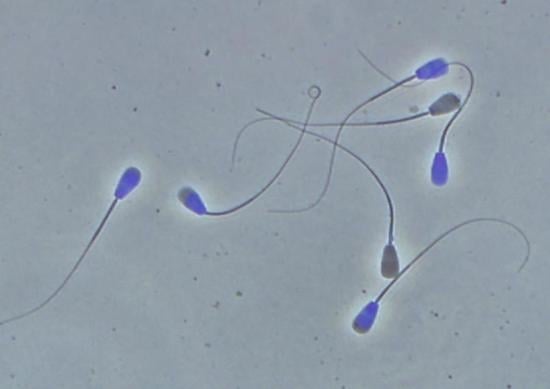
Assessment of Sperm Binding Capacity in the Tubal Reservoir Using a Bovine Ex Vivo Oviduct Culture and Fluorescence Microscopy
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- Semen straws (2–15 million spermatozoa/0.25 mL straw)
- Distilled water
- Sorensen’s buffer (see Reagents Setup)
- Hepes buffer (see Reagents Setup)
- Glutaraldehyde (25% solution), (Sigma-Aldrich, Arklow, Wicklow, Ireland; Cat. no.: G5882)
- KH2PO4 (Sigma-Aldrich, Arklow, Wicklow, Ireland; Cat. no.: P5655)
- Na2HPO4-2H2O (Sigma-Aldrich, Arklow, Wicklow, Ireland; Cat. no.: P71642)
- KCl (Sigma-Aldrich, Arklow, Wicklow, Ireland; Cat. no.: P3911)
- NaCl (Sigma-Aldrich, Arklow, Wicklow, Ireland; Cat. no.: S9888)
- MgCl2-6H2O (Sigma-Aldrich, Arklow, Wicklow, Ireland; Cat. no.: M9272)
- CaCl2-2H2O (Sigma-Aldrich, Arklow, Wicklow, Ireland; Cat. no.: 223506)
- D-(+)-Glucose (Sigma-Aldrich, Arklow, Wicklow, Ireland; Cat. no.: 49139)
- Hepes (Sigma-Aldrich, Arklow, Wicklow, Ireland; Cat. no.: H3375)
- Hoechst 33342 (ThermoFisher Scientific, Dublin, Ireland; Cat. no.: H3570)
- Fluo-4 AM (ThermoFisher Scientific, Dublin, Ireland; Cat. no.: F14217)
- Sterile disposable scalpels (ThermoFisher Scientific, Dublin, Ireland, Cat. no.: 11798343)
- Superfrost Plus slides (ThermoFisher Scientific, Dublin, Ireland; Cat. no.: 22-037-246)
- 1.5 mL Eppendorf tubes (Sigma-Aldrich, Arklow, Wicklow, Ireland, Cat. no.: T6649)
- Cover slips (Sigma-Aldrich, Arklow, Wicklow, Ireland, Cat. no.: BR470045)
- Pipette tips (P20), (Starlab, Milton Keynes, UK; Cat. no.: S1110-3710)
- Pipette tips (P200), (Starlab, Milton Keynes, UK; Cat. no.: S1111-1716)
- Pipette tips (P1000), (Starlab, Milton Keynes, UK; Cat. no.: S1111-6701)
- Lint-free tissue wipes (VWR, Dublin, Ireland, Cat. no.: 115-0202)
2.2. Equipment
- Olympus BX51 Fluorescence Microscope (Olympus, Hamburg, Germany)
- DP71 camera (Olympus, Hamburg, Germany)
- Olympus CKX3-SLP phase contrast filter (Olympus, Hamburg, Germany)
- Olympus Filter Cube (Edmund Optics, York, UK; Cat. no.: 86-371)
- DAPI dichroic filter (Edmund Optics, York, UK; Cat. no.: 86-330)
- MyBlock™ Mini Dry Bath (Benchmark Scientific, Sayreville, NJ, USA; Cat. no.: BSH200)
- Heating block (Benchmark Scientific, Sayreville, NJ, USA; Cat. no.: BSH100-15)
- Thermometer (ThermoFisher Scientific, Dublin, Ireland, Cat. no.: 15350684)
- Dissecting scissors (ThermoFisher Scientific, Dublin, Ireland, Cat. no.: 12847622)
- Neubauer counting chamber (ThermoFisher Scientific, Dublin, Ireland; Cat. no.: 02-671-54).
