1. Introduction
Measuring electrical responses in plants is common practice. As with animals, the approaches used for measuring electrical signals are very diverse. Various approaches and potential applications of measuring electrical responses, which are applicable to many plants, have been reviewed [
1,
2,
3]. In brief, plant electrical measurements include (1) extracellular signals around a plant (i.e., field potentials in the air or soil or in other mediums), (2) potential differences, in and out of a plant, to an environment (i.e., differences between a fluid compartment within a plant and external surfaces such as the soil or a moist surface), and (3) intracellular recordings across a cell wall to fluid compartments (i.e., xylem or phloem) or to the surface of a plant.
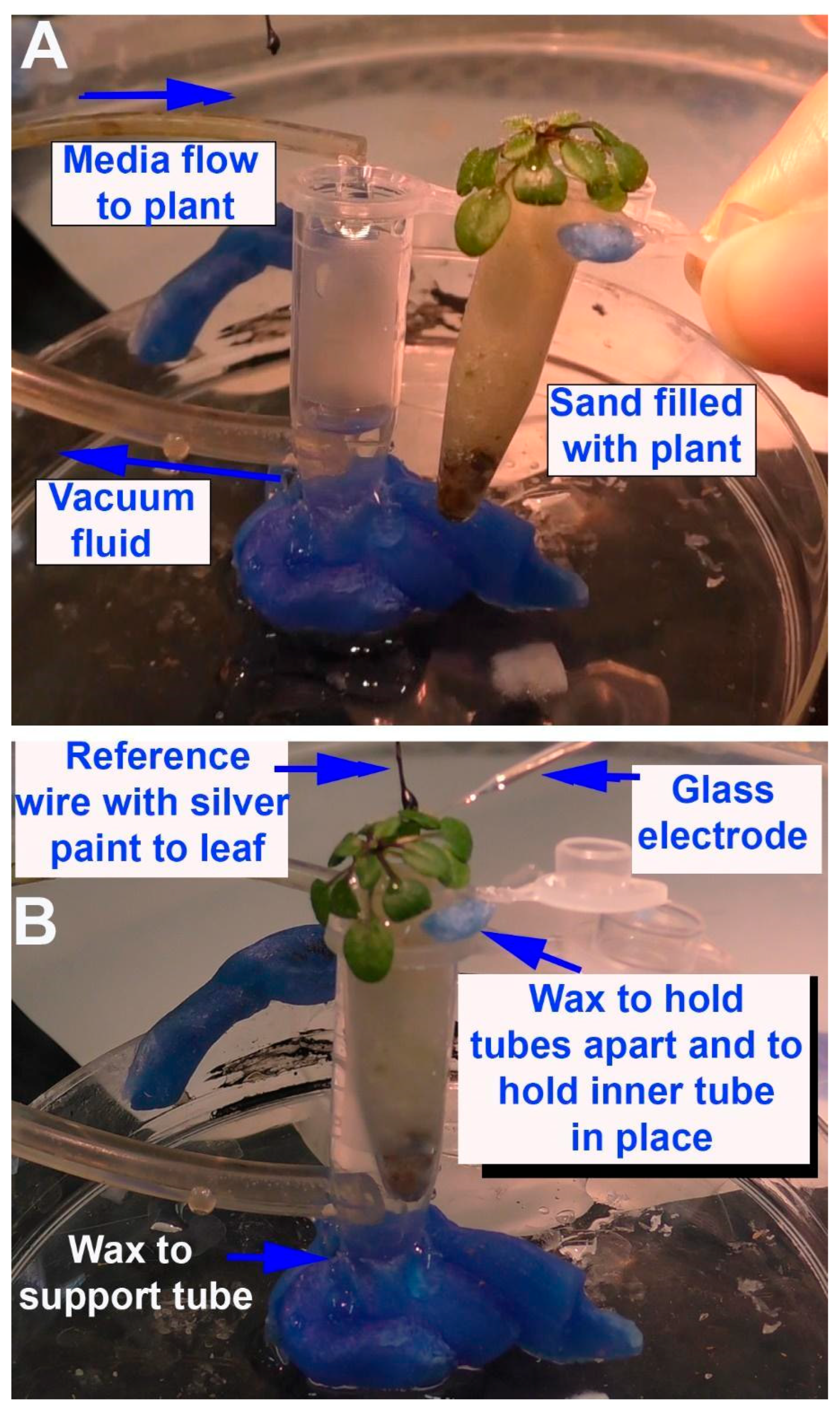
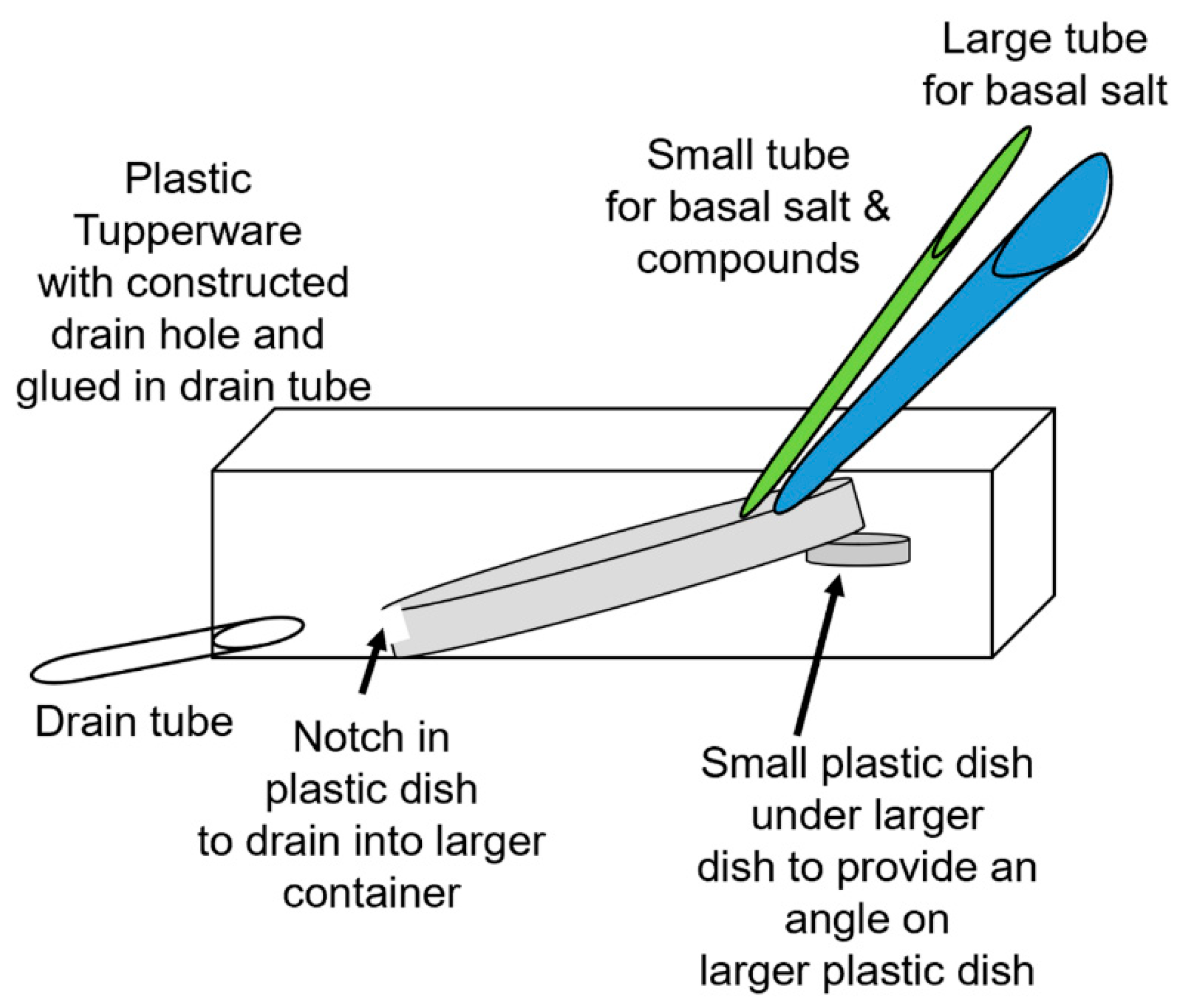
