An Experimental Approach to Address the Functional Relationship between Antioxidant Enzymes and Mitochondrial Respiratory Complexes
Abstract
1. Introduction
2. Procedure and Experimental Design
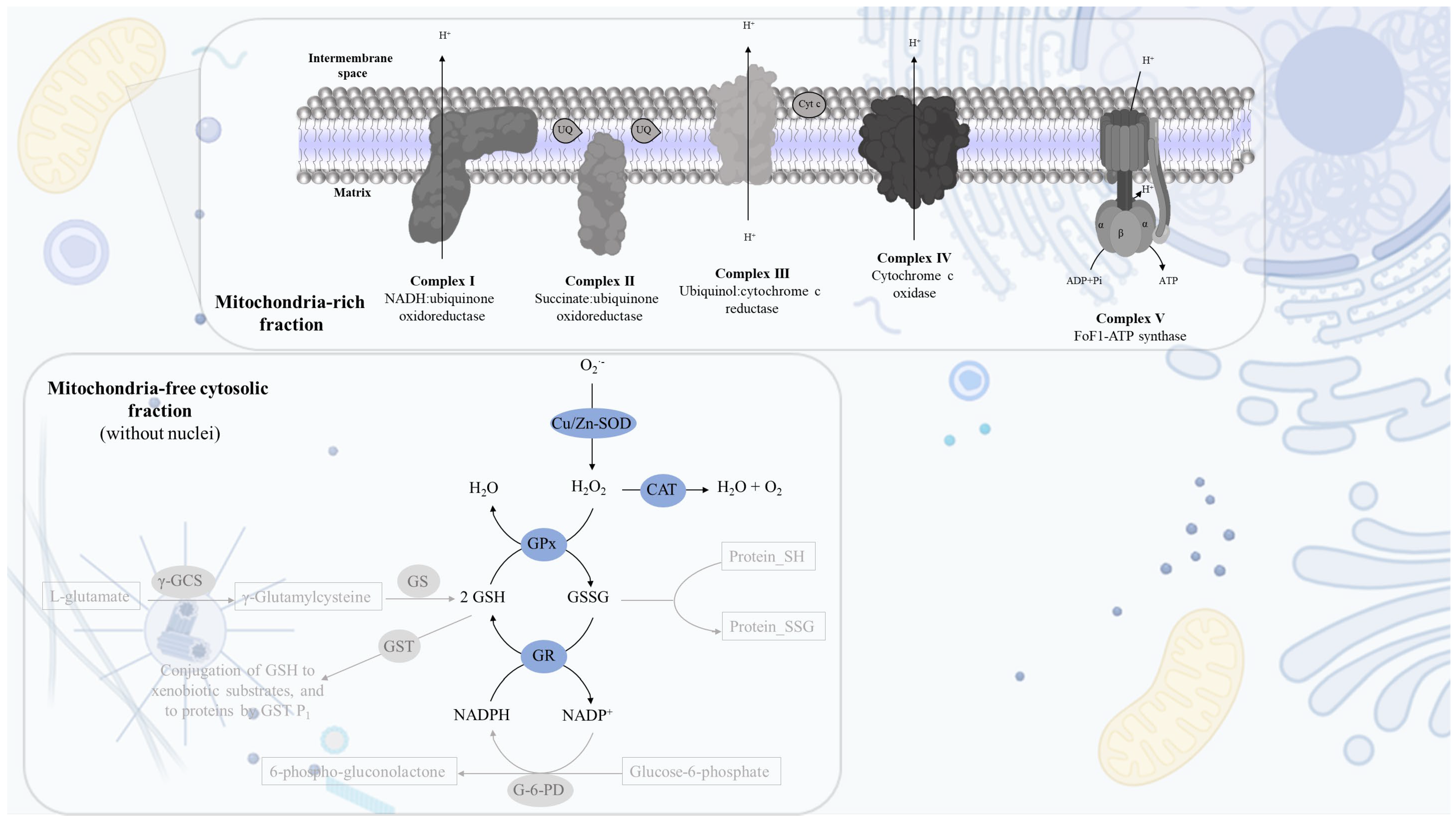
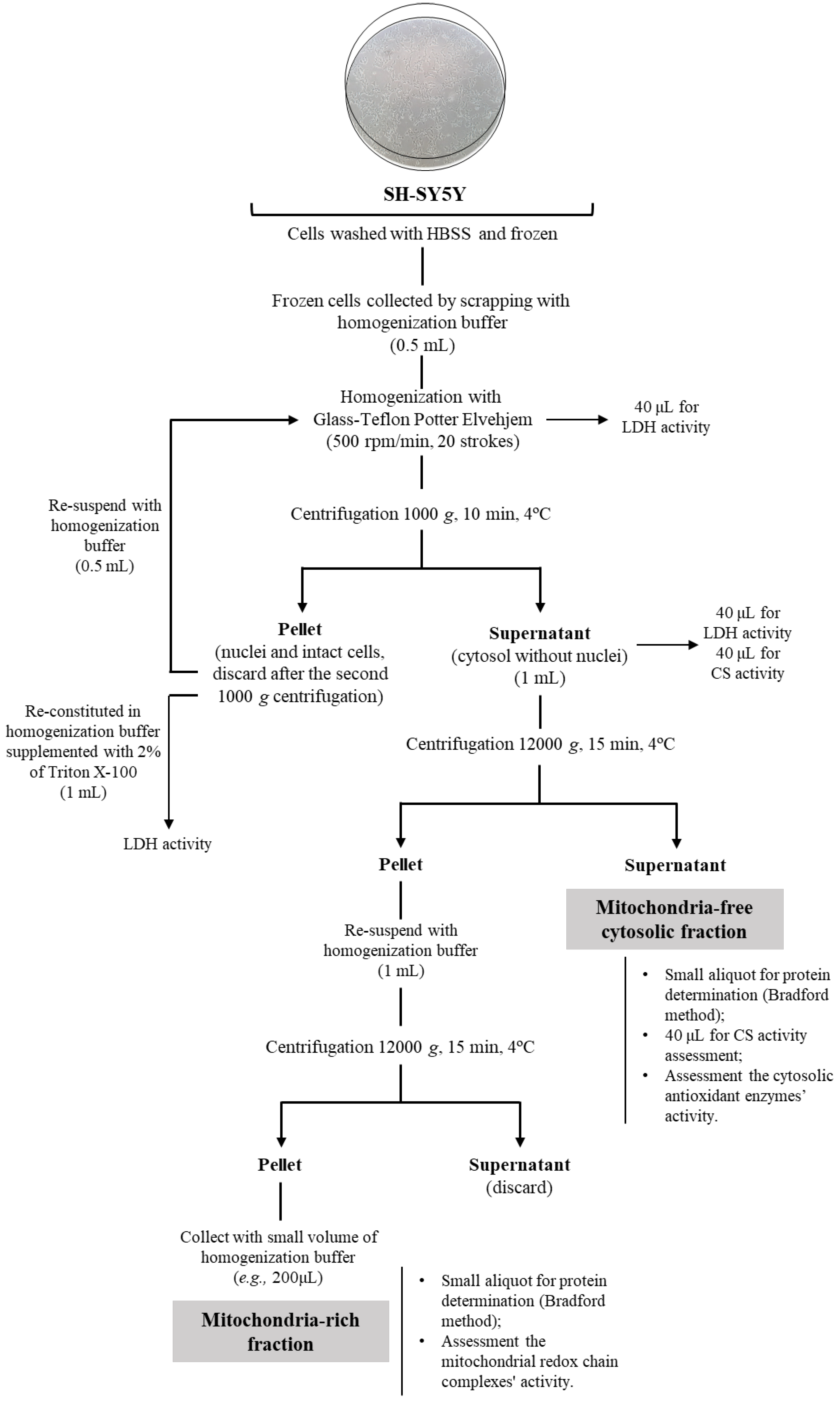
2.1. Cell Fractionation: Mitochondria-Free Cytosolic Fraction and Mitochondria-Rich Fraction
2.2. Mitochondria-Free Cytosolic Fraction: Assessment of the Activity of the Cytosolic Antioxidant Enzymes
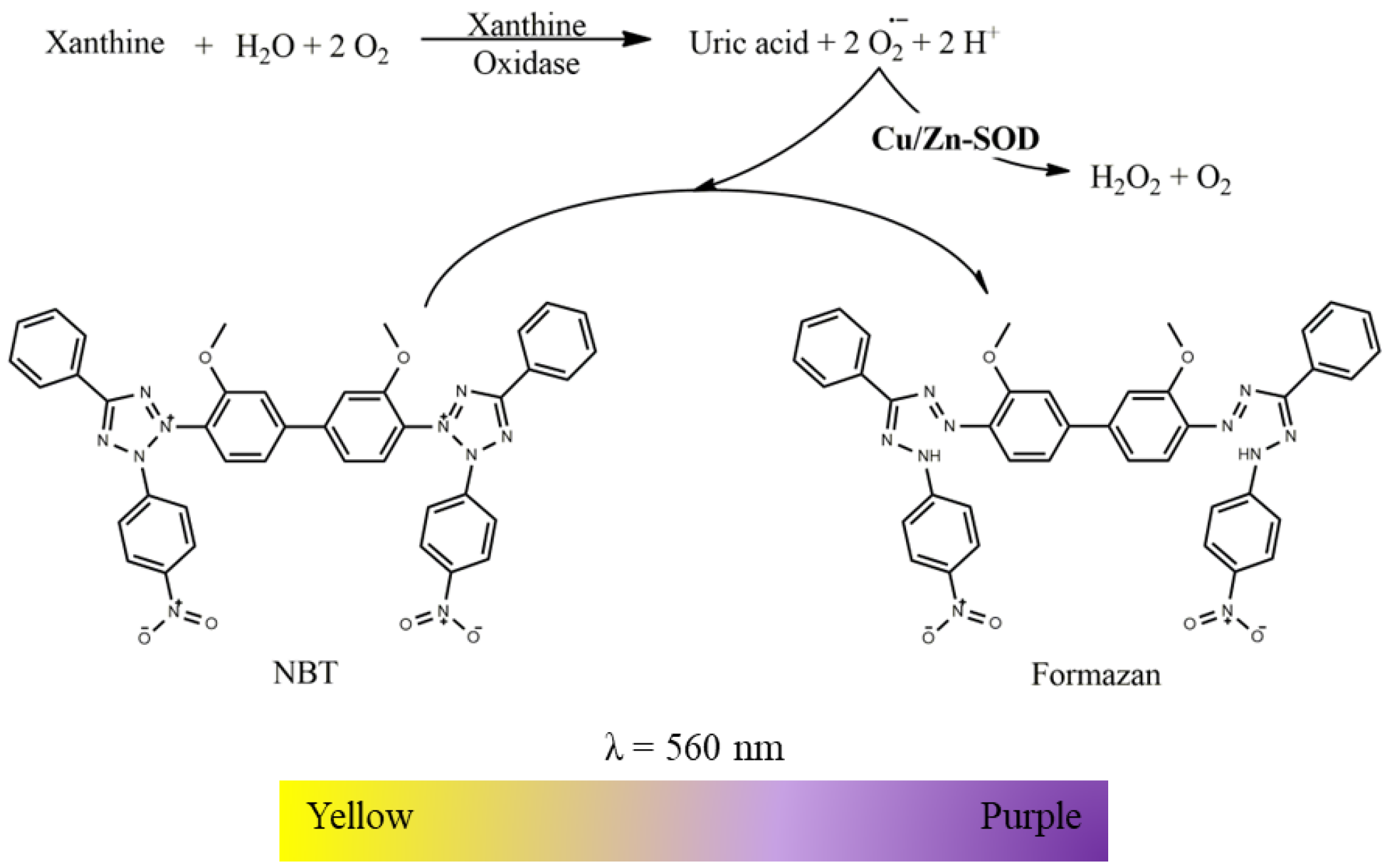
2.2.1. Superoxide Dismutase (Cu/Zn) Activity
