Streamlining Culture Conditions for the Neuroblastoma Cell Line SH-SY5Y: A Prerequisite for Functional Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of SH-SY5Y Cells
2.2. Expansion of SH-SY5Y Cells
2.3. Data Acquisition and Statistical Analysis
3. Results and Discussion
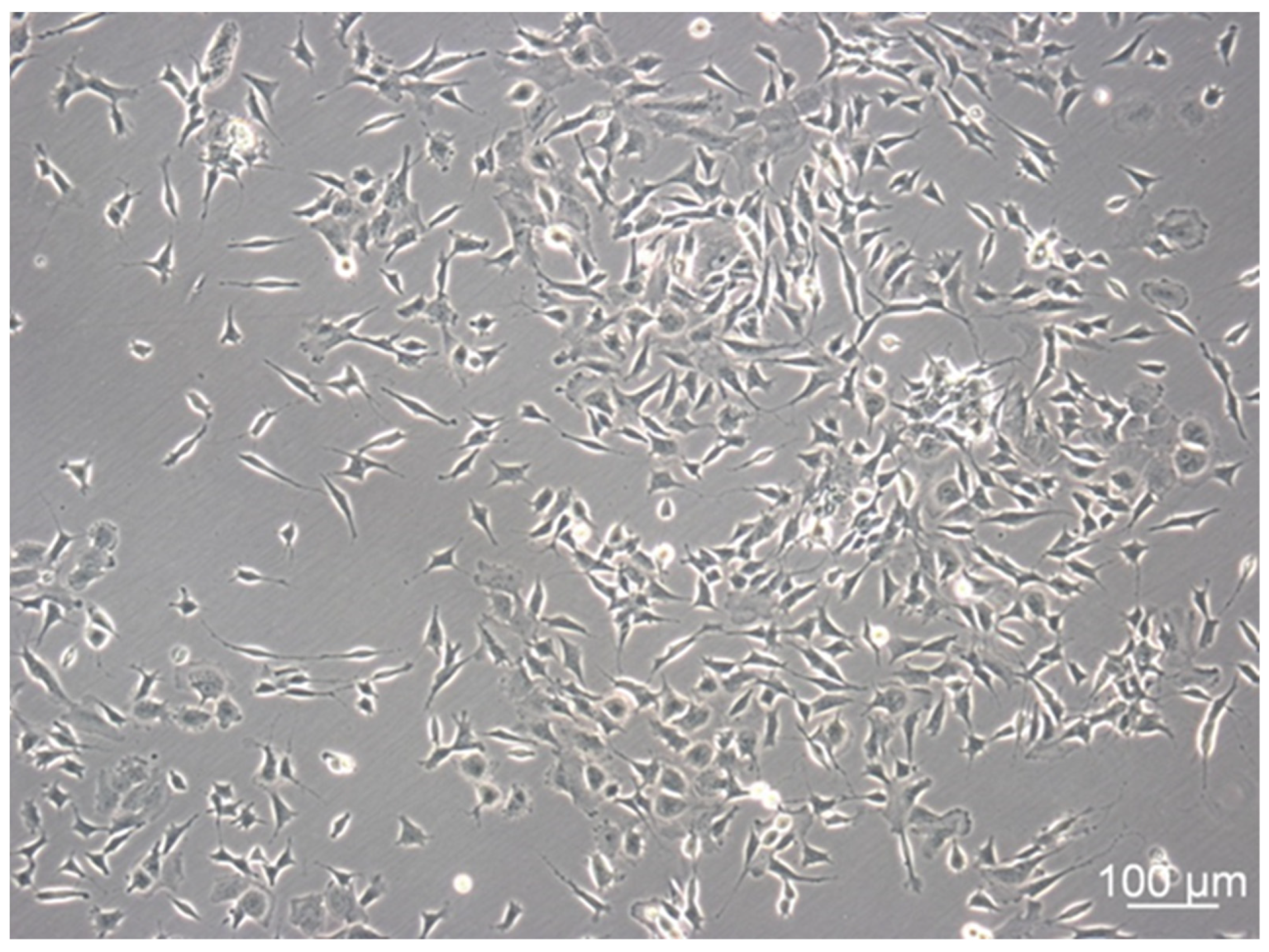
3.1. SH-SY5Y Cells Show Distinctive Cell Subtypes
3.2. Verification of Experimental Comparability between Different Culture Samples
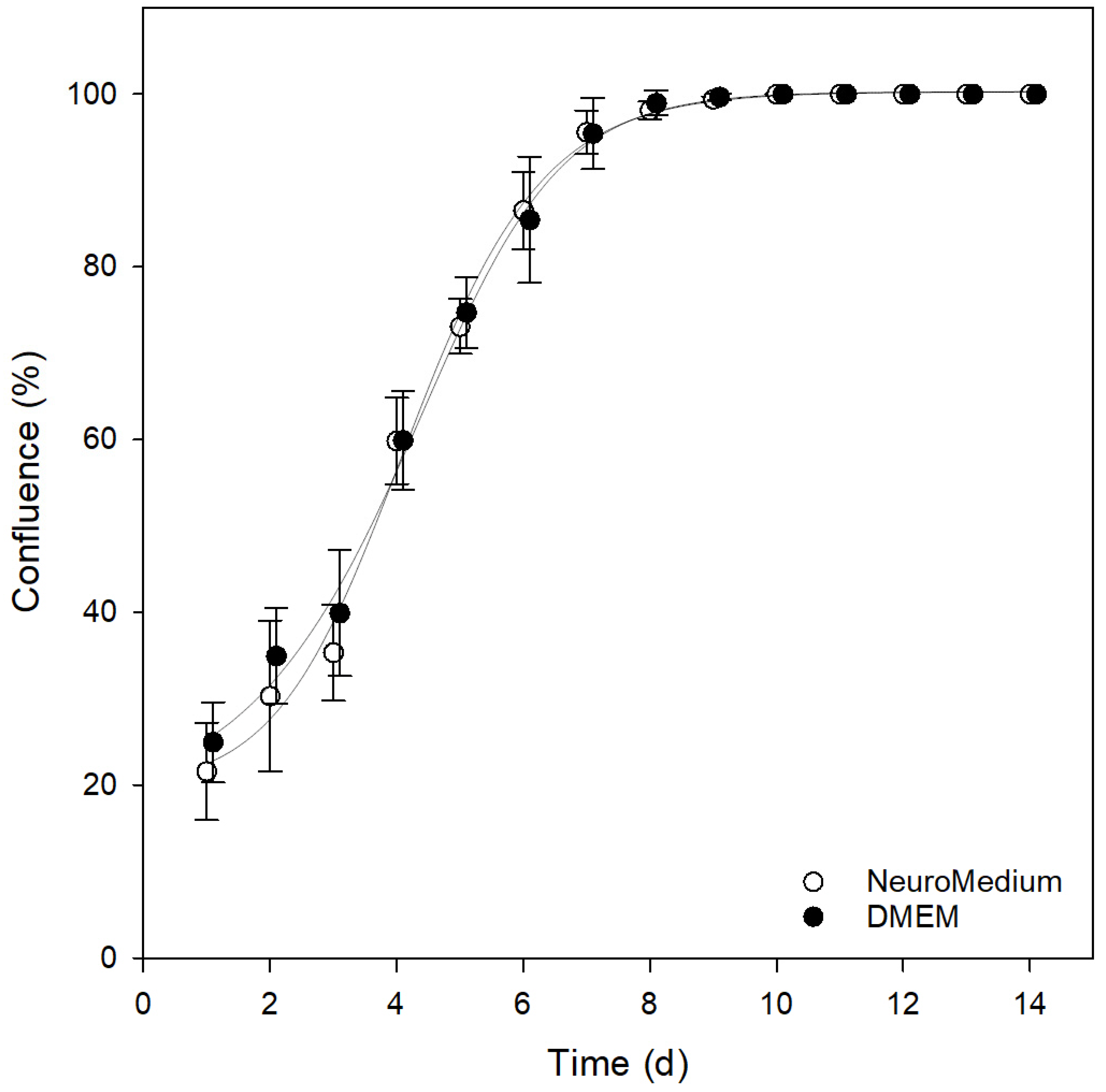
3.3. Comparison of Different Cultivation Media
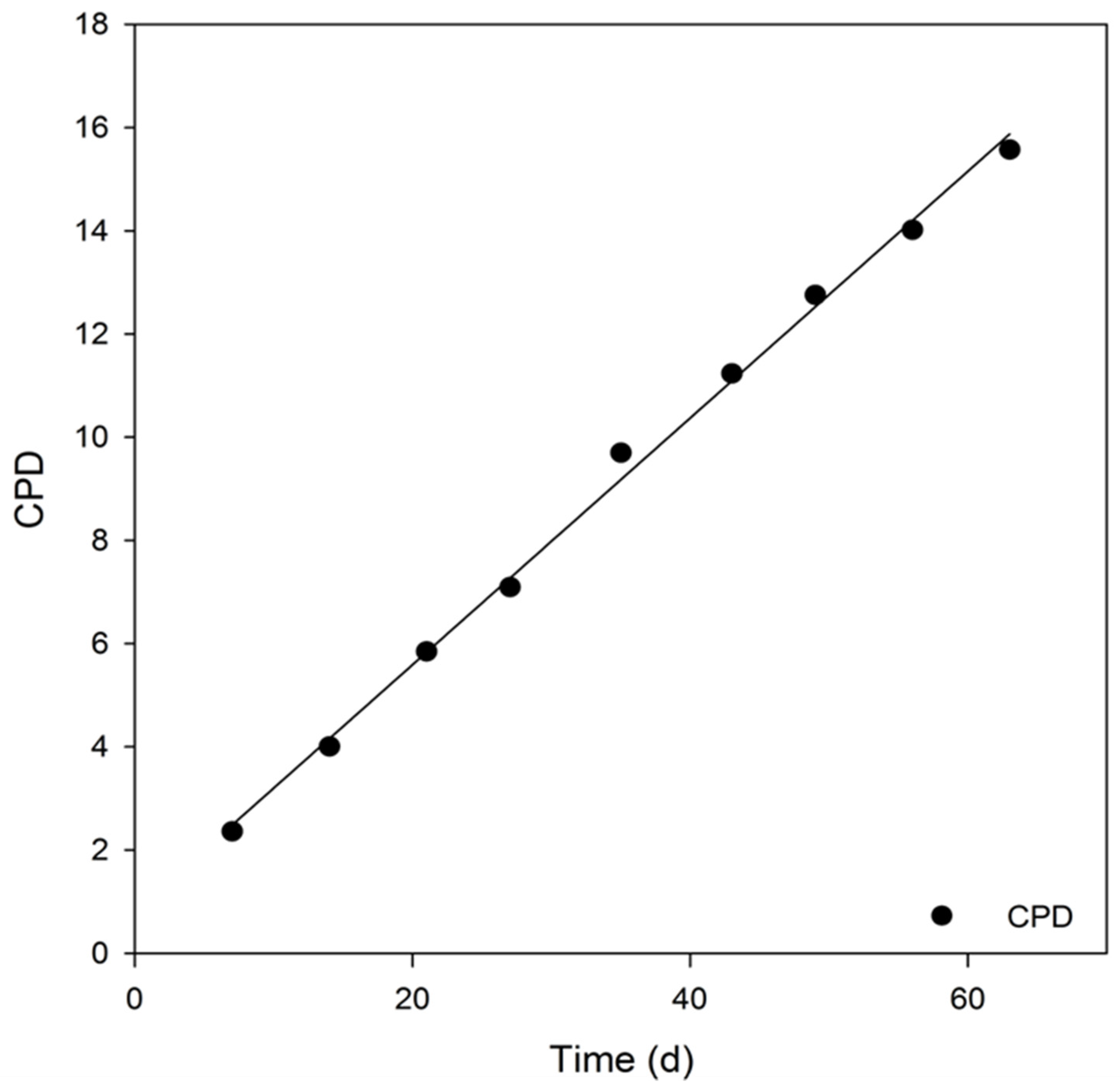
3.4. Analysis of Key Cell Line Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. In Neuronal Cell Culture; Springer: New York, NY, USA, 2013; pp. 9–21. [Google Scholar]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016, 108, 56193. [Google Scholar] [CrossRef] [PubMed]
- Um, M.; Lodish, H.F. Antiapoptotic effects of erythropoietin in differentiated neuroblastoma SH-SY5Y cells require activation of both the STAT5 and AKT signaling pathways. J. Biol. Chem. 2006, 281, 5648–5656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biedler, J.L.; Helson, L.; Spengler, B.A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973, 33, 2643–2652. [Google Scholar] [PubMed]
- Biedler, J.L.; Roffler-Tarlov, S.; Schachner, M.; Freedman, L.S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978, 38, 3751–3757. [Google Scholar]
- Ross, R.A.; Spengler, B.A.; Biedler, J.L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J. Natl. Cancer Inst. 1983, 71, 741–747. [Google Scholar] [PubMed]
- Lopes, F.M.; Schröder, R.; da Frota, M.L.C., Jr.; Zanotto-Filho, A.; Müller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, P.F.; Peers, C.; Walker, J.H. The use of the human neuroblastoma SH-SY5Y to study the effect of second messengers on noradrenaline release. Gen. Pharmacol. Vasc. Syst. 1995, 26, 1191–1201. [Google Scholar] [CrossRef]