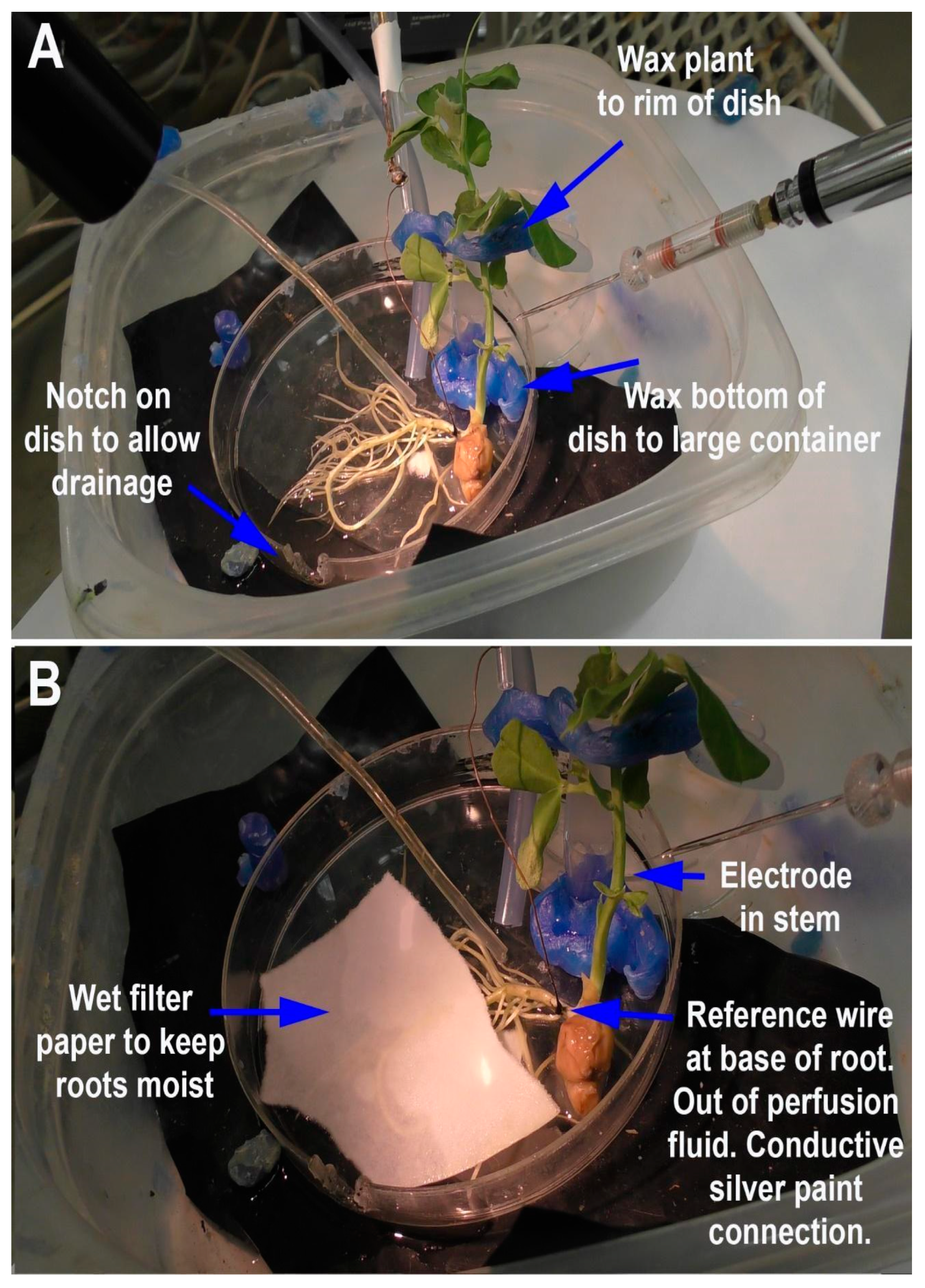
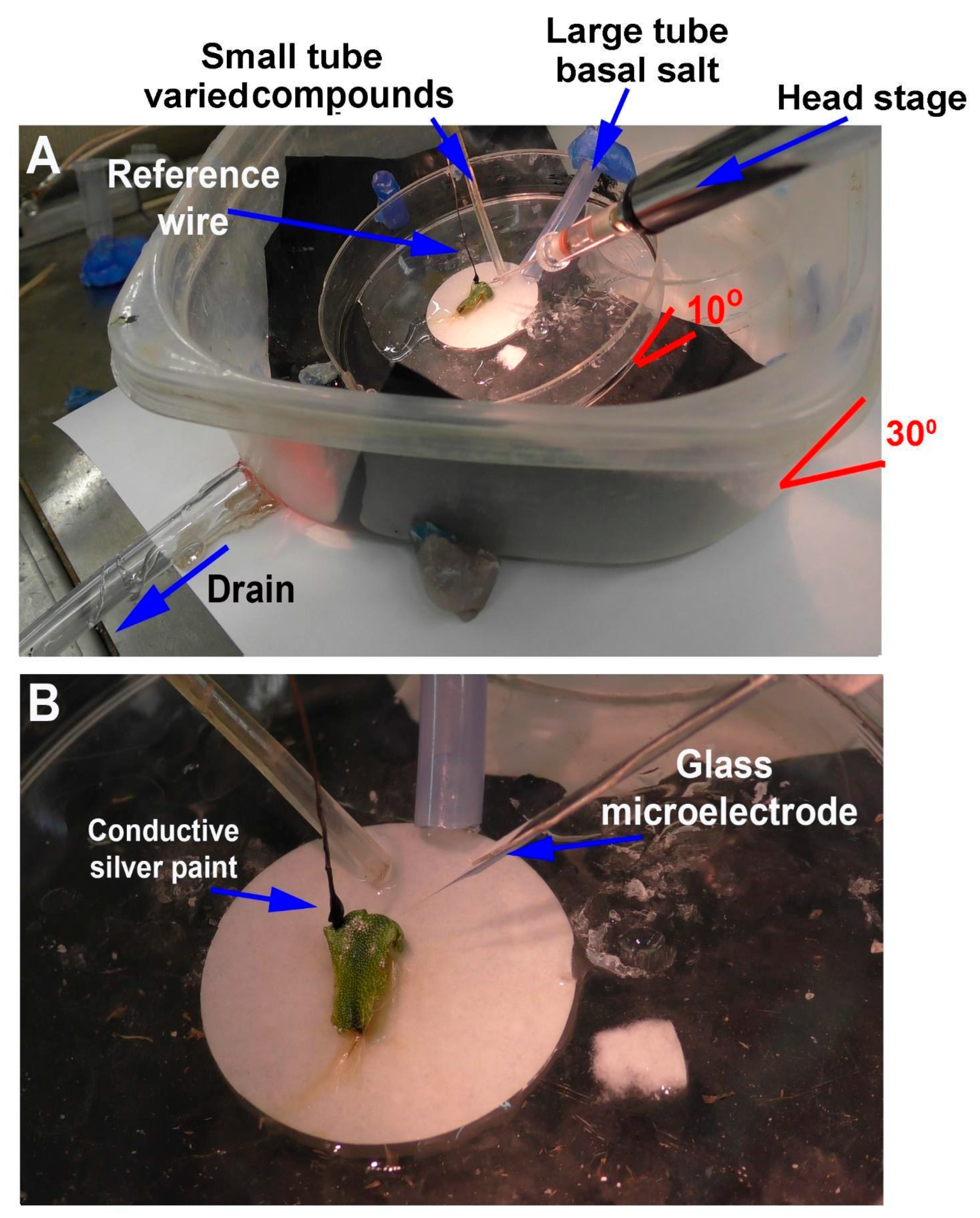
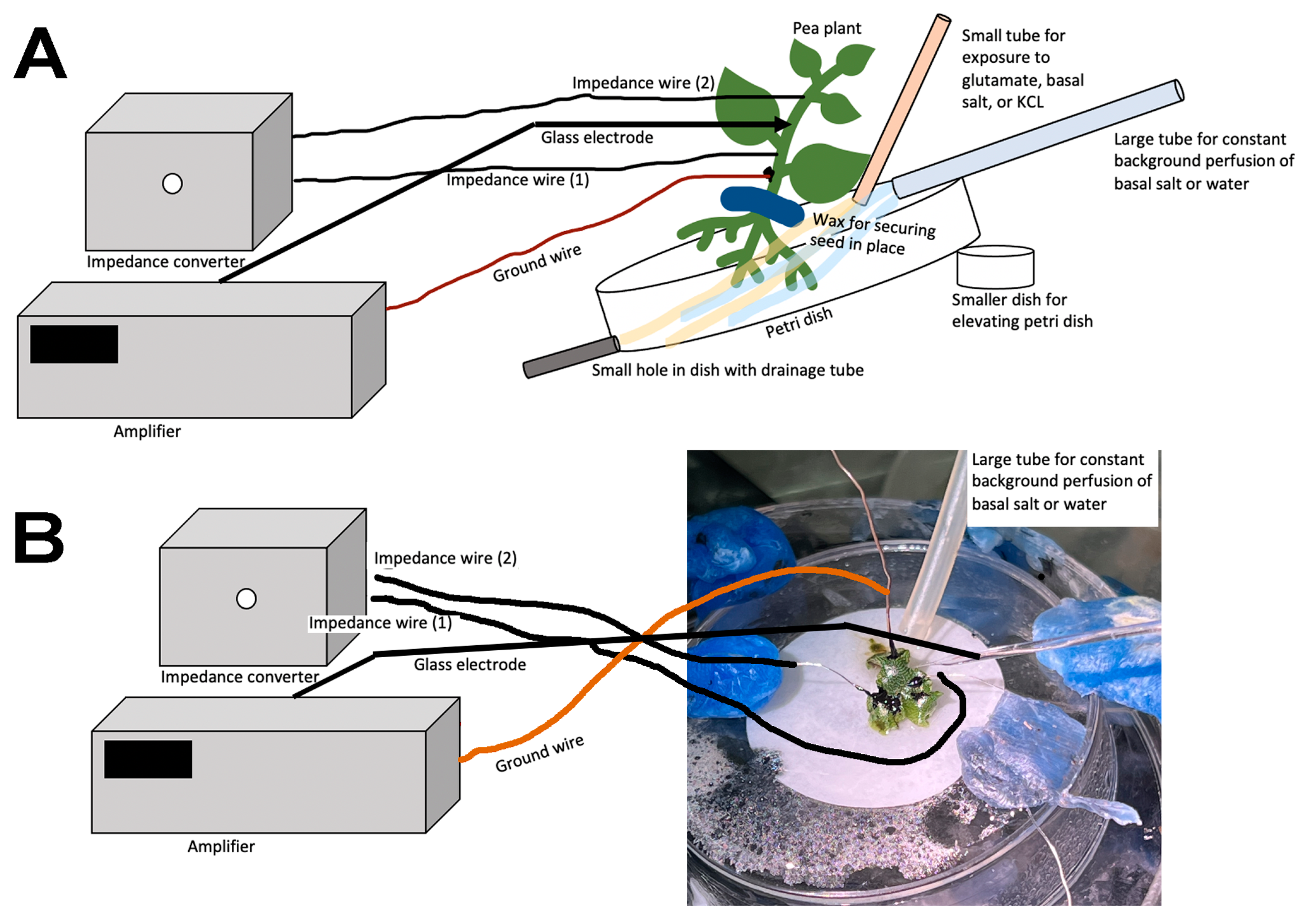
Depending on the research questions, and for ease of reproducibility, a standardized recording method is optimal. In this report, two general methods are presented to monitor the responses of intact seedling roots (or rhizoids of a liverwort) to acute exposure to chemical agents. There are various approaches used in placing a reference electrode (i.e., a ground for completing the electrical circuit) to measure potential differences and changes within a plant. However, depending on where the reference electrode is placed, the signals may be dampened in amplitude due to dissipation of the electrical field. The approach of placing a second glass electrode as a reference or ground lead into the plant may provide the largest response to detect a change in the electrical potential within a plant. Dipping a leaf into a beaker of KCl with a reference lead placed inside the beaker is another approach. This is an approach for larger plants where a beaker can be placed within the recording area; however, spatially, this is cumbersome for seedlings. Growing seedlings on a moist filter paper and placing a reference lead on the moist filter paper in contact with the roots is an alternative approach. Depending on the concentration of the salts bathing the filter paper, the amount of contact the roots have with the filter paper and the distance from the roots to the ground wire can result in varied amplitudes of electrical responses. The filter paper approach also assumes that an ionic change can be measured through the roots to the bathing salt solution on the filter paper. In addition, if various compounds are introduced on the roots, the ionic nature of the compound can produce an electrical potential change which can result in an electrical artifact; thus, this possible electrical change must be accounted for. One challenge of working with small plants is ensuring the circuit is complete. We found that placing a ground wire on the base of the root from the sprout, with a small amount of conductive paint containing a silver paste, provides a good electrical connection with a plant. A solution can be perfused over the roots without a change in the environment in direct contact with the reference lead which serves as a ground, thus illustrating how a plant responds to alterations in the environment, selectively on the roots. There are various approaches used for detecting the electrical signals of plants, depending on the plant and responses being examined [
4].