- Suspension of mitochondria-free cytosolic fraction (1000 µg protein/mL);
- Phosphate buffer (KH2PO4 100 mM, EDTA 5 mM, pH 7.4);
- Nitroblue tetrazolium chloride (NBT, 2 mM in phosphate buffer);
- Xanthine oxidase (12.5 U/mL in phosphate buffer);
- Xanthine (2 mM in phosphate buffer).
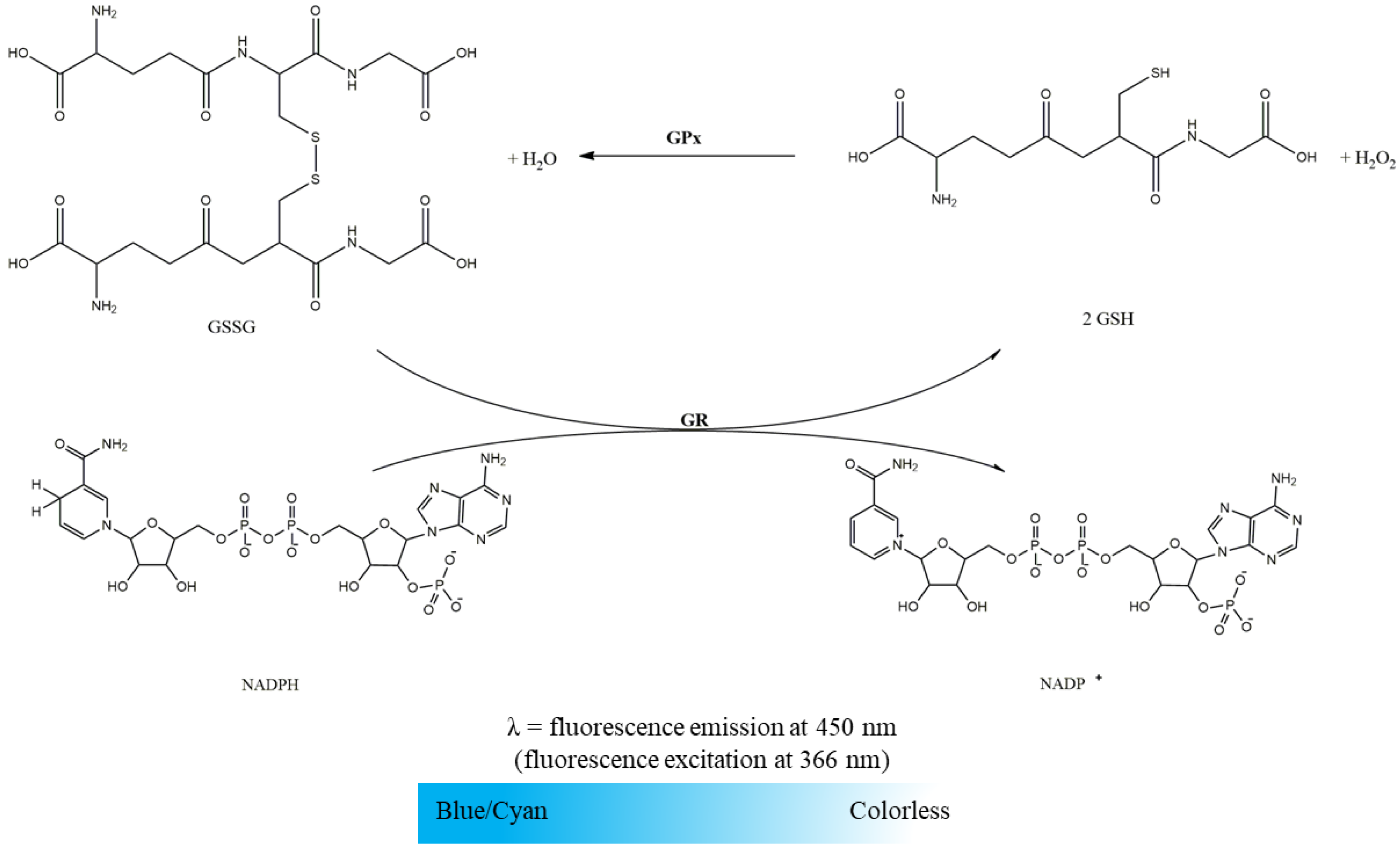
2.2.2. Glutathione Reductase Activity
- Suspension of mitochondria-free cytosolic fraction (1000 µg protein/mL);
- Phosphate buffer (KH2PO4 100 mM, EDTA 5 mM, pH 7.4);
- GSSG (10 mM in water);
- NADPH (10 mM in water).
