1. Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is an essential neurotransmitter underlying both neurological and gastrointestinal (GI) functions, making it a target of study for these functions and their related disorders. In the brain, 5-HT helps regulate behaviors such as sleep and learning [
1,
2] as well as onset of anxiety and depression [
3]. 5-HT is produced at even higher levels in the mammalian GI tract in pathways responsible for peristalsis [
4], visceral sensation [
5], and symptoms such as nausea [
6], and has been implicated in a number of functional GI disorders [
7,
8,
9,
10]. Often, the goal is to quantify 5-HT concentrations within tissues or bodily fluids and correlate this with biological processes, especially underlying disease. To this end, there is a long history of electrochemical 5-HT detection methods, which provide real-time, label-free quantification of 5-HT within these biological environments, given adequate accessibility for the insertion of electrodes [
11,
12,
13] in benchtop and in vivo environments.
5-HT electrochemical detection is an adsorption-limited reaction [
14], where 5-HT molecules adsorb to an electroactive surface and are oxidized, producing a measurable current signal. This implies that 5-HT binding and detection are time-dependent, requiring strict control over the measurement frequency to record an accurate concentration [
15,
16]. Furthermore, electrochemical detection leads to downstream fouling as spontaneous 5-HT oxidative polymerization results in the formation of polymeric byproducts [
17], which cover the electrode and decrease electroactive surface area over successive measurements [
14,
18,
19]. 5-HT fouling causes progressive loss of sensitivity over time, which limits the reliability of 5-HT quantification. There is significant research towards optimizing electrode materials to resist fouling (e.g., carbon nanotubes (CNTs) [
20,
21], diamond [
22,
23]) or electrochemical protocols that discourage fouling (e.g., fast-scan cyclic voltammetry [
13,
24,
25]). However, these methods rarely fully eliminate fouling, and thus there remains a level of unreliability in the repeatability of 5-HT sensing. The Langmuir isotherm model of adsorption [
26] is used to study adsorption of molecules, such as 5-HT, onto substrates, such as electrodes. This type of model is commonly used to quantify electrode sensitivity (slope of linear region) and linear range, but is less frequently used to determine fundamental binding parameters underlying electrochemical detection [
27]. Furthermore, the Langmuir model of adsorption has never been directly combined with adsorption-limited electrochemical equations which describe the direct transfer of electrons from redox molecule to electrode, measuring current in direct correlation with molecular concentration [
28].
Here, we aim to develop a simple but effective method to model 5-HT electrochemical detection, based on experimentally-determined binding parameters with a given electrode, derived from a combination of Langmuir and electrochemical adsorption equations. 5-HT measurements made with a CNT-modified electrode, used here to demonstrate the method, were fitted with both equilibrium and time-dependent Langmuir adsorption models to characterize 5-HT binding affinity with the electrode. Langmuir equations combined with adsorption-limited electrochemical equations were then used to model 5-HT binding and subsequent current signal generation, which could effectively predict electrochemical signal output based on 5-HT concentration and accumulation time between measurements. These equations and binding parameters were also used to track the process of 5-HT fouling at the electrode, constituting a computational model of 5-HT adsorption, detection, and fouling. This model also aids in optimizing sensing parameters for a given application, such as detection of 5-HT from RIN14B cells, an immortalized cell line which expresses a similar 5-HT secretion pathway to enterochromaffin cells in the GI tract [
29]. The model demonstrated here could be applied to any electrode system given characterization of its unique binding parameters with 5-HT, or other adsorption-limited redox molecules, and could be combined with models of complex biological environments to improve understanding of molecular behavior in tissues of interest.
2. Materials and Methods
2.1. Adsorption Model Development
Langmuir Adsorption Kinetics are generally used to define the interaction of a ligand and a substrate in order to understand the formation of a product under equilibrium and time-dependent conditions. Here, they can describe the adsorption-limited electrochemical detection of 5-HT, which here will be demonstrated using cyclic voltammetry (CV). Detection begins with 5-HT adsorption onto the electrode surface to form the product 5-HT
ads, the number of moles of 5-HT adsorbed on the electrode, as illustrated in
Figure 1. This binding reaction states:
where 5-HT is the concentration of free 5-HT in aqueous solution (M), ELD is the number of available binding sites on the electrode surface (#mol sites), and 5-HT
ads is the number of adsorbed 5-HT (#mol). Modeling the electrode surface with a discrete number of binding sites, rather than surface area, allows for modeling of one-to-one binding with molecules, as is described by Langmuir kinetic processes.