- Centrifuge 5702 (Eppendorf, Stevenage, UK; Cat. no.: 5702000329)
- Dewar (KGW Isotherm, Karlsruhe, Germany; Cat. no.: 1211)
- General application forceps (30 cm) (ThermoFisher Scientific, Dublin, Ireland, Cat. no.: 16-100-107)
- Micropipette (P2.5), (Eppendorf, Stevenage, UK; Cat. no.: 3123000012)
- Micropipette (P20), (Eppendorf, Stevenage, UK; Cat. no.: 3124000032)
- Micropipette (P100), (Eppendorf, Stevenage, UK; Cat. no.: 3124000075)
- Micropipette (P1000), (Eppendorf, Stevenage, UK; Cat. no.: 3123000063)
3. Procedure
3.1. Collection and Preparation of Fallopian Tubes (1–2 hrs)
- Collect heifer/cow female reproductive tracts from an abattoir immediately after slaughter. Wrap them in a plastic bag and keep them on ice (4 °C) during transport back to a lab;
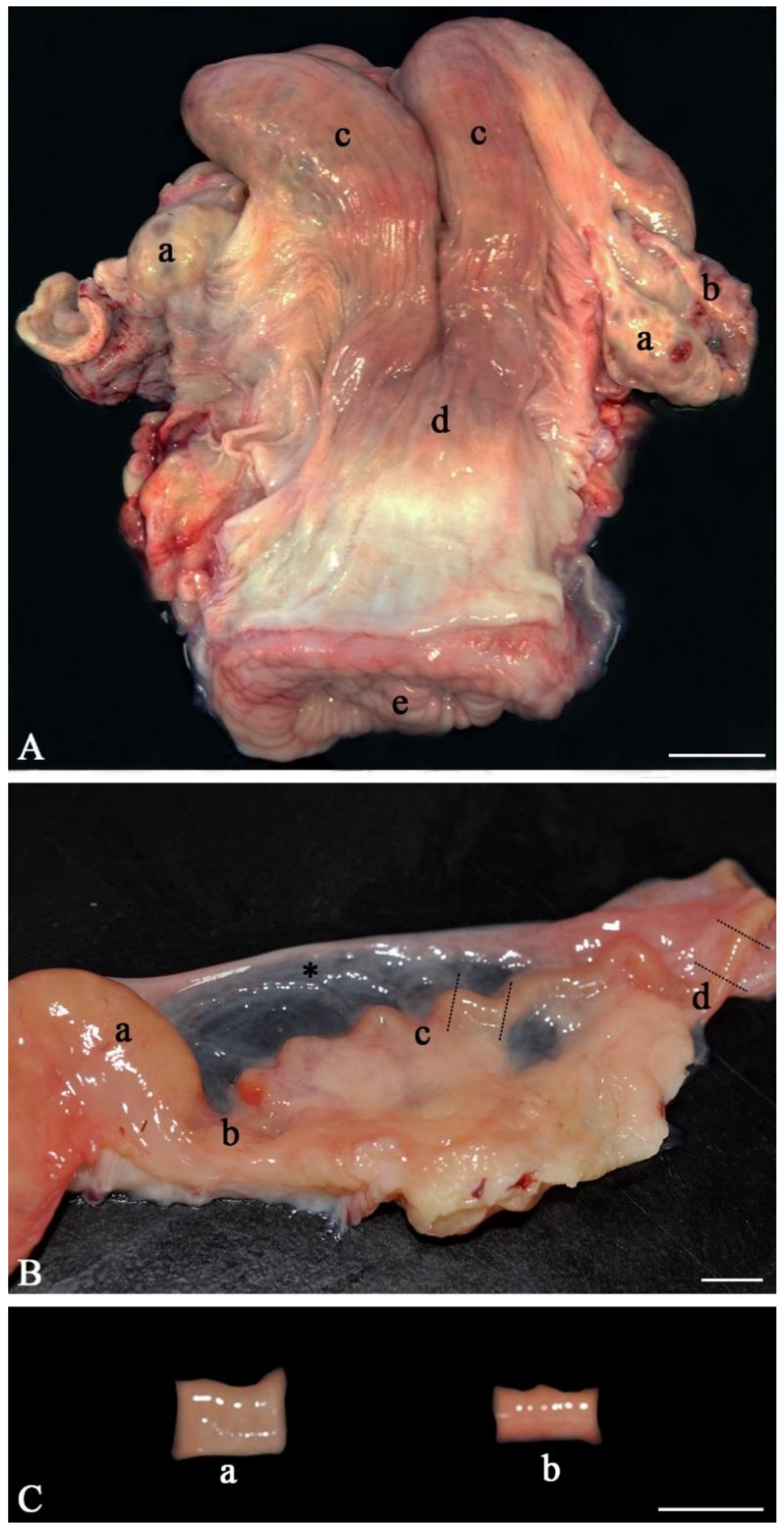
- Anatomically inspect the whole female genital tract. Only use healthy genital tracts (Figure 1A). Exclude genital tracts with signs of inflammation such as reddening of the mucosa and accumulation of fluid or pus. Be aware that an infection/inflammation in the uterus also means inflammation of the oviduct as all parts of the genital organs are in contact with the fluid of the genital tract, which is moved up and down by smooth muscle contractions. Clearly distinguish between the different parts of the oviduct (infundibulum, ampulla, isthmus, Figure 1B). Diagnose the stage of the estrous cycle by macroscopic assessment of the ovaries, uterine body and cervix. For example, features of the diestrus stage include the presence of a corpus luteum on the ovary as well as a flaccid muscle tone, a lack of secretions and a closed cervix. In contrast, estrus is characterized by the presence of a Graafian follicle on one of the ovaries, as well as a high muscle tone, high amount of secretions and an open cervix;
- With anatomical scissors, carefully separate both oviducts from the rest of the tract and pin them onto a dissection board with the pins on both ends of the tube;
- Using a scalpel, dissect and remove the surrounding mesosalpinx from the oviduct;
- Carefully cut the isthmus or the ampulla in 1 cm pieces (Figure 1C). As shown by live cell imaging in the native oviduct, sperm binding occurs both in the ampulla (Figure 2A) and in the isthmus (Figure 2B). In the isthmus, the lumen is narrower than in the ampulla so that it is advisable to use surgical eye scissors to open it up. Always use the same part of the ampulla or isthmus (e.g. the middle third of the isthmus or the junction between ampulla and isthmus) for comparative analyses of sperm binding in individual males or before and after specific treatments. Our studies revealed that—if more tissue is needed—the same sites of right and left oviducts can be used.
