Isolation of Live Leukocytes from Human Inflammatory Muscles
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- 10 cm Petri dishes (NUNC, ThermoFisher Scientific, Malaga, WA, Australia, Cat. no.: 150318).
- Scalpel blades (Merck, Bayswater, VIC, Australia, Cat. no.: S2646).
- Pipette tips 1000 μL (ART1000, ThermoFisher Scientific, Malaga, WA, Australia, Cat. no.: MBP2179-05-HR).
- Pipette tips 10 μL (ART10, ThermoFisher Scientific, Malaga, WA, Australia, Cat. no.: MBP2140-05-HR).
- Disposable transfer pipettes (SamcoTM, ThermoFisher Scientific, Malaga, WA, Australia, Cat. no.: 212-1S).
- Nylon Wool fiber (PolySciences, Gymea, NSW, Australia, Cat. no.: 18369).
- 10 mL Luer-Lock Syringes (Beckton Dickinson, Macquarie Park, NSW, Australia, Cat. no.: 302149).
- Sterilization roll (SterikingTM, ThermoFisher Scientific, Malaga, WA, Australia, Cat. no.: R125PK).
- ConnectaTM 3-way stopcocks (Beckton Dickinson, Macquarie Park, NSW, Australia, Cat. no.: 394600).
- 15 ml tubes (Greiner, Heidelberg West, VIC, Australia, no.: 188271S).
- 5 mL disposable serological pipettes (Greiner, Heidelberg West, VIC, Australia, Cat. no.: NUN159625N).
- 10 mL disposable pipettes (Greiner, Heidelberg West, VIC, Australia, Cat. no.: NUN170356N).
- PBS, pH 7.4, 500 mL bottle (Invitrogen; Malaga, WA, Australia, Cat. no.: 10010-049).
- RPMI 1640, 500 mL bottle (Invitrogen; Malaga, WA, Australia, Cat. no.: 11875093).
- Collagenase P (Roche Diagnostics Australia, North Ryde, NSW, Australia, Cat. no.: 11213865001).
- DNase I (Roche Diagnostics Australia, North Ryde, NSW, Australia, Cat. no.: 10104159001).
- Fetal Calf Serum = FCS (Fisher Biotec Australia, Wembley, WA, Australia, Cat. no.: S-FBS-AU-015).
- Ficoll-Paque Plus (Bio-Strategy, Campbellfield, VIC, Australia, Cat. no.: GEHE17-1440-03).
- Trypan Blue Solution 0.4% (Gibco, Malaga, WA, Australia, Cat. no.: 15250061).
2.2. Equipment
- P1000 Pipette.
- P10 Pipette.
- Electronic pipette controller.
- Tube Rotator MACSmixTM, (Miltenyi Biotec, Macquarie Park, NSW, Australia) or other model with similar specifications.
- Incubator set at 37 °C, 5% CO2.