The forward and reverse reaction rates are k
on (M
−1 s
−1) and k
off (s
−1), respectively. The dissociation constant, K
D (M), can be found from the ratio of k
off/k
on, and will be used here to define binding affinity. Of note, we can assume the binding reaction has reached equilibrium when sufficient accumulation time (t
acc) is given to achieve 5-HT binding saturation on the electrode. One can follow the derivation included in the
Supplementary Information (Supplementary Figure S1) to determine the following Langmuir isotherm equation at equilibrium [
26]:
where ELD
tot is the total number of available binding sites on the electrode surface. This equation can be fitted to experimental data in order to calculate key values for any given electrode and analyte: K
D and ELD
tot.
The following equations describe time-dependent adsorption and desorption, respectively, based on the same Langmuir isotherm model [
30]:
where 5-HT
ads is calculated for a given applied t
acc (s) of binding time. The adsorption regime is considered when a constant non-zero [5-HT] is applied to the electrode, resulting in progression of the binding reaction toward equilibrium. The desorption regime is when [5-HT] = 0, resulting in unbinding toward full dissociation of 5-HT from the surface. Here, Equation (3) is fitted with experimental cyclic voltammetry (CV) data measuring a constant [5-HT] over increasing t
acc to determine k
on and k
off, given the solution of 5-HT
ads,eq from (2) under equilibrium conditions. Equations (3) and (4) are later modeled using these calculated binding parameters to predict 5-HT binding behavior at various binding times and concentrations.
Adsorption-controlled electrochemical processes are governed by the binding of a given redox-active analyte (such as 5-HT) to the electrode surface, wherein the surface coverage of these redox molecules leads to direct electron transfer that causes an increase in anodic peak current (Ipa) signal. This interaction can be described by the following equation [
28]:
where n is the number of electrons transferred per molecule, F is Faraday’s constant, R is the ideal gas constant, T is temperature (K), ν is scan rate, A is surface area, and Γ is molecular surface coverage. Here, A can be described as the surface area of the electrode (mm
2) and Γ is the molecular surface coverage of 5-HT
. The product of A∙Γ reduces to 5-HT
ads, which linearly scales with Ipa. Therefore, Equation (5) reduces to Equation (6):
This equation serves as a conversion between the number of adsorbed moles of 5-HT (5-HTads), which can be calculated using Equations (2)–(4), and electrochemical signal output (Ipa). It should be noted that this equation is time-dependent in that 5-HTads depends on tacc, and thus so does Ipa. If the applied tacc is enough to achieve equilibrium binding, then 5-HTads(tacc) = 5-HTads,eq.
If one were modeling electrochemical detection of an adsorbed analyte, they could follow the above equations in the presented order to estimate the signal that may be obtained from detection of a given analyte, with known concentration over a given tacc. Conversely, the equations may be used in reverse order to calculate the analyte concentration measured from a given electrochemical experiment, given its Ipa signal and tacc. Furthermore, the intermediate calculations of 5-HTads allows for more in depth modeling of molecular behavior, as might be useful in complex biological environments.
5-HT Fouling can also be modeled using these equations. The fouling process can be identified as when CV signal decreases, even in a constant 5-HT concentration solution, due to incremental covering of the electrode surface with polymerized 5-HT material. This can be approximated as a decrease in available ELD binding sites after each 5-HT CV measurement, wherein some fraction of bound 5-HT molecules do not unbind. The following equation is proposed to describe this fouling of the ELD surface:
where i denotes CV cycle number, ELD
1 = ELD
tot, and
a is a proportionality factor describing the fraction of 5-HT
ads that does not unbind and therefore fouls ELD sites. In this way, the rate of fouling is proportional to 5-HT
ads, which depends on 5-HT concentration and applied t
acc, as has been found experimentally (
Supplementary Figure S2).
A model was established to fit this fouling process to experimental CV data. For each CV cycle, Equation (2) was used to calculate the equilibrium 5-HTads,eq for the given [5-HT] and available ELD sites (ELDi), which was used in Equation (3) to calculate the time-dependent 5-HTads given the applied tacc. Then, Equation (7) was used to calculate the decrease in total available ELD sites, where a was solved to fit the experimental data, and the calculated ELDi+1 value was used for the next CV cycle. Finally, 5-HTads was converted to Ipa for each cycle using Equation (6), and both were plotted over CV cycle.