In nature, the chemical concentrations in the soil are dynamic and roots/rhizoids are exposed to many compounds in different concentrations and for different periods of time. How a plant responds to a particular substance can be determined by growing seedlings in a media and adding or removing a substance of interest. To address whether plants respond to repetitive exposure, as well as whether the responses show desensitization to a compound, an approach using repetitive acute exposure needs to be considered. The amino acid L-glutamate is known to alter root growth and causes Ca
2+ flux inside roots, and such fluxes are detected by electrical changes [
5,
6]. Using L-glutamate, two different flow-through approaches are presented as a proof of concept for detecting the acute responses of the roots of seedlings or rhizoids of a liverwort.
The purpose of this report is to illustrate the flow-through approaches we found useful for monitoring acute chemical exposure to roots/rhizoids by detecting the electrical signals within plants.
4. Discussion
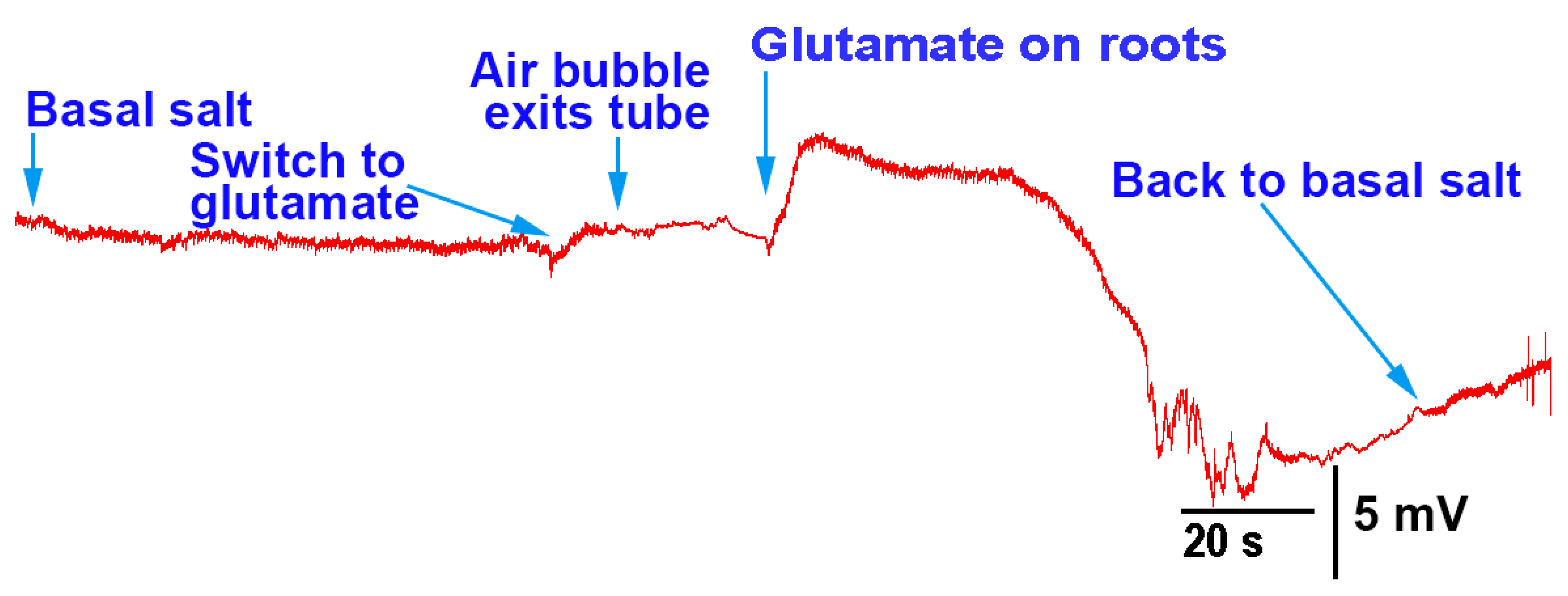
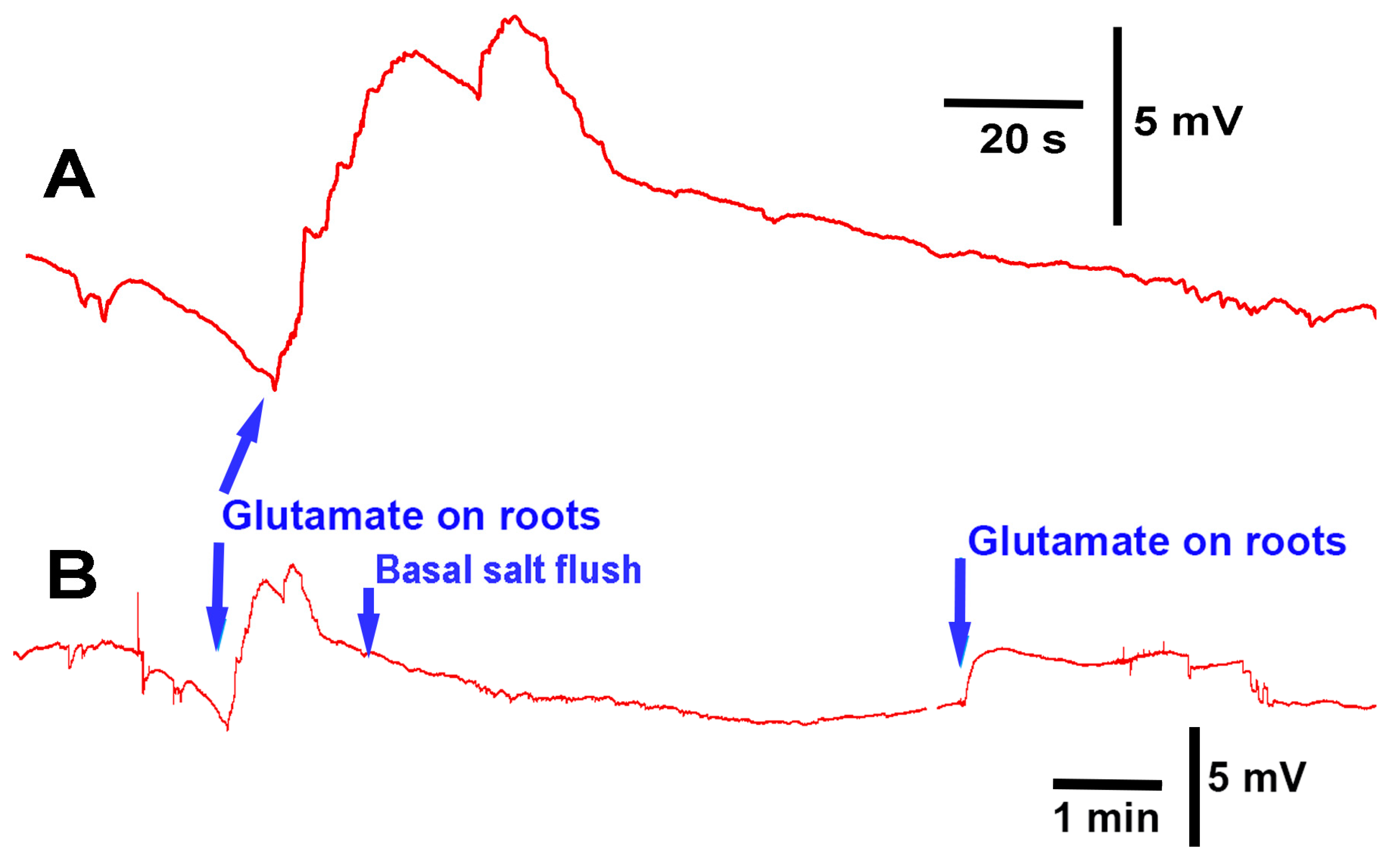
The methods presented can be used to investigate how roots/rhizoids may sense and respond to short- or long-term exposure to compounds in a controlled environment. As a proof of concept, three model plants were examined. In two of the three species, acute exposure to glutamate resulted in immediate electrical depolarization. The responses indicate the receptors to glutamate are present on the roots of A. thaliana and P. sativum, but not M. inflexa, under the experimental conditions used in this study. However, it was expected that the roots and rhizoids of plants would respond to a high extracellular concentration of KCl, similar to many animal cells. Cells are generally driven by potassium concentrations to maintain their resting membrane potentials; with exposure to extracellular KCl cells, they will typically depolarize cells, as was the case for P. sativum and M. inflexa. Simultaneous electrical potential recordings using the impedance technique and a glass microelectrode were obtained, illustrating that both techniques are possible to use for detecting responses to compounds.
There was substantial variation in the electrical response among the same species of plant with regard to the same stimulus. This is likely due to the variation in root structure and contact area with the tested compounds. Different recording techniques may be required to monitor electrical signals, depending on the plant structure, such as stems with large leaves or sprouts. In addition, if the surface of the stem is easy to penetrate with an impedance lead or glass electrode, one might choose these approaches to maximize the detection of an electrical signal. A woody stem with high resistance to the internal fluid may not be well electrically connected to a lead placed on the surface, even with conductive