2.2.3. Glutathione Peroxidase Activity
- Suspension of mitochondria-free cytosolic fraction (1000 µg protein/mL);
- Phosphate buffer (KH2PO4 100mM, EDTA 5 mM, pH 7.4);
- GSH (10 mM in water);
- GR (116.48 U/mL in phosphate buffer);
- NADPH (10 mM in water);
- H2O2 (88 mM in water).
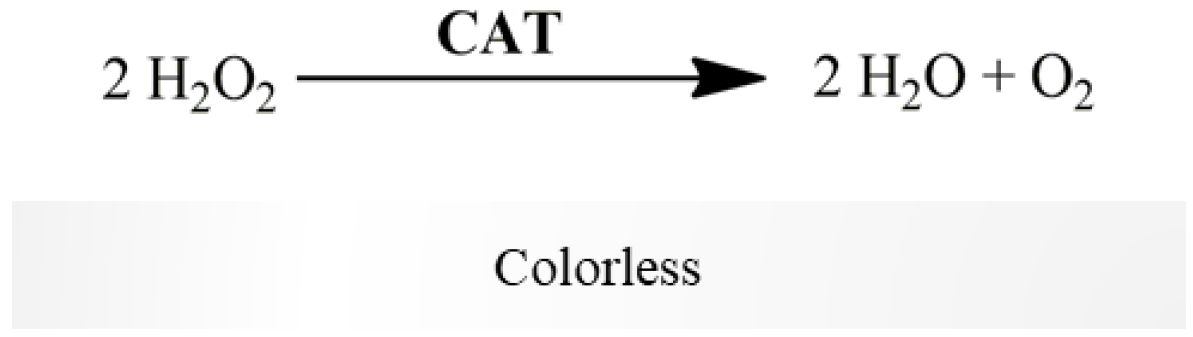
2.2.4. Catalase Activity
- Suspension of mitochondria-free cytosolic fraction (1000 µg protein/mL);
- Phosphate buffer (KH2PO4 100 mM, EDTA 5 mM, pH 7.4);
- H2O2 (88 mM in water).
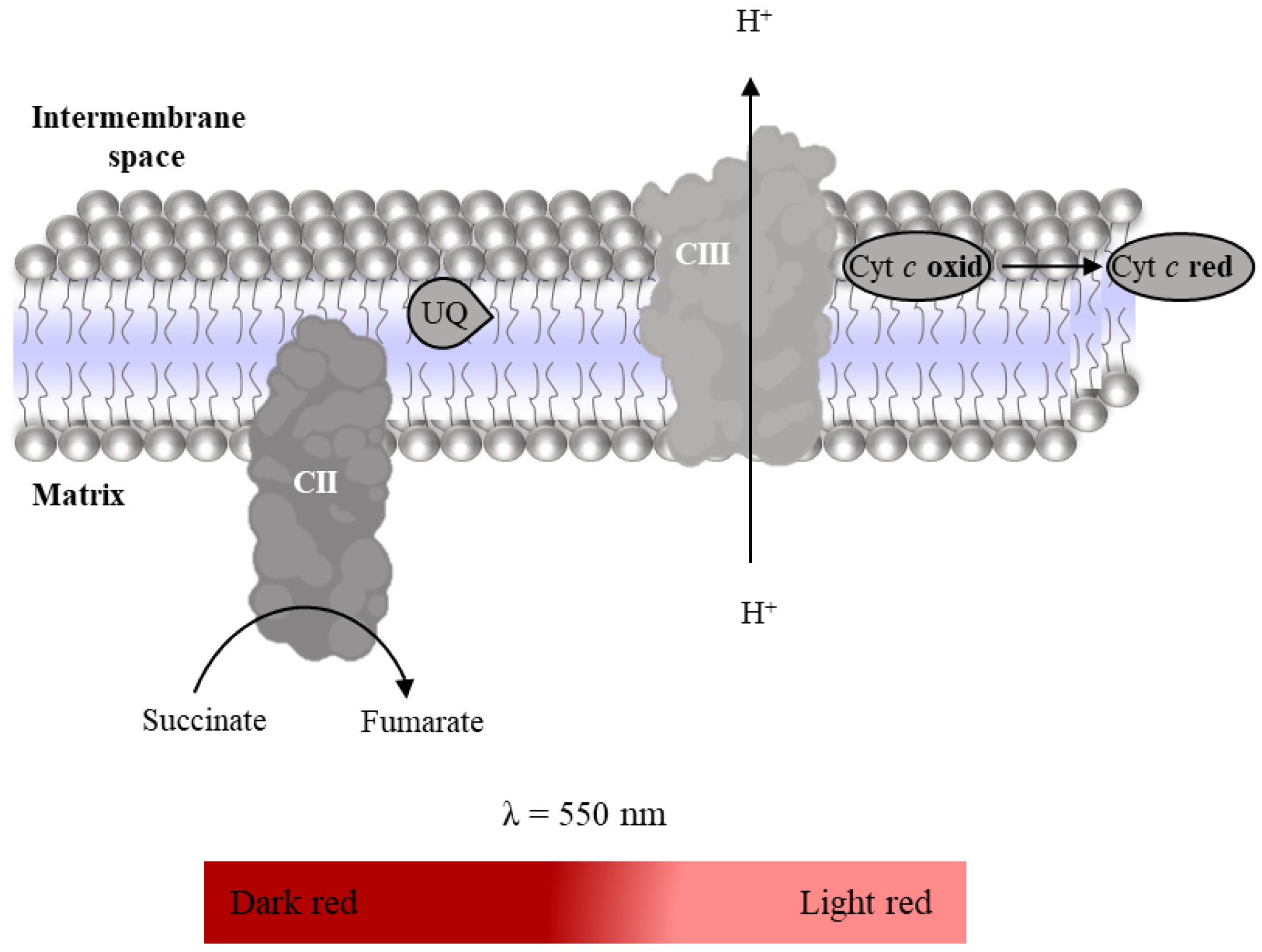
2.3. Mitochondria-Rich Fraction: Assessment of the Mitochondrial Respiratory Chain Complexes Activities
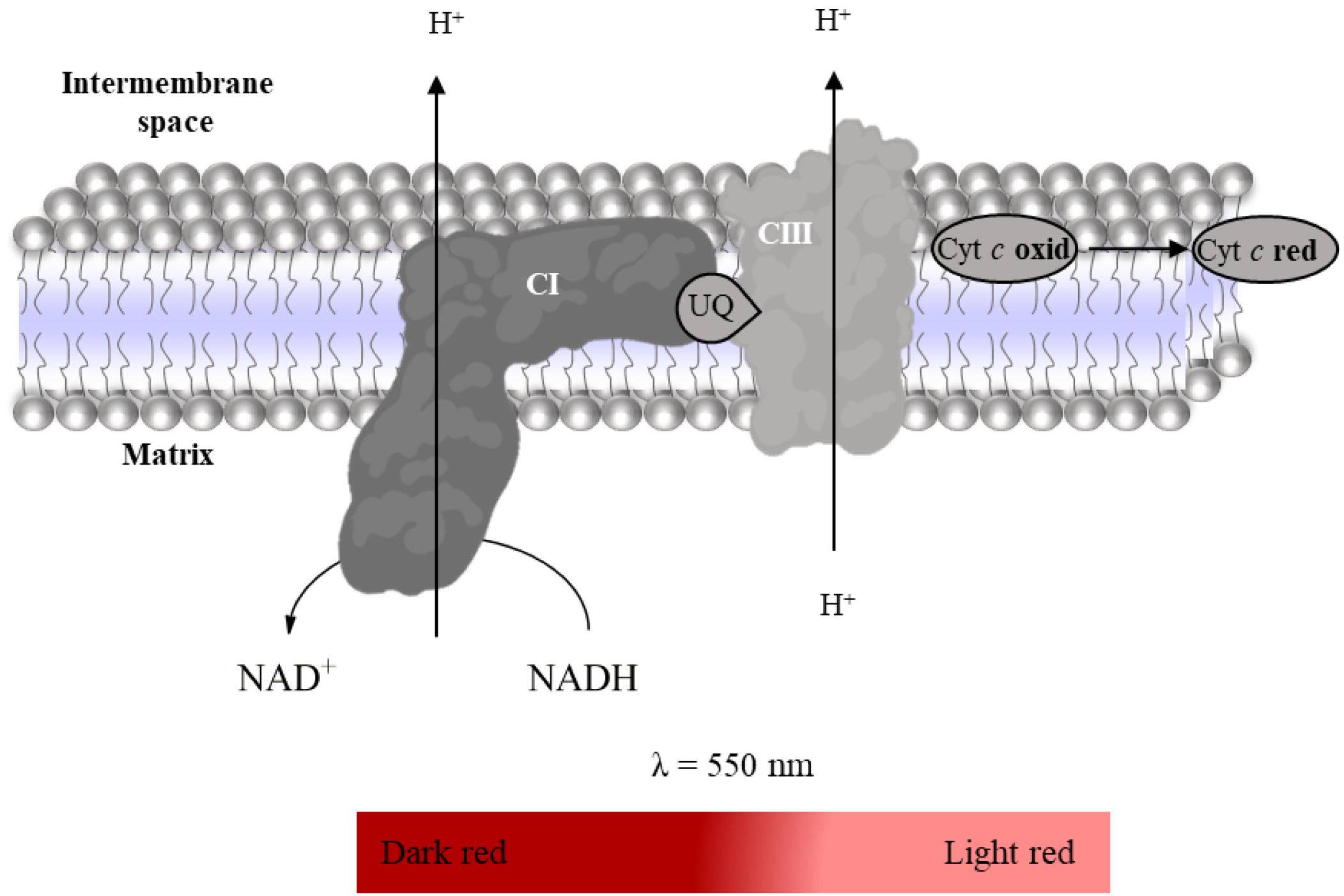
2.3.1. Complex I: NADH:Ubiquinone Oxidoreductase Activity