- Centrifuge with swinging-bucket rotor.
- Microscope.
- Neubauer Hematocytometer and coverslip.
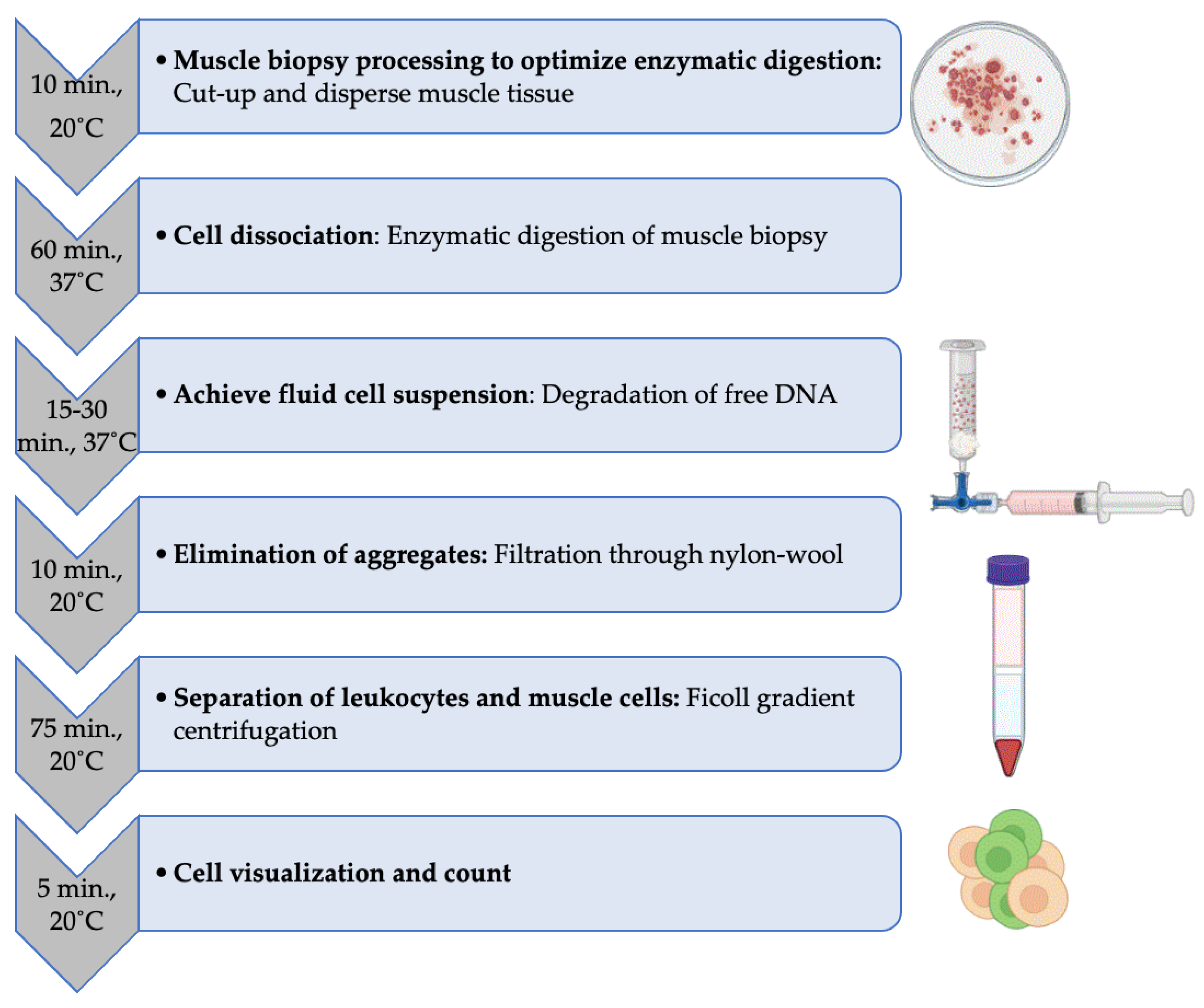
3. Procedure
3.1. Dissection of the Muscle Biopsy
- Place the muscle biopsy in a 10 cm Petri dish (Video S1).
- If necessary, separate the adipose tissue that may be attached to the muscle tissue using a sterile scalpel blade, while holding the biopsy in place using sterile dissecting forceps.
- 3.
- Discard the resected adipose tissue fraction.
- 4.
- Cut the muscle tissue into small pieces and disperse using the scalpel blade while holding in place with the dissecting forceps.
- 5.
- Using a sterile disposable transfer pipette, add 2 mL of sterile PBS onto the muscle fragments, resuspend and transfer into a 15 mL tube.
- 6.
- Add another 2 mL of PBS in the Petri dish to rinse and optimize sample recovery; then, resuspend and transfer into the same 15 mL tube.
3.2. Enzymatic Digestion of the Muscle Tissue
- Add 1 mL of Collagenase P (stock solution concentration: 6 mg/mL) onto the 4 mL of muscle tissue suspension in the 15 mL tube, thereby, obtaining a final concentration of 1.2 mg/mL.
- 2.
- Place the tube on a tube rotator. Place another tube of similar weight on the rotator for balancing purpose.
- 3.
- Place the rotator in a 37 °C incubator and set to rotate at 10–15 revolutions/minute for 60 min.
- 4.
- Resuspend the suspension using a disposable transfer pipette to improve tissue dissociation.
3.3. Digestion of the DNA Released in the Medium
- Take the tube off the rotator.
- Under the safety cabinet, add 50 μL of DNAse I (stock solution 20 mg/mL; 50 μL = 1 mg; final concentration 0.2 mg/mL).
- Place back on the rotator and leave to rotate at 37 °C for an additional 15 min.
- If the buffer remains viscous, add another 50 μL of DNAse I and place back to rotate for another 15 min, otherwise, proceed to the next step.
- Use a disposable transfer pipette to homogenize the cell suspension by aspirating up and down several times in the 15 mL tube.
- Using a 5 mL disposable serological pipette mounted on an electronic pipette controller, top up to 10 mL with PBS 2% FCS.