2.2. Electrode Fabrication
Here, an electrochemical platform was fabricated consisting of Au electrodes modified with CNTs (Au-CNT) to increase electrode sensitivity to 5-HT, and potentially limit fouling [
16,
19]. This Au-CNT electrode was previously demonstrated to exhibit adsorption-limited detection of 5-HT, given by a linear relation between measured Ipa and applied scan rate [
15]. The electrochemical testing platform was assembled by fabricating Au-CNT electrodes on a standard 25.4 × 76.2 mm glass slide and then affixed with a 3D-printed well housing, materials which are relatively inert and robust (
Figure 2). The glass slides were solvent cleaned with acetone, methanol, and isopropanol (AMI), followed by a DI water rinse. Au working and counter electrodes (WE, CE) were patterned on the glass via electron beam evaporation (20 nm Ti/100 nm Au) through a laser-cut shadow mask, as previously described [
15,
31]. The WE was modified with 1 mg/mL purified single walled CNTs (Carbon Solutions Inc., Riverside, CA, USA) dispersed in a 1:1 mixture of ethanol and N-methyl-2-pyrrolidone (NMP) (Thermo Fisher Scientific, Waltham, MA, USA). The solution was ultrasonicated to improve uniformity, and then 3 µL was drop cast on the WE after being heated on a 200 °C hotplate, which functions to evaporate the edge of the CNT droplet before it spreads across the surface of the glass, containing the coating to the WE. This method was previously shown to produce a thin, uniform mesh of CNTs on the electrode surface, which did not visually degrade over the process of experimentation [
15]. The whole slide was then cleaned again with AMI before assembly with the 3D printed wells.
The wells were designed in Autodesk
® Inventor™ and printed with an Objet500 Connex3 3D printer (Stratasys, Eden Prairie, MN, USA) with MED610, a photopolymer using PolyJet technology. MED610 is a bioinert 3D printing material, characterized to not show significant cytotoxicity by ISO standards if proper cleaning procedures are taken [
32]. This cleaning included support material removal by sonication in 2% NaOH and 1% Na
2SiO
3 for up to 24 h, followed by thorough leeching of any further photoreactive materials by alternating sonication in isopropanol and diH
2O for 2 h each [
33]. Once dried, the rim of the 3D printed well structure was coated with polydimethylsulfoxide (PDMS) in a 10:1 ratio (Sylgard
®184, Dow Corning, Midland, MI, USA), affixed to the glass slide, and thermally cured on an 80 °C hotplate for 1 h. This leaves a 10 mm space in the rim of the 3D printed wells to provide access to the contact pads for wiring via microalligator clips.
2.3. Electrochemical Methods
5-HT was purchased from Alfa Aesar (Ward Hill, MA, USA) and dissolved in 1× phosphate buffered saline (PBS, pH = 7.4) (Thermo Fisher Scientific) for electrode characterization. All electrochemical experiments were carried out using the VSP-300 potentiostat, and data was recorded using the EC-Lab software, both from Bio-Logic Science Instruments (Seyssinet-Pariset, France). Cyclic voltammetry (CV) measurements of 5-HT were performed between 0.1–0.5 V to center around the 5-HT redox potential at ~0.33 V. These measurements were performed using the glass slide-fabricated Au CE and Au-CNT WE, and a standard Ag/AgCl reference electrode (RE) in saturated KCl (CH Instruments, Austin, TX, USA) that was manually inserted into the fluid during electrochemical measurements. This electrode was chosen for its superior longevity compared to thin-film Ag electrodes, which can drift over time [
34].
Because of the adsorption-limited nature of 5-HT electrochemical detection, an accumulation time (t
acc) was applied before each CV cycle, holding the electrode at open circuit potential for a given time to allow for sufficient 5-HT adsorption to produce a measurable peak, or to reach equilibrium binding.
Supplementary Figure S3 shows a comparison of different scan rates and t
accs used to detect 100 nM 5-HT. While a more in-depth analysis of t
acc-dependent detection is performed later, this analysis showed that compared to lower scan rates, 400 mV/s resulted in the least amount of noise in the current while still maintaining peak sharpness at low concentrations. Thus, CV measurements were performed at 400 mV/s, unless otherwise stated.