- Suspension of mitochondria-rich fraction (0.5 mg protein/mL);
- Phosphate buffer (KH2PO4 25 mM, MgCl2 5 mM, pH 7.5);
- Potassium cyanide (KCN, 100 mM in water);
- Antimycin A (2 mM in ethanol);
- Decylubiquinone (38.75 mM in DMSO);
- Rotenone (600 µM in ethanol);
- NADH (5 mM in water).
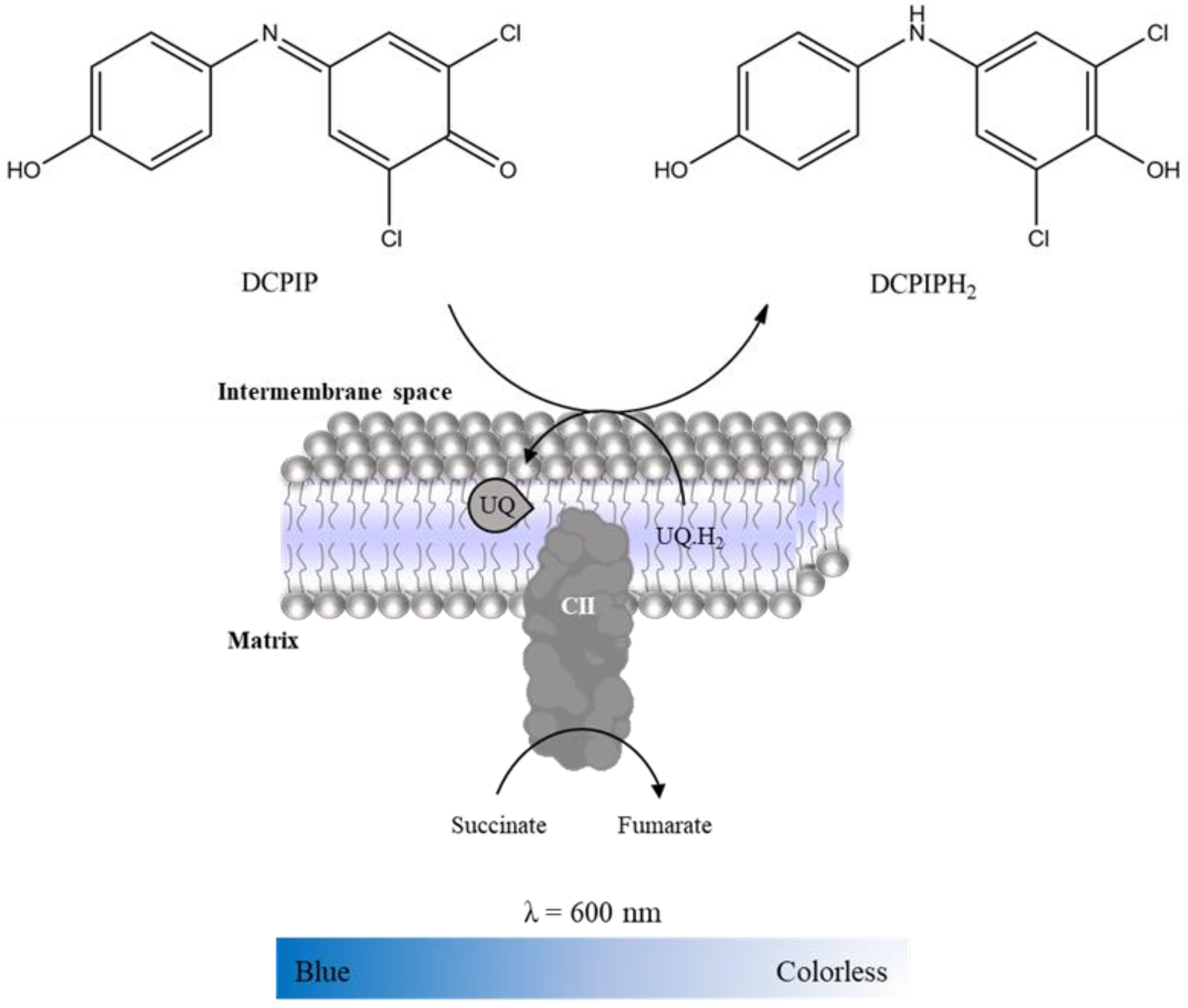
2.3.2. Complex II: Succinate:Ubiquinone Oxidoreductase
- Suspension of mitochondria-rich fraction (0.5 mg protein/mL);
- Phosphate buffer (KH2PO4 25 mM, pH 7.5);
- 2,6-dichlorophenolindophenol (DCPIP, 25 mM in water);
- KCN (100 mM in water);
- Antimycin A (2mM in ethanol);
- Decylubiquinone (38.75 mM in DMSO);
- Rotenone (600 µM in ethanol);
- Oxaloacetate (500 mM in water);
- Succinate (100 mM in water).