- Khwanraj, K.; Phruksaniyom, C.; Madlah, S.; Dharmasaroja, P. Differential Expression of Tyrosine Hydroxylase Protein and Apoptosis-Related Genes in Differentiated and Undifferentiated SH-SY5Y Neuroblastoma Cells Treated with MPP+. Neurol. Res. Int. 2015, 2015, 734703. [Google Scholar] [CrossRef] [Green Version]
- Oyarce, A.M.; Fleming, P.J. Multiple forms of human dopamine beta-hydroxylase in SH-SY5Y neuroblastoma cells. Arch. Biochem. Biophys. 1991, 290, 503–510. [Google Scholar] [CrossRef]
- Oe, T.; Sasayama, T.; Nagashima, T.; Muramoto, M.; Yamazaki, T.; Morikawa, N.; Okitsu, O.; Nishimura, S.; Aoki, T.; Katayama, Y.; et al. Differences in gene expression profile among SH-SY5Y neuroblastoma subclones with different neurite outgrowth responses to nerve growth factor. J. Neurochem. 2005, 94, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.H.; Li, J.-Y.; Ahlman, H.; Dahlström, A. SSR2(a) receptor expression and adrenergic/cholinergic characteristics in differentiated SH-SY5Y cells. Neurochem. Res. 2003, 28, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Jankovic, J.; Guo, Y.; Xie, W.; Le, W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem. Biophys. Res. Commun. 2005, 337, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.S. Characterization of SH-SY5Y Neurons Subject to 92kHz Ultrasound Stimulation. Int. J. Morphol. 2021, 39, 1109–1115. [Google Scholar] [CrossRef]
- Cohen, M.A.; Zhang, S.; Sengupta, S.; Ma, H.; Bell, G.W.; Horton, B.; Sharma, B.; George, R.E.; Spranger, S.; Jaenisch, R. Formation of human neuroblastoma in mouse-human neural crest chimeras. Cell Stem Cell 2020, 26, 579–592. [Google Scholar] [CrossRef]
- Hu, X.; Guo, X.; Ni, J.; Wang, H.; Cao, N.; Liang, Z.; Wang, X. High homocysteine promotes telomere dysfunction and chromosomal instability in human neuroblastoma SH-SY5Y cells. Mutat. Res. Toxicol. Environ. Mutagen. 2020, 854-855, 503197. [Google Scholar] [CrossRef]