3.4. Filtration of the Individualized Cells from the Remaining Tissue Aggregates
- Screw-in a sterile nylon wool-packed 10 mL syringe to the top connection of a 3-way stopcock (Video S2).
- Screw-in a 10 mL Luer-Lock syringe to the bottom connection of tap; check that the valve is set correctly and that the flow is only possible between the two syringes.
- 4.
- Gently pull the plunger of the syringe connected to the bottom connection of the 3-way stopcock to aspirate and filter the cell suspension through the nylon wool into this bottom syringe.
- 5.
- Unscrew the bottom syringe and gently flush its content (filtered cell suspension) into a new 15 mL tube.
- 6.
- Using a 5 mL disposable serological pipette, top up to 15 mL using PBS 2% FCS.
- 7.
- Place the tube in a centrifuge, balance the rotor accordingly and spin at 300× g for 7 min.
- 8.
- Aspirate the supernatant using a 10 mL disposable serological pipette and discard.
- 9.
- Resuspend the cell pellet in 15 mL PBS to wash off the FCS remnant prior to Ficoll separation.
- 10.
- Again, place the tube in a centrifuge and spin at 300 × g for 7 min.
- 11.
- Aspirate the supernatant using a 10 mL disposable serological pipette and discard.
- 12.
- Using a 5 mL disposable serological pipette, resuspend the cell pellet in 3 mL a phenol red tainted medium (e.g., RPMI or DMEM).
3.5. Separation of Leukocytes from Muscle Fiber Cells
- Using a 5 mL disposable pipette mounted on an electronic pipette controller, place 3 mL of Ficoll into a new 15 mL tube (Video S3).
- Using a 5 mL disposable pipette mounted on an electronic pipette controller set on low-speed, carefully overlay the 3 mL of cell suspension onto the Ficoll (3 mL) in order to obtain two clearly defined phases.
- Place the tube in a centrifuge and spin at 800 × g for 15 min with the brake turned off.
- 4.
- Aspirate the leukocyte layer using a disposable transfer pipette and transfer into new a 15 mL tube.
- 5.
- Using a 10 mL disposable pipette mounted on an electronic pipette controller, top up to 15 mL using PBS 2% FCS.
- 6.
- Place the tube in a centrifuge and spin at 300 × g for 7 min.
- 7.
- Using a 10 mL disposable pipette mounted on an electronic pipette controller aspirate the supernatant and discard.
- 8.
- Using a P1000 pipette, gently resuspend the cell pellet by aspirating up and down in 1 mL PBS 2% FCS if the pellet is barely visible.
3.6. Cell Count of the Isolated Leukocytes
- Using a P10 pipette, pipet 10 μL of the leukocyte suspension and mix with 10 μL trypan blue.
- Place 10 μL of the cell/trypan blue mix onto a Neubauer hematocytometer and view under a microscope using the 10X objective lens for counting.
4. Expected Results
4.1. Number of Immune Cells Recovered
4.2. Phenotypic Characterization of the Isolated Cells by Flow Cytometry
- Transfer 25,000 cells (or less) into a well of a U bottom 96 well-plate.
- Stain the cells by adding 50 μL of PBS 2% FCS containing the following antibodies: CD3ε BV510 (UCHT1, Becton Dickinson, Macquarie Park, NSW, Australia), CD4 FITC (OKT4, BioLegend, Wangara, WA, Australia), CD8 APC-H7 (SK1, Becton Dickinson), CD19 APC-FIRE750 (HIB19, Becton Dickinson) and leave during 30 min between 4–20 °C protected from direct light.
- At the end of the incubation period, top up to 250 μL using PBS 2% FCS to wash-off the unbound antibodies. Place the plate in a centrifuge and spin for 5 min at 300× g.
- Discard the supernatant without disturbing the cell pellet, which might not be visible.
- Resuspend the cell pellet in 300 μL of PBS 2% FCS and transferred into a FACs tube for analysis on a Beckman Coulter (Lane Cove West, NSW, Australia) Gallios® Flow cytometer using the Kaluza® acquisition software.
5. Reagents Setup
5.1. Nylon Wool-Packed Syringes
- Weigh 400 mg of nylon wool fiber on a precision scale.
- Take a 10 mL Luer-Lock syringe; remove and discard the plunger.
- Gently pull the fiber until obtaining uniform thin strands.
