoms or Disorders in Adults? A Systematic Review of Observational Studies. Crit. Rev. Food Sci. Nutr. 2019, 60, 3653–3668. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Intake and Mental Health in Adults: A Systematic Review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef]
- Opie, R.S.; O’Neil, A.; Itsiopoulos, C.; Jacka, F.N. The Impact of Whole-of-Diet Interventions on Depression and Anxiety: A Systematic Review of Randomised Controlled Trials. Public Health Nutr. 2015, 18, 2074–2093. [Google Scholar] [CrossRef]
- Salari-Moghaddam, A.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. Glycemic Index, Glycemic Load, and Depression: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2018, 73, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy Dietary Indices and Risk of Depressive Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Mol. Psychiatry 2018, 24, 965–986. [Google Scholar] [CrossRef]
- Arab, A.; Mehrabani, S.; Moradi, S.; Amani, R. The Association between Diet and Mood: A Systematic Review of Current Literature. Psychiatry Res. 2019, 271, 428–437. [Google Scholar] [CrossRef]
- Shafiei, F.; Salari-Moghaddam, A.; Larijani, B.; Esmaillzadeh, A. Adherence to the Mediterranean Diet and Risk of Depression: A Systematic Review and Updated Meta-Analysis of Observational Studies. Nutr. Rev. 2019, 77, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Altun, A.; Brown, H.; Szoeke, C.; Goodwill, A.M. The Mediterranean Dietary Pattern and Depression Risk: A Systematic Review. Neurol. Psychiatry Brain Res. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Chen, K.; Jing, Y.; He, J.; Sun, H.; Hu, X. Dietary Inflammatory Index and Depression: A Meta-Analysis. Public. Health Nutr. 2019, 22, 654–660. [Google Scholar] [CrossRef]
- Tolkien, K.; Bradburn, S.; Murgatroyd, C. An Anti-Inflammatory Diet as a Potential Intervention for Depressive Disorders: A Systematic Review and Meta-Analysis. Clin. Nutr. 2019, 38, 2045–2052. [Google Scholar] [CrossRef]
- Nicolaou, M.; Colpo, M.; Vermeulen, E.; Elstgeest, L.E.M.; Cabout, M.; Gibson-Smith, D.; Knuppel, A.; Sini, G.; Schoenaker, D.A.J.M.; Mishra, G.D.; et al. Association of a priori Dietary Patterns with Depressive Symptoms: A Harmonised Meta-Analysis of Observational Studies. Psychol. Med. 2020, 50, 1872–1883. [Google Scholar] [CrossRef]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and Veganism Compared with Mental Health and Cognitive Outcomes: A Systematic Review and Meta-Analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Lin, M.Y.; Tsai, P.S. Alternate Healthy Eating Index and Risk of Depression: A Meta-Analysis and Systemematic Review. Nutr. Neurosci. 2018, 23, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Daneshzad, E.; Darooghegi Mofrad, M.; Bellissimo, N.; Suitor, K.; Azadbakht, L. Vegetarian diet and the risk of depression, anxiety, and stress symptoms: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S. Systematic Reviews and Meta-Analysis in Nutrition Research. Br. J. Nutr. 2019, 122, 1279–1294. [Google Scholar] [CrossRef]
- Ioannidis, J. Next-Generation Systematic Reviews: Prospective Meta-Analysis, Individual-Level Data, Networks and Umbrella Reviews. Br. J. Sports Med. 2017, 51, 1456–1458. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-Analyses. Milbank Q. 2016, 94, 485–514. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary Pattern Analysis: A New Direction in Nutritional Epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, E.M.; Hu, F.B. Dietary Patterns: From Nutritional Epidemiologic Analysis to National Guidelines. Am. J. Clin. Nutr. 2015, 101, 899–900. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Estarli, M.; Barrera, E.S.A.; et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Rev. Esp. Nutr. Humana Diet. 2016, 20, 148–160. [Google Scholar] [CrossRef]
- Lunny, C.; Brennan, S.E.; McDonald, S.; McKenzie, J.E. Toward a Comprehensive Evidence Map of Overview of Systematic Review Methods: Paper 1—Purpose, Eligibility, Search and Data Extraction. Syst. Rev. 2017, 6, 231. [Google Scholar] [CrossRef]
- Lunny, C.; Brennan, S.E.; McDonald, S.; McKenzie, J.E. Toward a Comprehensive Evidence Map of Overview of Systematic Review Methods: Paper 2—Risk of Bias Assessment; Synthesis, Presentation and Summary of the Findings; And A sessment of the Certainty of the Evidence. Syst. Rev. 2018, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Tianjing, L.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; The Cochrane Collaboration & Wiley-Blackwell: Glasgow, UK, 2019. [Google Scholar]
- Li, T.; Higgins, J.P.T.; Deeks, J.J. Collecting Data. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Tianjing, L., Page, M., Welch, V., Eds.; The Cochrane Collaboration & Wiley-Blackwell: Glasgow, UK, 2019; pp. 109–141. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Tianjing, L., Page, M., Welch, V., Eds.; The Cochrane Collaboration & Wiley-Blackwell: Glasgow, UK, 2019; pp. 205–228. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, 4008. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, V.; Beaudart, C.; Tirelli, E.; Bruyère, O. Psychometric Measurements of AMSTAR 2 in a Sample of Meta-Analyses Indexed in PsycINFO. J. Clin. Epidemiol. 2020, 119, 144–145. [Google Scholar] [CrossRef]
- Lorenz, R.C.; Matthias, K.; Pieper, D.; Wegewitz, U.; Morche, J.; Nocon, M.; Rissling, O.; Schirm, J.; Jacobs, A. A Psychometric Study Found AMSTAR 2 to Be a Valid and Moderately Reliable Appraisal Tool. J. Clin. Epidemiol. 2019, 114, 133–140. [Google Scholar] [CrossRef]
- National Heart Lung and Blood Institute National Heart Lung and Blood Institute (NHLBI). NHLBI Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 4 July 2023).