- Peng, Y.; Chu, S.; Yang, Y.; Zhang, Z.; Pang, Z.; Chen, N. Neuroinflammatory In Vitro Cell Culture Models and the Potential Applications for Neurological Disorders. Front. Pharmacol. 2021, 12, 671734. [Google Scholar] [CrossRef]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res. Notes 2013, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Baboo, J.; Kilbride, P.; Delahaye, M.; Milne, S.; Fonseca, F.; Blanco, M.; Meneghel, J.; Nancekievill, A.; Gaddum, N.; Morris, G.J. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019, 9, 3417. [Google Scholar] [CrossRef]
- Mazur, P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. Physiol. 1984, 247, C125–C142. [Google Scholar] [CrossRef]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2013, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tiffany-Castiglioni, E.; Koh, H.C.; Son, I.-H. Paraquat activates the IRE1/ASK1/JNK cascade associated with apoptosis in human neuroblastoma SH-SY5Y cells. Toxicol. Lett. 2009, 191, 203–210. [Google Scholar] [CrossRef]
- Puck, T.T.; Marcus, P.I.; Cieciura, S.J. Clonal growth of mammalian cells in vitro: Growth characteristics of colonies from single HeLa cells with and without a" feeder" layer. J. Exp. Med. 1956, 103, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.S.; Forrester, J.V.; McInnes, C.R.; Lawrie, F. Adhesion of cells to polystyrene surfaces. J. Cell Biol. 1983, 97, 1500–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinescu, R.; Constantinescu, A.T.; Reichmann, H.; Janetzky, B. Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. In Neuropsychiatric Disorders an Integrative Approach; Springer: New York, NY, USA, 2007; pp. 17–28. [Google Scholar]
- Masters, J.R.; Stacey, G.N. Changing medium and passaging cell lines. Nat. Protoc. 2007, 2, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Hsing, H.-W.; Lai, T.-C.; Chen, Y.-W.; Lee, T.-R.; Chan, H.-T.; Lyu, P.-C.; Wu, C.-L.; Lu, Y.-C.; Lin, S.-T.; et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 2010, 17, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Quaglia, M.P.; Manchester, K.M. A comparative analysis of neuroblastic and substrate-adherent human neuroblastoma cell lines. J. Pediatr. Surg. 1996, 31, 315–318. [Google Scholar] [CrossRef]