- Insert the nylon wool into the syringe and loosely pack to approximately 4 mL graduation
- Seal inside a sterilization pouch
- Autoclave at 121 °C for 30 min under at least 15 psi of saturated steam pressure.
5.2. Fetal Bovine Serum Inactivation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dalakas, M.C. Inflammatory muscle diseases. N. Engl. J. Med. 2015, 372, 1734–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, C.; Paramalingam, S.; Stevenson, B.; Brusch, A.; Needham, M. Idiopathic inflammatory myopathies: A review. Intern. Med. J. 2021, 51, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Allenbach, Y.; Chaara, W.; Six, A.; Prevel, N.; Mingozzi, F.; Wanschitz, J.; Musset, L.; Charuel, J.-L.; Eymard, B.; Salomon, B.; et al. Th1 response and systemic treg deficiency in inclusion body myositis. PLoS ONE 2014, 9, e88788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandya, J.M.; Fasth, A.E.R.; Zong, M.; Arnardottir, S.; Dani, L.; Lindroos, E.; Malmström, V.; Lundberg, I.E. Expanded T cell receptor Vβ-restricted T cells from patients with sporadic inclusion body myositis are proinflammatory and cytotoxic CD28null T cells. Arthritis Rheum. 2010, 62, 3457–3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rygiel, K.A.; Miller, J.; Grady, J.P.; Rocha, M.C.; Taylor, R.W.; Turnbull, U.M. Mitochondrial and inflammatory changes in sporadic inclusion body myositis. Neuropathol. Appl. Neurobiol. 2015, 41, 288–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Needham, M.; Mastaglia, F.L. Inclusion body myositis: Current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet Neurol. 2007, 6, 620–631. [Google Scholar] [CrossRef]
- Schmidt, J.; Dalakas, M.C. Pathomechanisms of inflammatory myopathies: Recent advances and implications for diagnosis and therapies. Expert Opin. Med. Diagn. 2010, 4, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.; Preusse, C.; Hathazi, D.; Goebel, H.-H.; Stenzel, W. Proteomic profiling unravels a key role of specific macrophage subtypes in sporadic inclusion body myositis. Front. Immunol. 