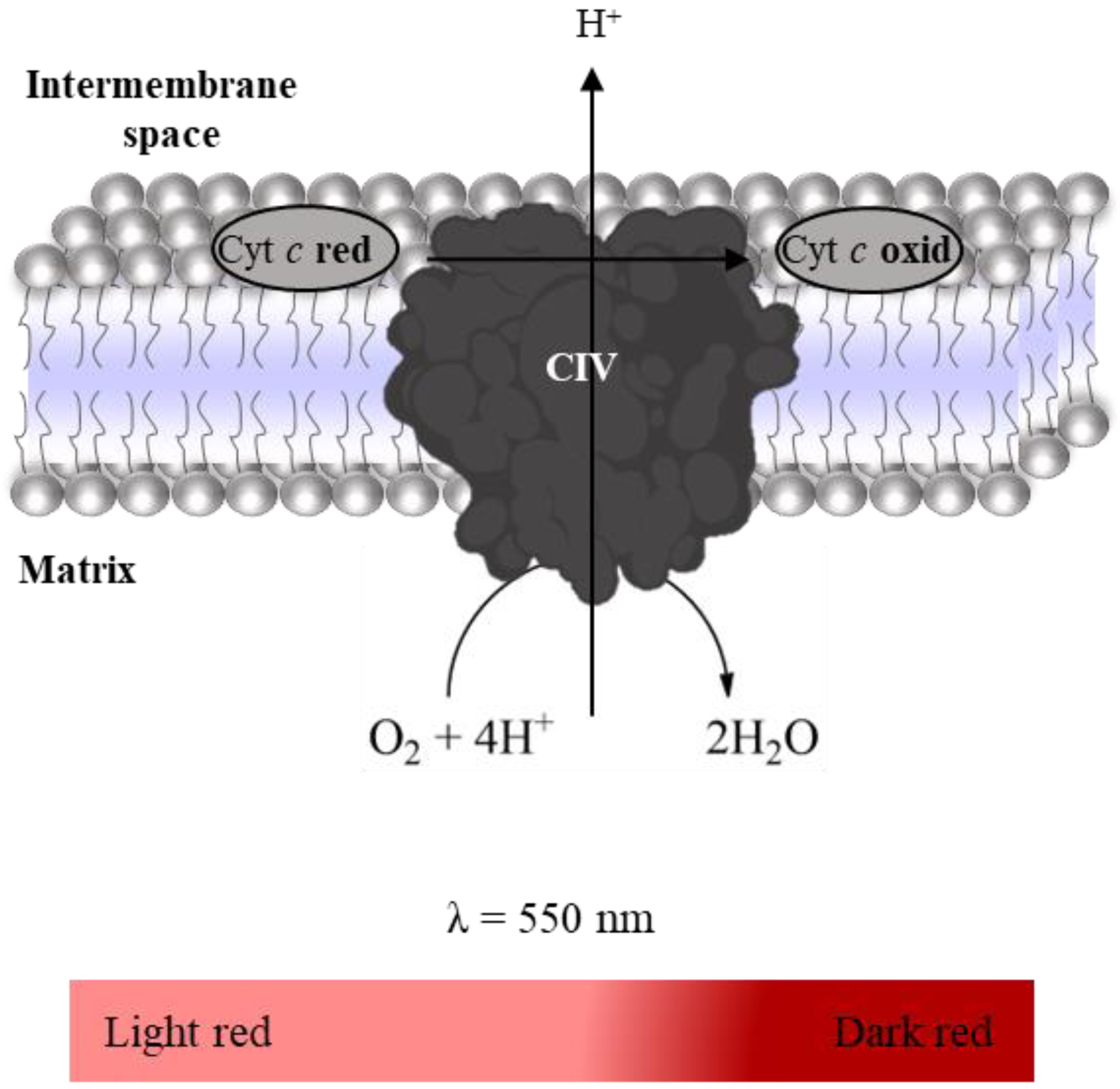
2.3.3. Complex IV: Cytochrome c Oxidase Activity
- Suspension of mitochondria-rich fraction (0.5 mg protein/mL);
- Phosphate buffer (KH2PO4 25 mM, pH 7.5);
- Antimycin A (2 mM in ethanol);
- Rotenone (600 µM in ethanol);
- KCN (100 mM in water);
- Reduced Cyt c (200 µM in phosphate buffer).
2.3.4. Super-Complex I–III Activity
- Suspension of mitochondria-rich fraction (0.5 mg protein/mL);
- Phosphate buffer (KH2PO4 25 mM, MgCl2 5 mM, pH 7.5);
- KCN (100 mM in water);
- Antimycin A (2 mM in ethanol);
- Rotenone (600 µM in ethanol);
- Oxidized Cyt c (200 µM in phosphate buffer);
- NADH (5 mM in water).
2.3.5. Complex II–III Conjugated Activity
- Suspension of mitochondria-rich fraction (0.5 mg protein/mL);
- Phosphate buffer (KH2PO4 25 mM, pH 7.5);
- KCN (100 mM in water);
- Antimycin A (2 mM in ethanol);
- Rotenone (600 µM in ethanol);
- Oxidized Cyt c (200 µM in phosphate buffer);
- Succinate (100 mM in water).
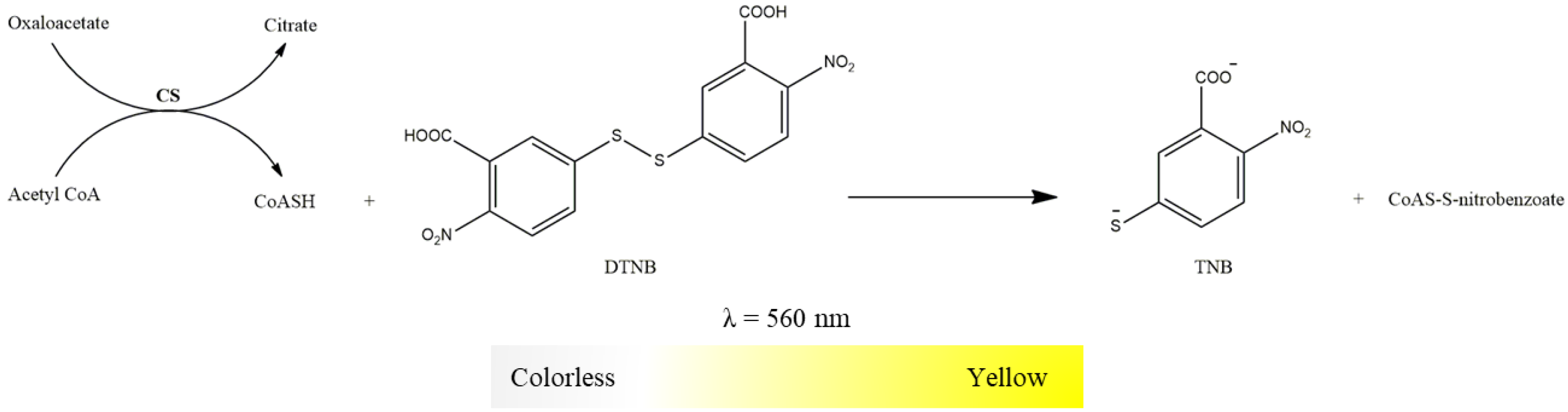
2.3.6. Citrate Synthase Activity
- Suspension of mitochondria-rich fraction (0.5 mg protein/mL);
- Buffer (Tris-HCl 20 mM, pH 8, Triton X-100 0.02%);
- 5,5-dithio-bis-2-nitrobenzoic acid (DTNB, 10 mM in water);
- Oxaloacetate (100 mM in water);
- Acetyl-CoA (6.1 mM in water);
- Succinyl-CoA (60 mM in water).