- Bell, N. Store-operated Ca2+ entry in proliferating and retinoic acid-differentiated N-and S-type neuroblastoma cells. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential Treatment of SH-SY5Y Cells with Retinoic Acid and Brain-Derived Neurotrophic Factor Gives Rise to Fully Differentiated, Neurotrophic Factor-Dependent, Human Neuron-Like Cells. J. Neurochem. 2002, 75, 991–1003. [Google Scholar] [CrossRef]
- Bouché, M.; Senni, M.; Grossi, A.; Zappelli, F.; Polimeni, M.; Arnold, H.; Cossu, G.; Molinaro, M. TPA-Induced Differentiation of Human Rhabdomyosarcoma Cells: Expression of the Myogenic Regulatory Factors. Exp. Cell Res. 1993, 208, 209–217. [Google Scholar] [CrossRef]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An In Vitro Model for Neuroscience: Differentiation of SH-SY5Y Cells into Cells with Morphological and Biochemical Characteristics of Mature Neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari-Toninelli, G.; Paccioretti, S.; Francisconi, S.; Uberti, D.; Memo, M. TorsinA negatively controls neurite outgrowth of SH-SY5Y human neuronal cell line. Brain Res. 2004, 1012, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.I.; Köglsberger, S.; Trefois, C.; Boyd, O.; Baumuratov, A.S.; Buck, L.; Balling, R.; Antony, P.M.A. Characterization of Differentiated SH-SY5Y as Neuronal Screening Model Reveals Increased Oxidative Vulnerability. SLAS Discov. Adv. Sci. Drug Discov. 2016, 21, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Korecka, J.A.; van Kesteren, R.; Blaas, E.; Spitzer, S.O.; Kamstra, J.; Smit, A.B.; Swaab, D.; Verhaagen, J.; Bossers, K. Phenotypic Characterization of Retinoic Acid Differentiated SH-SY5Y Cells by Transcriptional Profiling. PLoS ONE 2013, 8, e63862. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto, R.; Hiraiwa, T.; Nakai, Y.; Shindo, Y.; Oka, K.; Hiroi, N.; Funahashi, A. Detection of Temperature Difference in Neuronal Cells. Sci. Rep. 2016, 6, 22071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Monica, N.; Racaniello, V.R. Differences in replication of attenuated and neurovirulent polioviruses in human neuroblastoma cell line SH-SY5Y. J. Virol. 1989, 63, 2357–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Um, M.; Gross, A.W.; Lodish, H.F. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell. Signal. 2007, 19, 634–645. [Google Scholar] [CrossRef]
- Tennant, J.R. Evaluation of the trypan blue technique for determination of cell viability. Transplantation 1964, 2, 685–694. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feles, S.; Overath, C.; Reichardt, S.; Diegeler, S.; Schmitz, C.; Kronenberg, J.; Baumstark-Khan, C.; Hemmersbach, R.; Hellweg, C.E.; Liemersdorf, C. Streamlining Culture Conditions for the Neuroblastoma Cell Line SH-SY5Y: A Prerequisite for Functional Studies. Methods Protoc. 2022, 5, 58. https://doi.org/10.3390/mps5040058
Feles S, Overath C, Reichardt S, Diegeler S, Schmitz C, Kronenberg J, Baumstark-Khan C, Hemmersbach R, Hellweg CE, Liemersdorf C. Streamlining Culture Conditions for the Neuroblastoma Cell Line SH-SY5Y: A Prerequisite for Functional Studies. Methods and Protocols. 2022; 5(4):58. https://doi.org/10.3390/mps5040058
Chicago/Turabian StyleFeles, Sebastian, Christian Overath, Sina Reichardt, Sebastian Diegeler, Claudia Schmitz, Jessica Kronenberg, Christa Baumstark-Khan, Ruth Hemmersbach, Christine E. Hellweg, and Christian Liemersdorf. 2022. "Streamlining Culture Conditions for the Neuroblastoma Cell Line SH-SY5Y: A Prerequisite for Functional Studies" Methods and Protocols 5, no. 4: 58. https://doi.org/10.3390/mps5040058
APA StyleFeles, S., Overath, C., Reichardt, S., Diegeler, S., Schmitz, C., Kronenberg, J., Baumstark-Khan, C., Hemmersbach, R., Hellweg, C. E., & Liemersdorf, C. (2022). Streamlining Culture Conditions for the Neuroblastoma Cell Line SH-SY5Y: A Prerequisite for Functional Studies. Methods and Protocols, 5(4), 58. https://doi.org/10.3390/mps5040058