2019, 10, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, S.A.; Bradshaw, E.M.; Pinkus, J.L.; Pinkus, G.S.; Burleson, T.; Due, B.; Bregoli, L.S.; O’Connor, K.C.; Amato, A.A. Plasma cells in muscle in inclusion body myositis and polymyositis. Neurology 2005, 65, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Ieronimakis, N.; Balasundaram, G.; Reyes, M. Direct isolation, culture and transplant of mouse skeletal muscle derived endothelial cells with angiogenic potential. PLoS ONE 2008, 3, e0001753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammers, D.W.; Rybalko, V.; Merscham-Banda, M.; Hsieh, P.-L.; Suggs, L.J.; Farrar, R.P. Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia-reperfusion. J. Appl. Physiol. 2015, 118, 1067–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiglioni, A.; Corna, G.; Manfredi, A.A.; Rovere-Querini, P.; Rigamonti, E.; Basso, V.; Vezzoli, M.; Monno, A.; Almada, A.E.; Mondino, A.; et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS ONE 2015, 10, e0128094. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, N.; Asakura, Y.; Asakura, A. Isolation, culture, and transplantation of muscle satellite cells. J. Vis. Exp. 2014, 86, e50846. [Google Scholar] [CrossRef] [PubMed]
- Autengruber, A.; Gereke, M.; Hansen, G.; Hennig, C.; Bruder, D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur. J. Microbiol. Immunol. 2012, 2, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
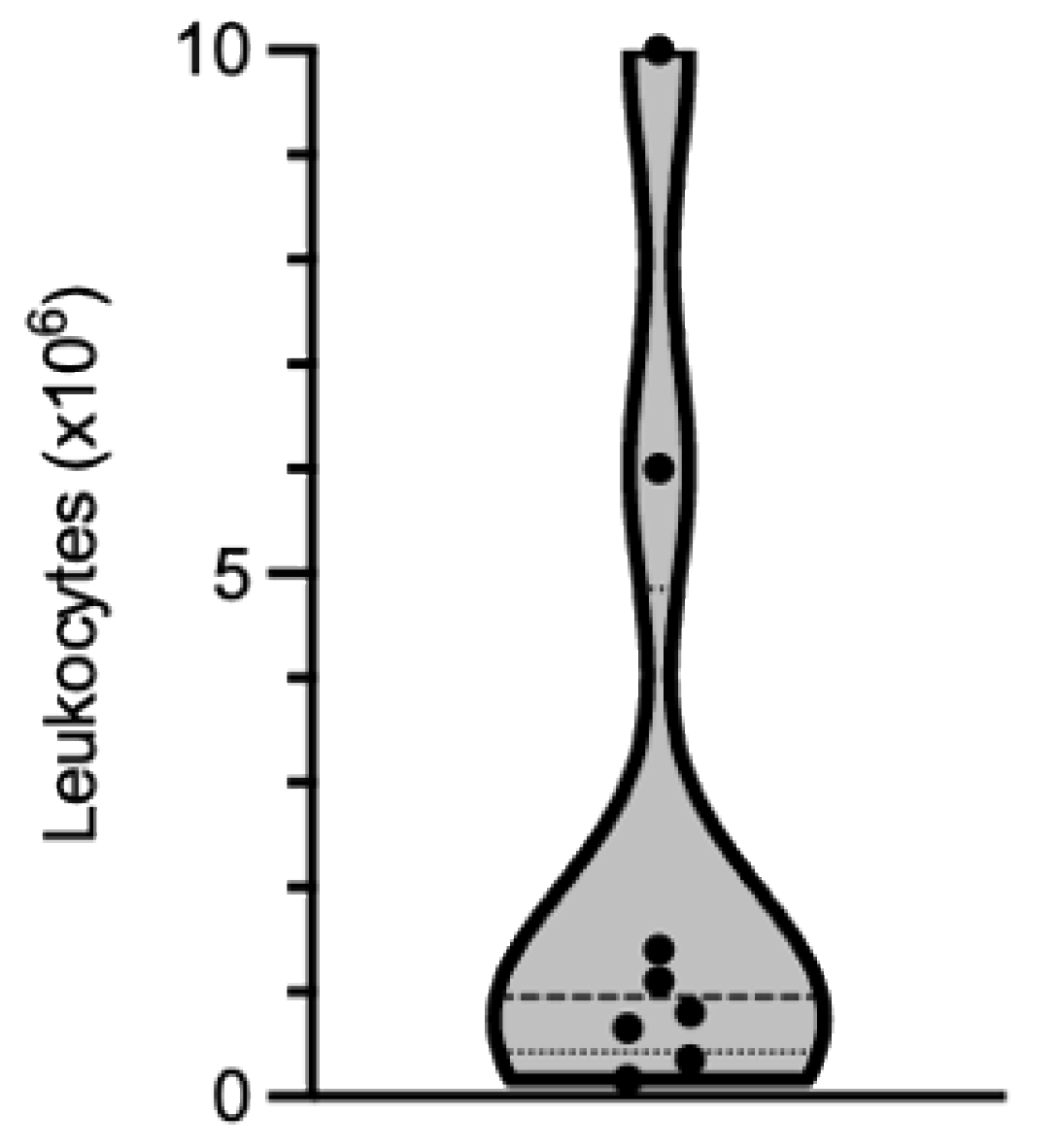

| Diagnosis | Year of Diagnosis | Leukocyte Isolated (×106) |
|---|---|---|
| IBM | 2017 | 1.1 |
| IBM | 2008 | 10 |
| IBM | 2017 | 6 |
| IBM | 2020 | 0.65 |
| IBM | 2017 | 0.8 |
| IBM | 2020 | 1.4 |
| histopathology inconclusive, weak inflammation | 2018 | 0.165 |
| histopathology inconclusive, weak inflammation | 2020 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coudert, J.D.; McLeish, E.; Sooda, A.; Slater, N.; Beer, K.; Paramalingam, S.; Lamont, P.J.; Needham, M. Isolation of Live Leukocytes from Human Inflammatory Muscles. Methods Protoc. 2021, 4, 75. https://doi.org/10.3390/mps4040075
Coudert JD, McLeish E, Sooda A, Slater N, Beer K, Paramalingam S, Lamont PJ, Needham M. Isolation of Live Leukocytes from Human Inflammatory Muscles. Methods and Protocols. 2021; 4(4):75. https://doi.org/10.3390/mps4040075
Chicago/Turabian StyleCoudert, Jerome D., Emily McLeish, Anuradha Sooda, Nataliya Slater, Kelly Beer, Shereen Paramalingam, Phillipa J. Lamont, and Merrilee Needham. 2021. "Isolation of Live Leukocytes from Human Inflammatory Muscles" Methods and Protocols 4, no. 4: 75. https://doi.org/10.3390/mps4040075