3. Expected Results
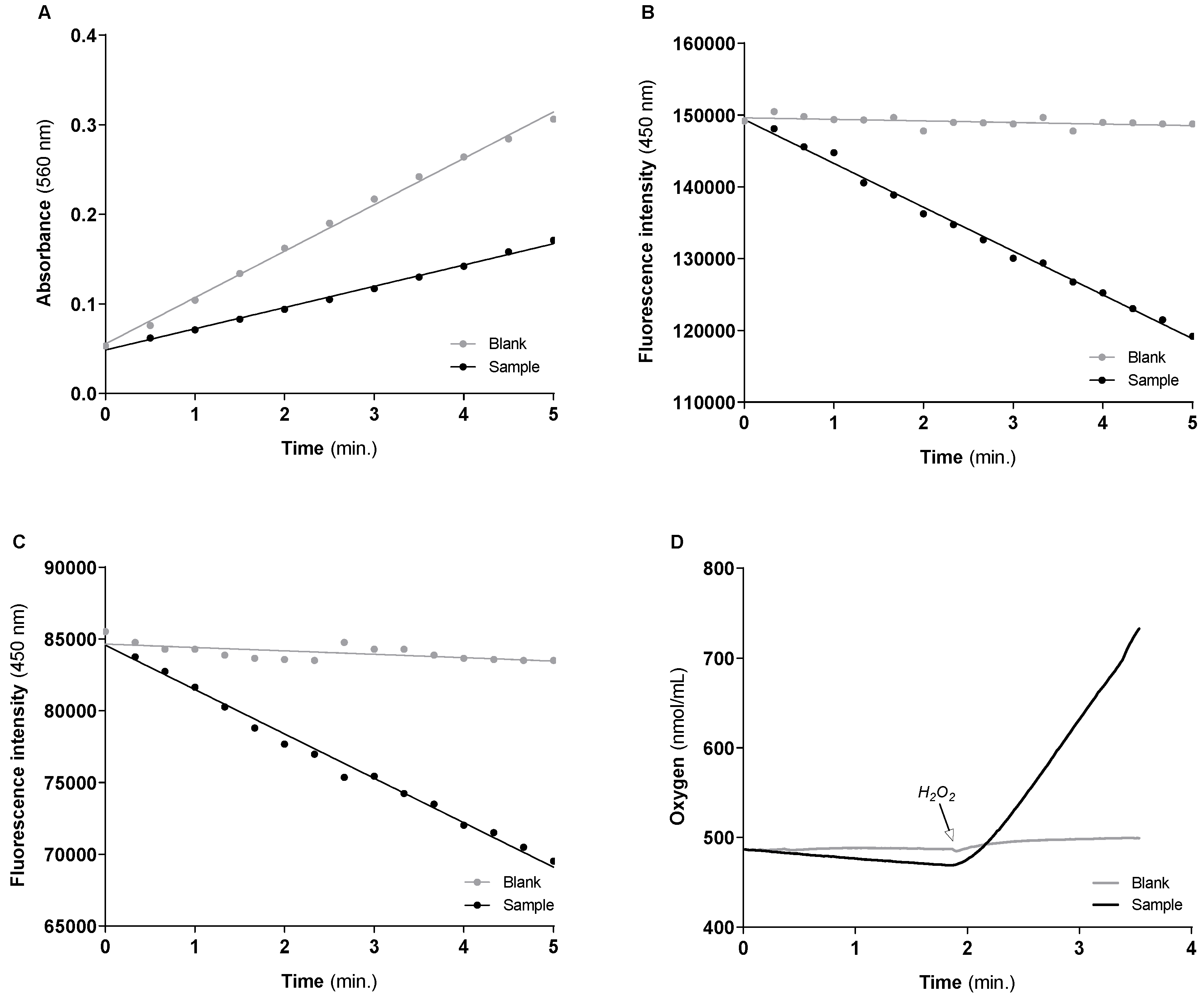
3.1. Activities of the Cytosolic Antioxidant Enzymes
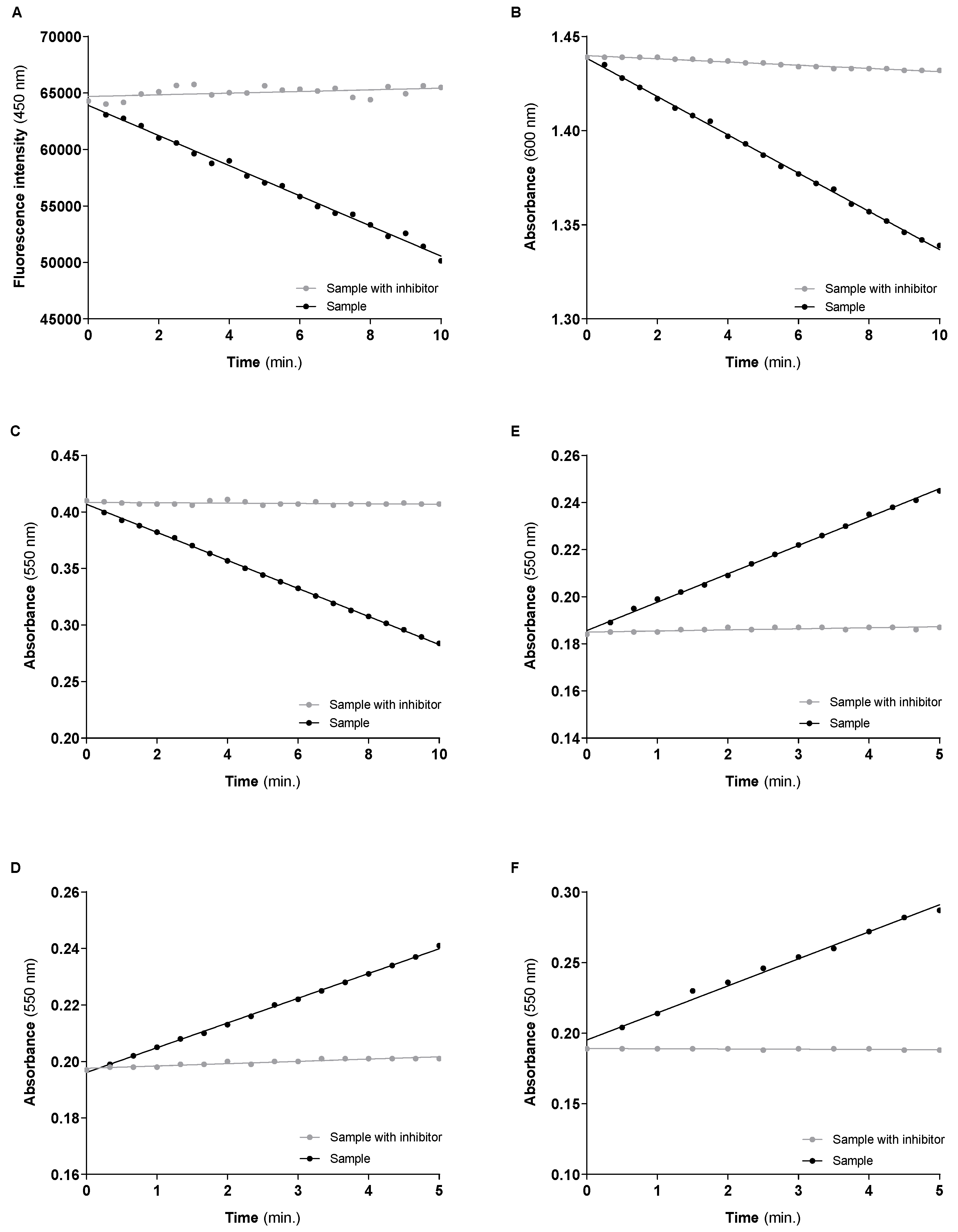
3.2. Mitochondrial Respiratory Chain Complexes Activities
3.3. Relationship between Mitochondrial Redox Chain Functionality and the Cytosolic Antioxidant Enzymes Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zolkipli-Cunningham, Z.; Falk, M.J. Clinical effects of chemical exposures on mitochondrial function. Toxicology 2017, 391, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, D.; Rodriguez-Lafrasse, C. Role of Mitochondria in Radiation Responses: Epigenetic, Metabolic, and Signaling Impacts. Int. J. Mol. Sci. 2021, 22, 11047. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Hospital, C.; Tête, A.; Brial, F.; Benoit, L.; Koual, M.; Tomkiewicz, C.; Kim, M.J.; Blanc, E.B.; Coumoul, X.; Bortoli, S. Mitochondrial Dysfunction as a Hallmark of Environmental Injury. Cells 2022, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552 Pt 2, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Larosa, V.; Remacle, C. Insights into the respiratory chain and oxidative stress. Biosci. Rep. 2018, 38, BSR20171492. [Google Scholar] [CrossRef]
- Ademowo, O.S.; Dias, H.K.I.; Burton, D.G.A.; Griffiths, H.R. Lipid (per) oxidation in mitochondria: An emerging target in the ageing process? Biogerontology 2017, 18, 859–879. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction, Oxidative Stress, and Neuroinflammation: Intertwined Roads to Neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef]
- Lin, M.M.; Liu, N.; Qin, Z.H.; Wang, Y. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol. Sin. 2022, 43, 2439–2447. [Google Scholar] [CrossRef]
- Neves, D.; Valentão, P.; Bernardo, J.; Oliveira, M.C.; Ferreira, J.M.G.; Pereira, D.M.; Andrade, P.B.; Videira, R.A. A new insight on elderberry anthocyanins bioactivity: Modulation of mitochondrial redox chain functionality and cell redox state. J. Funct. Foods 2019, 56, 145–155. [Google Scholar] [CrossRef]
- Videira, R.A.; Andrade, P.B.; Monteiro, L.S.; Valentão, P.; Ferreira, P.M.T.; Pereira, D.M. Toxicity and structure-activity relationship (SAR) of α, β-dehydroamino acids against human cancer cell lines. Toxicol. In Vitro 2018, 47, 26–37. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Worthington, D.J.; Rosemeyer, M.A. Human glutathione reductase: Purification of the crystalline enzyme from erythrocytes. Eur. J. Biochem. 1974, 48, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Fluorescence Sensing. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2010. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A.; Ortega, M.G.; López, A.L.; Gorgé, J.L. A more sensitive modification of the catalase assay with the Clark oxygen electrode. Application to the kinetic study of the pea leaf enzyme. Anal. Biochem. 1977, 80, 409–415. [Google Scholar] [CrossRef]
- Monteiro-Cardoso, V.F.; Oliveira, M.M.; Melo, T.; Domingues, M.R.; Moreira, P.I.; Ferreiro, E.; Peixoto, F.; Videira, R.A. Cardiolipin profile changes are associated to the early synaptic mitochondrial dysfunction in Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 1375–1392. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. Rapid kinetic microassay for catalase activity. J. Biomol. Tech. 2007, 18, 185–187. [Google Scholar]
- Dudkina, N.V.; Sunderhaus, S.; Boekema, E.J.; Braun, H.P. The higher level of organization of the oxidative phosphorylation system: Mitochondrial supercomplexes. J. Bioenerg. Biomembr. 2008, 40, 419–424. [Google Scholar] [CrossRef]
- Dudkina, N.V.; Kouril, R.; Peters, K.; Braun, H.P.; Boekema, E.J. Structure and function of mitochondrial supercomplexes. Biochim. Biophys. Acta 2010, 1797, 664–670. [Google Scholar] [CrossRef]
- Heerklotz, H. Membrane stress and permeabilization induced by asymmetric incorporation of compounds. Biophys. J. 2001, 81, 184–195. [Google Scholar] [CrossRef]
- Gellerich, F.N.; Deschauer, M.; Chen, Y.; Müller, T.; Neudecker, S.; Zierz, S. Mitochondrial respiratory rates and activities of respiratory chain complexes correlate linearly with heteroplasmy of deleted mtDNA without threshold and independently of deletion size. Biochim. Biophys. Acta 2002, 1556, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Duarte, A.I.; Moreno, A.; Gonçalves, L.M.; Providência, L.A. Carvedilol improves energy production during acute global myocardial ischaemia. Eur. J. Pharmacol. 2003, 482, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, Y.; Stiggall, D.L. Preparation and properties of succinate: Ubiquinone oxidoreductase (complex II). Methods Enzymol. 1978, 53, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.J.; Siedow, J.N.; Moreland, D.E. Succinate dehydrogenase: A partial purification from mung bean hypocotyls and soybean cotyledons. Plant Physiol. 1982, 70, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Margoliash, E.; Walasek, O.F. Cytochrome c from vertebrate and invertebrate sources. Methods Enzymol. 1967, 10, 339–348. [Google Scholar] [CrossRef]