MATLAB (MathWorks, Natick, MA, USA) was used to process the CV data, in which current was plotted against potential (I vs. E). The data was first smoothed using a low-pass filter with a cutoff frequency of 3 Hz and a sampling frequency of 2 kHz. To extract anodic peak currents (Ipa), a linear regression was fitted against the background signal preceding each CV peak and then subtracted from the measured current peak (
Supplementary Figure S4).
2.4. Cell Culture Testing
The RIN14B rat islet cell line (ATCC® CRL2059TM, Manassas, VA, USA) was used as a surrogate for 5-HT-secreting enterochromaffin cells in the mammalian gut epithelium. Cells were maintained in T25 polystyrene flasks with incubation at 37 °C and 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific). Cell passaging was performed every 3–5 days with 2.5% trypsin-EDTA (Sigma-Aldrich, St. Louis, MO, USA).
Cells were grown until confluency (~63 × 10
5 cell count) and then stimulated to release 5-HT using allyl isothiocyanate (AITC) and ionomycin (Thermo Fisher Scientific). AITC was purchased as an oil-based solution, which was first diluted in DMSO, then diluted further in diH
2O to result in a 3 mM AITC stock solution in 30% DMSO. To perform cell stimulation, cells were first washed with PBS pre-warmed to 37 °C to avoid premature stimulation. Then, 5-HT release was stimulated by incubating cells for 20 min in 1 mL stimulation solution containing either 300 µM AITC or 10 µM ionomycin. This stimulation solution consisted of 4 °C high-glucose DMEM, containing 1.8 mM CaCl
2, 25 mM glucose, and 2 µM fluoxetine, which are commonly used in RIN14B literature to boost the cells’ response to molecular triggers and maximize 5-HT secretion over the incubation period [
29,
35,
36]. Supernatant was then transferred to an Au-CNT electrode to detect 5-HT, using a 30 min t
acc and 50 mV/s scan rate. Electrodes were shown to have no response to AITC or ionomycin alone (
Supplementary Figure S5).
Supplementary Figure S6 shows the electrode sensitivity to 5-HT in comparison to other common redox molecules including uric acid, ascorbic acid, and dopamine (Sigma-Aldrich). In the performed potential range, the electrodes showed no response to uric acid or ascorbic acid. Dopamine produced an oxidation peak at 0.23 V, well-separated from 5-HT at 0.37 V, but with a lower Ipa (3.3 µA and 4.3 µA, respectively). When all molecules were combined, the 5-HT peak was still clearly distinguishable.
4. Discussion
In this work, we demonstrated the utility of an expanded adsorption model to predict and characterize electrode detection of 5-HT. To our knowledge, this is the first model which combines Langmuir and electrochemical adsorption models, and the first to calculate the binding affinity of a redox molecule to a fabricated electrode. Whereas most electrochemical electrodes are quantitatively compared by their sensitivity, LOD, and linear range, these parameters can depend on other factors such as tacc and fouling behavior and thus do not fully characterize their function. KD is a more fundamental parameter of the binding reaction, and thus may present a more useful quantitative factor for comparison between electrodes, such as when modifying a given electrode surface to deliberately increase molecular binding.
Using fabricated Au-CNT electrodes for this proof of concept, we experimentally determined adsorption parameters of 5-HT binding to this electrode. These parameters were then used to construct a computational model of 5-HT adsorption and desorption using adsorption-limited electrochemical and Langmuir adsorption equations. This model is useful for optimizing measurement conditions for a given application, such as determining electrode sensitivity across different tacc values. A model of electrochemical 5-HT fouling was then proposed, in which 5-HT adsorption and oxidation by CV results in some fraction of electrode binding sites being irrevocably covered by oxidized 5-HT byproducts. Although this is a simple model that does not account for specific chemical processes of fouling, the model was able to approximate the rate of fouling very closely when averaged across four electrodes. Interestingly, the electrodes exhibited an initial phase of signal increase over the first several CV cycles, which is not accounted for in the model and therefore was not fitted here. This could consist of a buildup of bound 5-HT that does not completely oxidize in the first several cycles, or a temporary increase in binding affinity of free 5-HT with a low density of oxidized 5-HT byproducts. This model could be expanded in the future to include this initial phase, given more precise understanding of the chemical interactions in this process.