paint; thus, penetration into a stem might be required. It is not expected that roots of the same species of plants would respond in the same manner as there is so much variation in root structure and fine processes of the root hairs. It may not be possible to expose the whole surface area equally and simultaneously; thus, it may be necessary to spread out the electrical signals, or even produce multiple deflections in the traces as the various roots come into contact with the solutions. However, a relative change in electrical potential can be measured due to introduced compounds. In addition, with the penetration of leads within a stem, it is difficult to know exactly which fluid compartment the leads are preferably measuring. Thus, variation in the responses between plants may occur due to comparing the responses in different compartments to a stimulus. Furthermore, penetrating a stem with a lead can induce an injury discharge to the plant, so some additional time may be required to obtain a stable baseline. It was shown in an aquatic plant (
Myriophyllum aquaticum) that inserting an electrode generated an electrical response [
7]. It is not surprising that an electrical response occurs when inserting an electrode into the stem of a plant. We also report that the insertion of the glass electrode induces an electrical change while first recording the electrical signals with leads for impedance and waiting for a stable response. However, in some cases, no differences in the impedance recordings were noted when the tip of the glass electrode penetrated a stem. In one recording, we did note a hyperpolarizing electrical signal from the stable baseline when the brief exposure to KCl (1 M) occurred for
M. inflexa. There are various possibilities for this spurious response, such as the compartment within the stem in which the tip of the glass electrode might have been placed. Since the glass electrode is filled with an electrically conductive solution, it is possible that a slow change in the electrical potential occurs as some of the fluid in the glass electrode may leak into a plant. Small-tip glass electrodes can reduce leakage, but there is a trade-off as the small-tip electrodes tend to clog. A physiological response of the plant may even add to the blockage of the tip, depending on the cellular reaction to the electrode. It is common to use a 10 to 20 µm tip diameter and 0.1 to 0.3 M KCl for the electrical conductor for recording using glass electrodes in plants. However, one may wish to try different recording arrangements to determine what is optimal for the experimental design and questions being addressed [
7]. It is known that an injury or physical disturbance to a plant, via a cut to a leaf or even the bending of a leaf, generates electrical signals which can be monitored using an impedance measure as well as with the insertion of a glass electrode in reference to the outside of the plant [
8].