- Barrientos, A.; Fontanesi, F.; Díaz, F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr. Protoc. Hum. Genet. 2009, 63, 19.3.1–19.3.14. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.; Garland, P.B. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem. J. 1969, 114, 597–610. [Google Scholar] [CrossRef]
- Mitchell, P. Chemiosmotic Coupling in Oxidative and Photosynthetic Phosphorylation. Biol. Ver. Camb. Philos. Soc. 1966, 41, 445–502. [Google Scholar] [CrossRef]
- Mendes, D.; Oliveira, M.M.; Moreira, P.I.; Coutinho, J.; Nunes, F.M.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Videira, R.A. Beneficial effects of white wine polyphenols-enriched diet on Alzheimer’s disease-like pathology. J. Nutr. Biochem. 2018, 55, 165–177. [Google Scholar] [CrossRef]
- Verstraete, K.; Verschueren, K.H.G.; Dansercoer, A.; Savvides, S.N. Acetyl-CoA is produced by the citrate synthase homology module of ATP-citrate lyase. Nat. Struct. Mol. Biol. 2021, 28, 636–638. [Google Scholar] [CrossRef]
- Kang, W.; Suzuki, M.; Saito, T.; Miyado, K. Emerging Role of TCA Cycle-Related Enzymes in Human Diseases. Int. J. Mol. Sci. 2021, 22, 13057. [Google Scholar] [CrossRef] [PubMed]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R. Mitochondria in health and disease: Perspectives on a new mitochondrial biology. Mol. Aspects. Med. 2004, 25, 365–451. [Google Scholar] [CrossRef] [PubMed]
- Bleier, L.; Dröse, S. Superoxide generation by complex III: From mechanistic rationales to functional consequences. Biochim. Biophys. Acta 2013, 1827, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef]
- Brischigliaro, M.; Frigo, E.; Fernandez-Vizarra, E.; Bernardi, P.; Viscomi, C. Measurement of mitochondrial respiratory chain enzymatic activities in Drosophila melanogaster samples. STAR Protoc. 2022, 3, 101322. [Google Scholar] [CrossRef]
- Mendes, D.; Valentão, P.; Oliveira, M.M.; Andrade, P.; Videira, R.A. A nanophytosomes formulation based on elderberry anthocyanins and Codium lipids to mitigate mitochondrial dysfunctions. Biomed. Pharmacother. 2021, 143, 112157. [Google Scholar] [CrossRef]
- Decleer, M.; Jovanovic, J.; Vakula, A.; Udovicki, B.; Agoua, R.E.K.; Madder, A.; De Saeger, S.; Rajkovic, A. Oxygen Consumption Rate Analysis of Mitochondrial Dysfunction Caused by Bacillus cereus Cereulide in Caco-2 and HepG2 Cells. Toxins 2018, 10, 266. [Google Scholar] [CrossRef]
- Silva, A.M.; Oliveira, P.J. Evaluation of Respiration with Clark-Type Electrode in Isolated Mitochondria and Permeabilized Animal Cells. Methods Mol. Biol. 2018, 1782, 7–29. [Google Scholar] [CrossRef]


| Sample | Blank | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 130 | 180 |
| Mitochondria-free cytosolic fraction | 50 | 0 |
| NBT | 20 | 20 |
| Xanthine oxidase | 10 | 10 |
| Incubate for 5 min, 37 °C | ||
| Xanthine | 40 | 40 |
| Read absorbance (560 nm) | Kinetics for 5 min, recording points every 30 s | |
| Sample | Blank | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 185 | 195 |
| Mitochondria-free cytosolic fraction | 50 | 50 |
| GSSG | 10 | 0 |
| Incubate for 5 min, 37 °C | ||
| NADPH | 5 | 5 |
| Read fluorescence (Ex 366 nm; Em 450 nm) | Kinetics for 5 min, recording points every 20 s | |
| Sample | Blank | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 155 | 165 |
| Mitochondria-free cytosolic fraction | 50 | 50 |
| GSH | 10 | 0 |
| GR | 10 | 10 |
| NADPH | 5 | 5 |
| Incubate for 5 min, 37 °C | ||
| H2O2 | 20 | 20 |
| Read fluorescence (Ex 366 nm; Em 450 nm) | Kinetics for 5 min, recording points every 20 s | |
| Sample | Blank | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 930 | 980 |
| Mitochondria-free cytosolic fraction | 50 | 0 |
| Incubate for 5 min, 37 °C | ||
| H2O2 | 20 | 20 |
| Read oxygen production | Kinetics for 2–3 min, recording points every 1 s | |
| Sample | Sample with Inhibitor | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 214 | 209 |
| Mitochondria-rich fraction | 20 | 20 |
| KCN | 2.5 | 2.5 |
| Antimycin A | 1 | 1 |
| Decylubiquinone | 5 | 5 |
| Rotenone | 0 | 5 |
| Incubate for 5 min, 37 °C | ||
| NADH | 7.5 | 7.5 |
| Read fluorescence (Ex 366 nm; Em 450 nm) | Kinetics for 10 min, recording points every 30 s | |
| Sample | Sample with Inhibitor | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 212.75 | 207.75 |