Furthermore, it was noted that because of this effect, fresh electrodes exhibited slightly lower affinity for 5-HT than electrodes which had been previously exposed to low concentrations of 5-HT. This may also explain why, when converting model-calculated 5-HT
ads to Ipa using Equation (6), a factor of 1.8× resulted in a precise fit of the modeled and experimental data for both
Figure 5 and
Figure 6. This would suggest that a higher Ipa signal is obtained than predicted for a given number of adsorbed 5-HT molecules. Here, this proportionality factor was able to account for the difference, but this has yet to be experimentally confirmed. In addition, this effect can be leveraged in the future to increase electrode sensitivity by pre-conditioning electrodes with 5-HT, wherein the experimental adsorption parameters can be compared to fresh electrodes.
This discrepancy between model-calculated 5-HT
ads and measured Ipa signal could also indicate that that an adsorption model cannot capture all possible mechanisms of 5-HT electrochemical detection. Electron transfer from a redox analyte to an electrochemical electrode can occur though quantum-mechanical electron tunneling across a nanometer-scale distance, bypassing the need for physical molecular adsorption. This can even occur across an insulating layer, such as fouled material [
40]. Our electrodes are modified with CNTs functionalized with carboxylic acid groups, both of which are capable of increasing electron transfer rates and decreasing the electron tunneling distance [
41,
42], suggesting this as a possible mechanism of detection for this electrode. Therefore, future work will be dedicated to evaluating the effect of any possible non-adsorptive 5-HT detection mechanisms, in addition to the adsorption mechanism addressed here. It should be noted, however, that 5-HT detection is reported to be adsorption-limited [
14], suggesting that this mechanism is predominant and can mostly predict electrochemical signal generation.
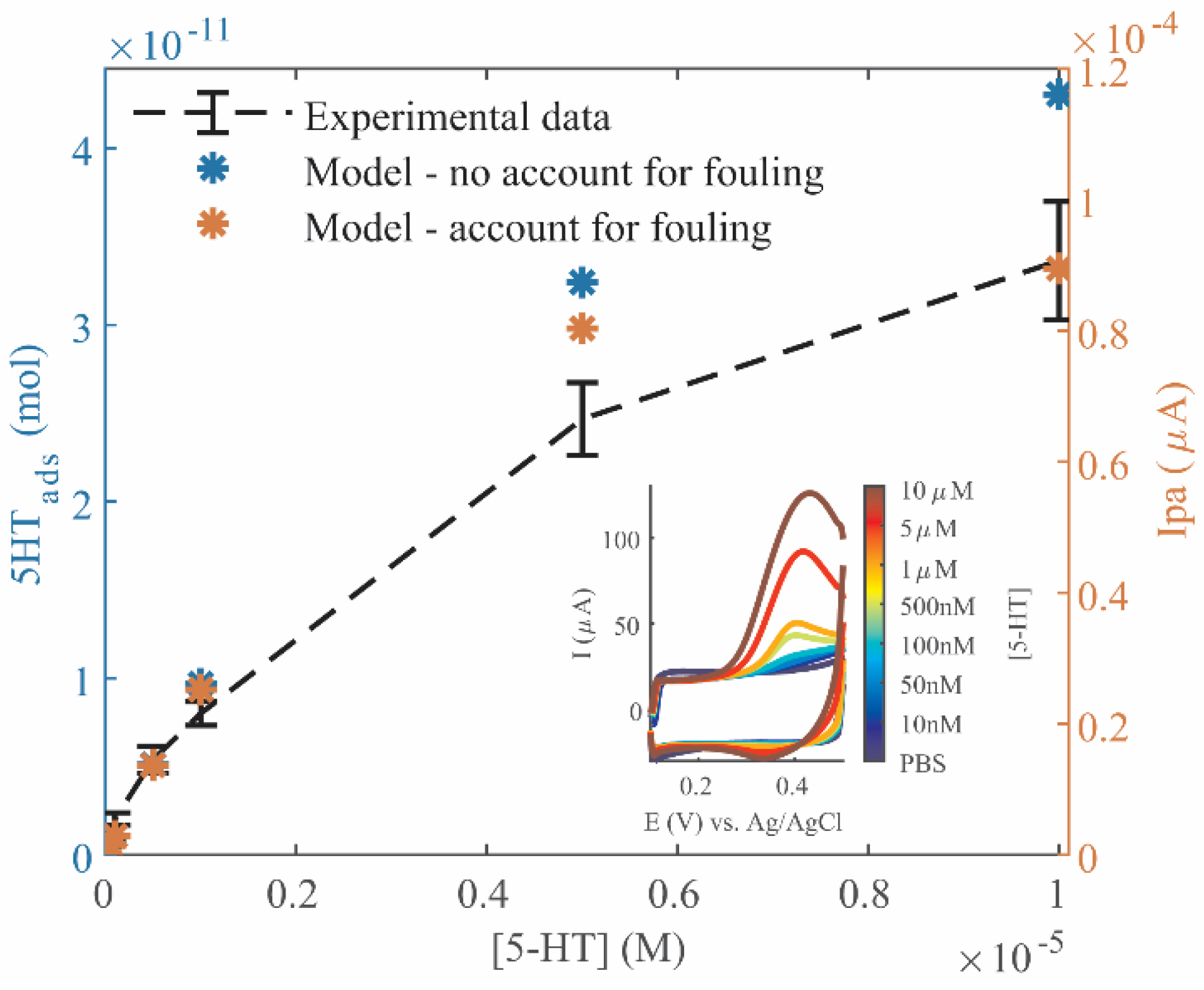
The model was also used to predict electrochemical signal over a range of 5-HT concentrations. When the model accounted for fouling, the fit of the model to the data increased. These results indicate that the model of 5-HT adsorption could accurately predict both electrochemical detection and fouling, to a useful extent, and that the calculated adsorption kinetic parameters worked for multiple experiments using different electrodes.
Measurement of 5-HT release from RIN14B cells, which has been commonly performed using ELISA as a gold standard in literature [
29,
35,
36], was successfully reproduced here using our electrochemical method. The model and the experimental sensitivity curve aided in quantifying 5-HT release from RIN14B cells, using the selected minimum t
acc of 30 min. The measurement of ~200–300 nM 5-HT lies above the electrode’s LOQ. This measurement corresponds to ~4–8 pmol 5-HT released per 10
5 cells, which is very similar to estimations from literature (~7 pmol/10
5 cells by Nozawa, et al. [