- National Heart Lung and Blood Institute National Heart Lung and Blood Institute (NHLBI). NHLBIQuality Assessment of Case-Control Studies. 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 4 July 2023).
- Smith, V.; Devane, D.; Begley, C.M.; Clarke, M. Methodology in Conducting a Systematic Review of Systematic Reviews of Healthcare Interventions. BMC Med. Res. Methodol. 2011, 11, 15. [Google Scholar] [CrossRef]
- McKenzie, J.E.; Brennan, S.E. Synthesizing and Presenting Findings Using Other Methods. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Tianjing, L., Page, M., Welch, V., Eds.; The Cochrane Collaboration & Wiley-Blackwell: Glasgow, UK, 2019; pp. 321–347. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Schwarzer, G. Package ‘Meta’. 2022. Available online: https://cran.r-project.org/web/packages/meta/meta.pdf (accessed on 4 July 2023).
- Schwarzer, G. Meta-Analysis in R. In Systematic Reviews in Health Research: Meta-Analysis in Context, 3rd ed.; Egger, M., Higgins, J.P.T., Smith, G.D., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 510–534. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101. [Google Scholar] [CrossRef]
- Deeks, J.; Higgins, J.; Altman, D. Chapter 10: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; John Wiley & Sons: Chichester, UK, 2019; p. 241. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A.; GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html#:~:text=The%20GRADE%20handbook%20describes%20the,www.gradeworkinggroup.org (accessed on 4 July 2023).
- McMaster University. Evidence Prime GRADEpro GDT: GRADEpro Guideline Development Tool 2022. Available online: https://www.gradepro.org (accessed on 4 July 2023).
| Search Terms | |
|---|---|
Theme #1
|
|
Theme #2
|
|
| Type of studies |
|
| Combining search terms |
|
| Language |
|
| Search period |
|
| Extraction Fields | |
|---|---|
| General information |
|
| Search strategy |
|
| Study selection |
|
| Population |
|
| Exposure(s) Intervention(s) |
|
| Comparators |
|
| Outcome(s) |
|
| Results |
|
| Extraction Fields | |
|---|---|
| General information |
|
| Study charactaristic |
|
| Population |
|
| Exposure(s) Intervention(s) |
|
| Comparator(s) |
|
| Outcome(s) |
|
| Results |
|
| Criteria for Judging the Overall Quality of SR | |||||
|---|---|---|---|---|---|
| Quality | Level of Confidence in the Results | Critical Weaknesses 1 (N=) | Non-Critical Weaknesses 2 (N=) | Score 3 Cut-Offs for Downgrading Due to Multiple Non-Critical Weaknesses | |
| SR without MA (__/13) | SR with MA (__/16) | ||||
| High | Provides an accurate and comprehensive summary of available studies. | 0 | 1 | - | - |
| Moderate | May provide an accurate and comprehensive summary of available studies | 0 | ≥2 | <8 | <11 |
| Low | May not provide an accurate and comprehensive summary of available studies | 1 | 0 or ≥1 | <8 | <9.5 |
| Critically low | Does not provide an accurate and comprehensive summary of available studies | ≥2 | 0 or ≥1 | - | - |
| Search Terms | |
|---|---|
Theme #1
|
|
Theme #2
|
|
| Combining search terms |
|
| Language |
|
| Search period |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodnaruc, A.M.; Vincent, C.; Soto, C.; Duquet, M.; Prud’homme, D.; Giroux, I. Gathering the Evidence on Diet and Depression: A Protocol for an Umbrella Review and Updated Meta-Analyses. Methods Protoc. 2023, 6, 78. https://doi.org/10.3390/mps6050078
Bodnaruc AM, Vincent C, Soto C, Duquet M, Prud’homme D, Giroux I. Gathering the Evidence on Diet and Depression: A Protocol for an Umbrella Review and Updated Meta-Analyses. Methods and Protocols. 2023; 6(5):78. https://doi.org/10.3390/mps6050078
Chicago/Turabian StyleBodnaruc, Alexandra M., Coralie Vincent, Carolina Soto, Miryam Duquet, Denis Prud’homme, and Isabelle Giroux. 2023. "Gathering the Evidence on Diet and Depression: A Protocol for an Umbrella Review and Updated Meta-Analyses" Methods and Protocols 6, no. 5: 78. https://doi.org/10.3390/mps6050078
APA StyleBodnaruc, A. M., Vincent, C., Soto, C., Duquet, M., Prud’homme, D., & Giroux, I. (2023). Gathering the Evidence on Diet and Depression: A Protocol for an Umbrella Review and Updated Meta-Analyses. Methods and Protocols, 6(5), 78. https://doi.org/10.3390/mps6050078