| Mitochondria-rich fraction | 20 | 20 |
| DCPIP | 2.5 | 2.5 |
| KCN | 2.5 | 2.5 |
| Antimycin A | 1 | 1 |
| Decylubiquinone | 1.25 | 1.25 |
| Rotenone | 5 | 5 |
| Oxaloacetate | 0 | 5 |
| Incubate for 5 min, 37 °C | ||
| Succinate | 5 | 5 |
| Read absorbance (600 nm) | Kinetics for 10 min, recording points every 30 s | |
| Sample | Sample with Inhibitor | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 189 | 186.5 |
| Mitochondria-rich fraction | 20 | 20 |
| Antimycin A | 1 | 1 |
| Rotenone | 5 | 5 |
| KCN | 0 | 2.5 |
| Incubate for 5 min, 37 °C | ||
| Reduced Cyt c | 35 | 35 |
| Read absorbance (550 nm) | Kinetics for 10 min, recording points every 30 s | |
| Sample | Sample with Inhibitor | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 202.5 | 196.5 |
| Mitochondria-rich fraction | 20 | 20 |
| KCN | 2.5 | 2.5 |
| Antimycin A | 0 | 1 |
| Rotenone | 0 | 5 |
| Oxidized Cyt c | 20 | 20 |
| Incubate for 5 min, 37 °C | ||
| NADH | 5 | 5 |
| Read absorbance (550 nm) | Kinetics for 5 min, recording points every 30 s | |
| Sample | Sample with Inhibitor | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 197.5 | 196.5 |
| Mitochondria-rich fraction | 20 | 20 |
| KCN | 2.5 | 2.5 |
| Antimycin A | 0 | 1 |
| Rotenone | 5 | 5 |
| Oxidized Cyt c | 20 | 20 |
| Incubate for 5 min, 37 °C | ||
| Succinate | 5 | 5 |
| Read absorbance (550 nm) | Kinetics for 5 min, recording points every 30 s | |
| Sample | Sample with Inhibitor | |
|---|---|---|
| Volume (μL) | Volume (μL) | |
| Buffer | 210 | 205 |
| Mitochondria-rich fraction | 20 | 20 |
| DTNB | 10 | 10 |
| Oxaloacetate | 5 | 5 |
| Succinyl-CoA | 0 | 5 |
| Incubate for 5 min, 37 °C | ||
| Acetyl-CoA | 5 | 5 |
| Read absorbance (412 nm) | Kinetics for 5 min, recording points every 30 s | |
| Linear Regression | Enzyme Activity | ||
|---|---|---|---|
| Cu/Zn-SOD | Blank | Y = 0.05178 × X + 0.05527 | 9494 nmol/min/mg of protein (9051.509 ± 377.986) # |
| Sample | Y = 0.02731 × X + 0.04855 | ||
| GR | Blank | Y = −216.9 × X + 149,618 | 47.486 nmol NADPH/min/mg of protein (47.572 ± 3.324) # |
| Sample | Y = −6108 × X + 149,379 | ||
| GPx | Blank | Y = −236.9 × X + 84,650 | 23.038 nmol H2O2/min/mg of protein (24.459 ± 1.083) # |
| Sample | Y = −3095 × X + 84,577 | ||
| CAT | Blank | Y = 2.835 × X + 446.5 | 560.217 nmol O2/min/mg of protein (583.549 ± 16.954) # |
| Sample | Y = 170.9 × X + 120.0 |
| Linear Regression | Enzyme Activity | Enzyme Activity/CS | ||
|---|---|---|---|---|
| CI | Sample | Y = −1334 × X + 63,904 | 54.629 nmol NADH/min/mg of protein (55.485 ± 4.313) # | 0.939 |
| Sample w/inhibitor | Y = 73.32 × X + 64,694 | |||
| CII | Sample | Y = −0.01016 × X + 1.438 | 18.736 nmol DCPIP reduced/min/mg of protein * (20.722 ± 1.796) # | 0.322 |
| Sample w/inhibitor | Y = −0.0008519 × X + 1.440 | |||
| CIV | Sample | Y = −0.01242 × X + 0.4068 | 17.319 nmol oxidized Cyt c/min/mg of protein (18.786 ± 1.181) # | 0.298 |
| Sample w/inhibitor | Y = −0.0001584 × X + 0.4085 | |||
| CI–III | Sample | Y = 0.01205 × X + 0.1856 | 16.372 nmol reduced Cyt c/min/mg of protein (18.808 ± 2.254) # | 0.281 |
| Sample w/inhibitor | Y = 0.0004588 × X + 0.1856 | |||
| CII–III | Sample | Y = 0.008762 × X + 0.1961 | 11.229 nmol reduced Cyt c/min/mg of protein (9.912 ± 1.011) # | 0.193 |
| Sample w/inhibitor | Y = 0.0008118 × X + 0.1976 | |||
| CS | Sample | Y = 0.01916 × X + 0.1952 | 58.200 nmol TNB/min/mg of protein ** (63.204 ± 5.699) # | - |
| Sample w/inhibitor | Y = −0.0001636 × X + 0.1891 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, D.; Silva, A.M.; Oliveira, M.M.; Andrade, P.B.; Videira, R.A. An Experimental Approach to Address the Functional Relationship between Antioxidant Enzymes and Mitochondrial Respiratory Complexes. Methods Protoc. 2023, 6, 32. https://doi.org/10.3390/mps6020032
Mendes D, Silva AM, Oliveira MM, Andrade PB, Videira RA. An Experimental Approach to Address the Functional Relationship between Antioxidant Enzymes and Mitochondrial Respiratory Complexes. Methods and Protocols. 2023; 6(2):32. https://doi.org/10.3390/mps6020032
Chicago/Turabian StyleMendes, Daniela, Ana Maria Silva, Maria Manuel Oliveira, Paula B. Andrade, and Romeu A. Videira. 2023. "An Experimental Approach to Address the Functional Relationship between Antioxidant Enzymes and Mitochondrial Respiratory Complexes" Methods and Protocols 6, no. 2: 32. https://doi.org/10.3390/mps6020032
APA StyleMendes, D., Silva, A. M., Oliveira, M. M., Andrade, P. B., & Videira, R. A. (2023). An Experimental Approach to Address the Functional Relationship between Antioxidant Enzymes and Mitochondrial Respiratory Complexes. Methods and Protocols, 6(2), 32. https://doi.org/10.3390/mps6020032