29]) This demonstrates the feasibility of using electrochemical methods for 5-HT detection from these cells, which will, in the future, be refined and validated in a comparative study side-by-side with ELISA upon the development of a desired measurement protocol. We aim to achieve temporal tracking of 5-HT release under applied conditions by leveraging shorter measurement times compared to ELISA, as this is an integral benefit of electrochemical methods. This would also require the anticipation of 5-HT fouling, which is demonstrated in this paper. Further cell measurements would require that additional redox-active cellular byproducts be tested for their potential interference with these 5-HT measurements, such as H
2O
2 and other reactive oxygen species that are markers of oxidative stress [
43,
44].
Future work should aim to improve upon these results to achieve a more robust understanding of adsorption-limited electrochemical detection mechanisms and improve sensor performance. The use of pulsed voltametric techniques (e.g., ACV, DPV) can increase resolution and sensitivity of detection, which may be necessary to further elucidate dynamics of molecular binding. Potential sources of error should be investigated as well to improve our model’s reliability. For instance, the stability of the CNT dropcast layer should be evaluated over the course of repeated electrochemical measurements, as CNTs can be shed in this environment. High-precision spectroscopic methods may be necessary to identify microscale-level changes to the CNT surface, which may be a source of variability.
The computational method developed here can be used to determine adsorption parameters of analyte binding to an electrode and conversion to electrochemical signal, aiding in precise modeling of molecular behavior. It can be used to evaluate adsorption-based electrochemical detection at any given electrode where binding with a redox molecule occurs, such as to quantitatively compare binding affinity between electrodes with experimentally modified surfaces. For instance, this could be used to quantify the difference in binding affinity between a bare Au electrode and one modified with carbon materials, as it has been shown that the presence of a modified carbon surface dramatically increases 5-HT binding on Au electrodes [
15]. This can also be applied to models of complex biological environments, taking into account environmental factors such as fluid flow, volumes, tissue porosity, etc. It is necessary, in these complex environments, to understand the context of measured molecular concentrations, since concentration depends on volume, which can vary drastically. Knowing the fundamental binding kinetics of a redox molecule with any given electrode, under an adsorption-limited scheme, allows back-calculation of unknown concentrations based on the physics of the interaction, given an understanding of the physics of the environment. Therefore, the methods presented here can act as a useful resource for many studies where molecular adsorption is the foundation of quantitative measurement.