The development of root structures in
A. thaliana is known to be altered by exposure to glutamate, a known agonist to glutamate receptors [
5,
9]. Developmental studies of roots with a compound embedded in a growth media, such as agar, presents constant exposure to the compound. Constant exposure could possibly result in a desensitization of the receptors. The family of glutamate receptors is well characterized physiologically, pharmacologically and by molecular make-up in animals. The metabotropic and ionotropic glutamate receptors in animals are generally classified by action of excitatory amino acids and by more selective agonists (i.e., kainic, N-methyl-D-aspartic (NMDA), and quisqualic acids, as well as selective antagonists) [
10,
11,
12].
L-glutamate decreases the long growth of roots in
A. thaliana and promotes branching, but the effects are not blocked by antagonist DNQX, MK-801 or AP-5, which are known to block ionotropic glutamate receptors in animals [
6,
13]. The exact classification of the glutamate receptor subtypes in the roots of various plants remains to be determined; however, for the plants examined, they appear similar to ionotropic glutamate receptors in animals [
9,
14]. Glutamate exposure produces a Ca
2+ flux in plant roots though a glutamate-like receptor, as noted in some types of glutamate receptors in animals [
15,
16,
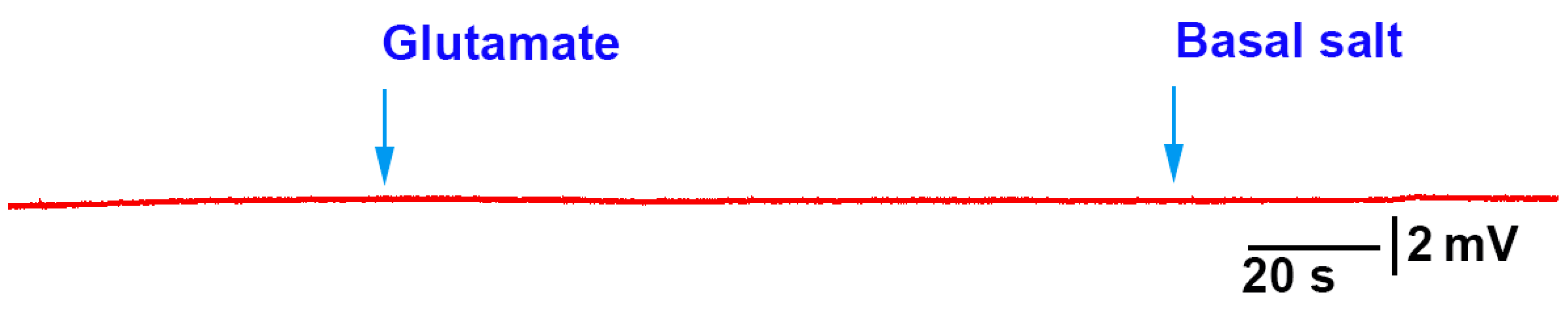
17]. The view is that this response may aid the plant in detecting a nitrogen source. The rhizoids of
M. inflexa did not appear express glutamate-like receptors in the culture conditions used in this study. Thus, glutamate responses do not appear to be universal in land plants. However, glutamate-like receptors might be expressed in other structures of
M. inflexa. The genomic and proteomic analysis of different tissues of
M. inflexa would help reveal the possibility of expression and location in a subtype of glutamate receptors.
The flow-through protocol presented allows acute and repetitive exposure to compounds. The ease of electrical recording with a reference electrode and conductive paint in contact with the plant, and a glass microelectrode placed within the plant, provides a means to measure ionic flow within the plant. In addition, cocktails of compounds can also be examined, as can agonists and antagonists, to examine potential types of receptors present on roots. The intracellular types of amplifiers for use in monitoring DC potentials over long periods are generally in the cost range of USD 5000, while measuring rapid changes or DC changes with the impedance converter, as used in this study, is in the range of USD 500–800. Both amplifiers require an analog-to-digital converter to be able to record to a computer. The glass electrode approach requires a manipulator and holder to aid in the placement of the glass electrode (~800 USD); thus, the impedance measures are cheaper to conduct with regard to equipment requirements.
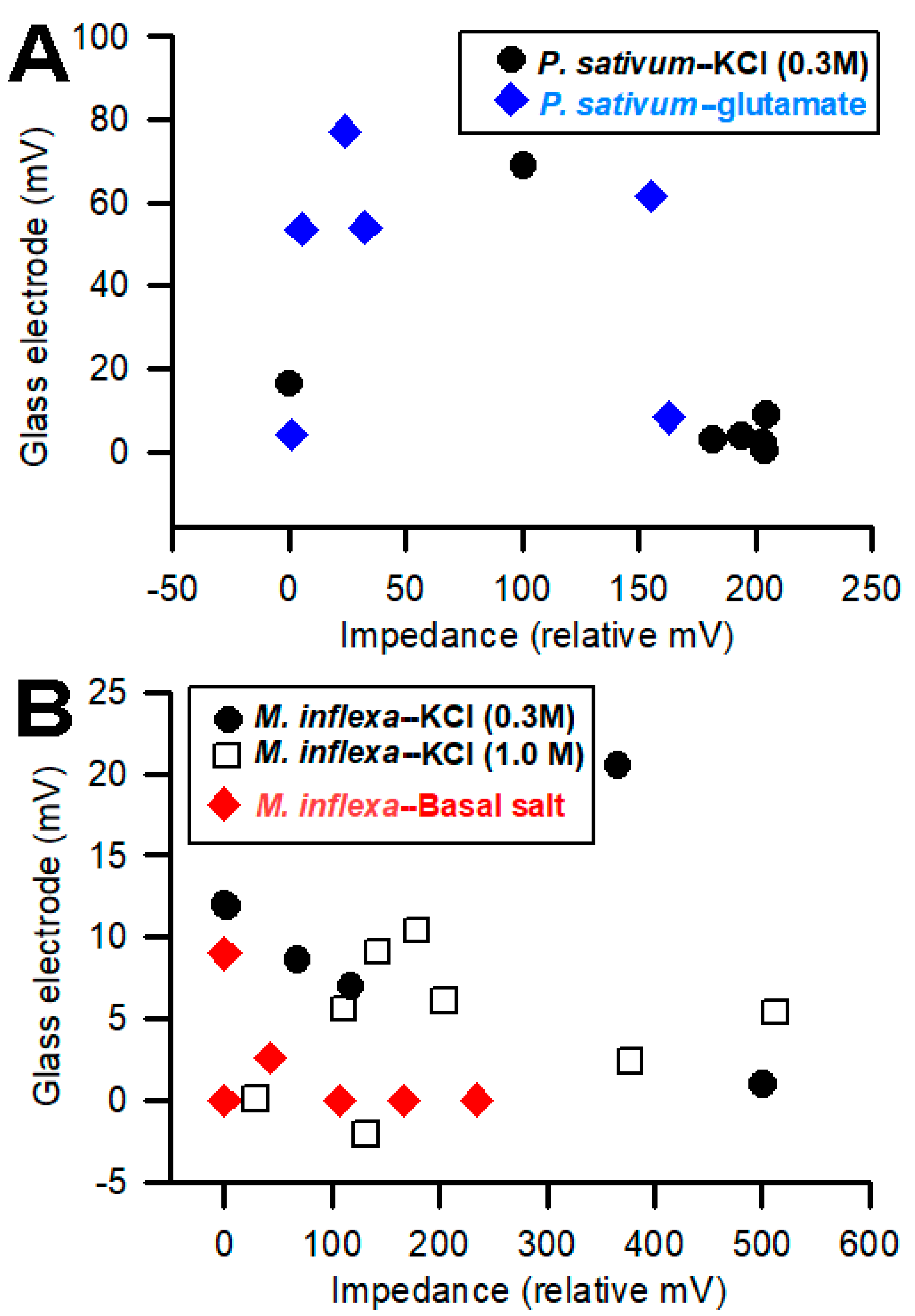
It was anticipated that larger impedance measures would be correlated with larger signals obtained with glass electrodes. However, the opposite correlation occurred in
P. sativum. In general, as the magnitude of the response increases for the impedance method, the magnitude of the glass electrode response decreases (
Figure 9). For
M. inflexa, there was no noticeable correlation in magnitude between the two techniques. This is surprising considering how close the two recording leads were to one another within the plant.
When observing the plot associated with
P. sativum (
Figure 9), the inverse correlation between the amplitudes/magnitudes for the two recording techniques allows us to pose interesting questions. If using two recording techniques simultaneously, will one of the recording methods present a more favorable path of conductivity—whether due to its proximity to the response site, or because of the corresponding compartment within which the recording electrode is placed—and, therefore, conduct a higher percentage of the plant’s total electrical response than the other recording electrode? Looking at the 0.3 M KCl flow-through for
P. sativum in
Figure 9, the four largest impedance measures for “
P. sativum-KCl”, are associated with the four smallest glass electrode measures for the same compound. Correspondingly, when looking at glutamate flow-through for
P. sativum, three of the four largest glass electrode measures produced three of the four smallest impedance measures for the same compound.
The exposure of roots/rhizoids to various compounds known in nature can occur for short periods of time with deluges and other natural phenomena, or over long periods of time as compounds containing organic materials decompose in the environment around a plant. These same methods could be used to study the long-term exposure of plants grown in soil, where soils might favor the retention of certain compounds over others. In addition, the examination of compounds exposed to roots while leaves may be photosynthesizing in different wavelengths, and/or being exposed to volatile substances, could be readily examined using the protocols and methods presented.