- Store the samples in Hepes buffer (4 °C) until stage 3.6.
3.2. Sperm Thawing (5 min)
- Transfer the semen straws (250 µL) from their storage tank to a liquid nitrogen-filled dewar using a suitable forceps;
- Prepare a water thawing bath with a temperature of 39 °C and transfer the semen straw to the water bath for 10 s;
- Remove the straw from the water bath and wipe it with lint-free tissue to remove excess water;
- Using scissors, cut the semen straw at the sealed end and place the newly exposed opening in a pre-heated (37 °C) 1.5 mL Eppendorf tube. Cut the semen straw below the cotton plug so that the semen is transferred to the Eppendorf tube.
 CRITICAL STEP: Ensure that the spermatozoa are kept at a constant temperature of 37 °C until the fixation step. Major deviations in temperature will reduce sperm motility and binding capacity.
CRITICAL STEP: Ensure that the spermatozoa are kept at a constant temperature of 37 °C until the fixation step. Major deviations in temperature will reduce sperm motility and binding capacity.3.3. Motility Analysis and Semen Washing (5 min)
- Transfer 7 µL of semen to a non-adhesive glass slide, cover slip the semen and examine the spermatozoa using a phase contrast microscope with a 20× or 40× lens;
- Estimate the total post-thaw motility of the spermatozoa. Only straws with >60% motility should be included in subsequent analyses;
- Wash the semen by suspending it in 1 mL Hepes buffer and centrifuge for 2 min at 200 rcf;
- Discard the supernatant and resuspend the resulting sperm pellet in 30 µL Hepes buffer;
- Estimate the post-wash motility of the spermatozoa. The overall motility should be >60%.
3.4. Adjustment of Concentrations (5 min)
- In an Eppendorf tube, dilute 2 µL of post-wash semen sample in 8 µL distilled water to create a 5× dilution;
- Load all 10 µL of the diluted sample into a cover slipped Neubauer counting chamber;
- Focusing on the center section of the grid, count the spermatozoa in five squares (top left, top right, bottom left, bottom right, and center square);
- Calculate the concentration of the post-wash samples using the following formula:
- 5.
- Repeat steps 2–5 with all samples;
- 6.
- Adjust the concentration of the samples by diluting accordingly with Hepes buffer. Normally, the final concentration should be 7000–8000 sperm/µL.
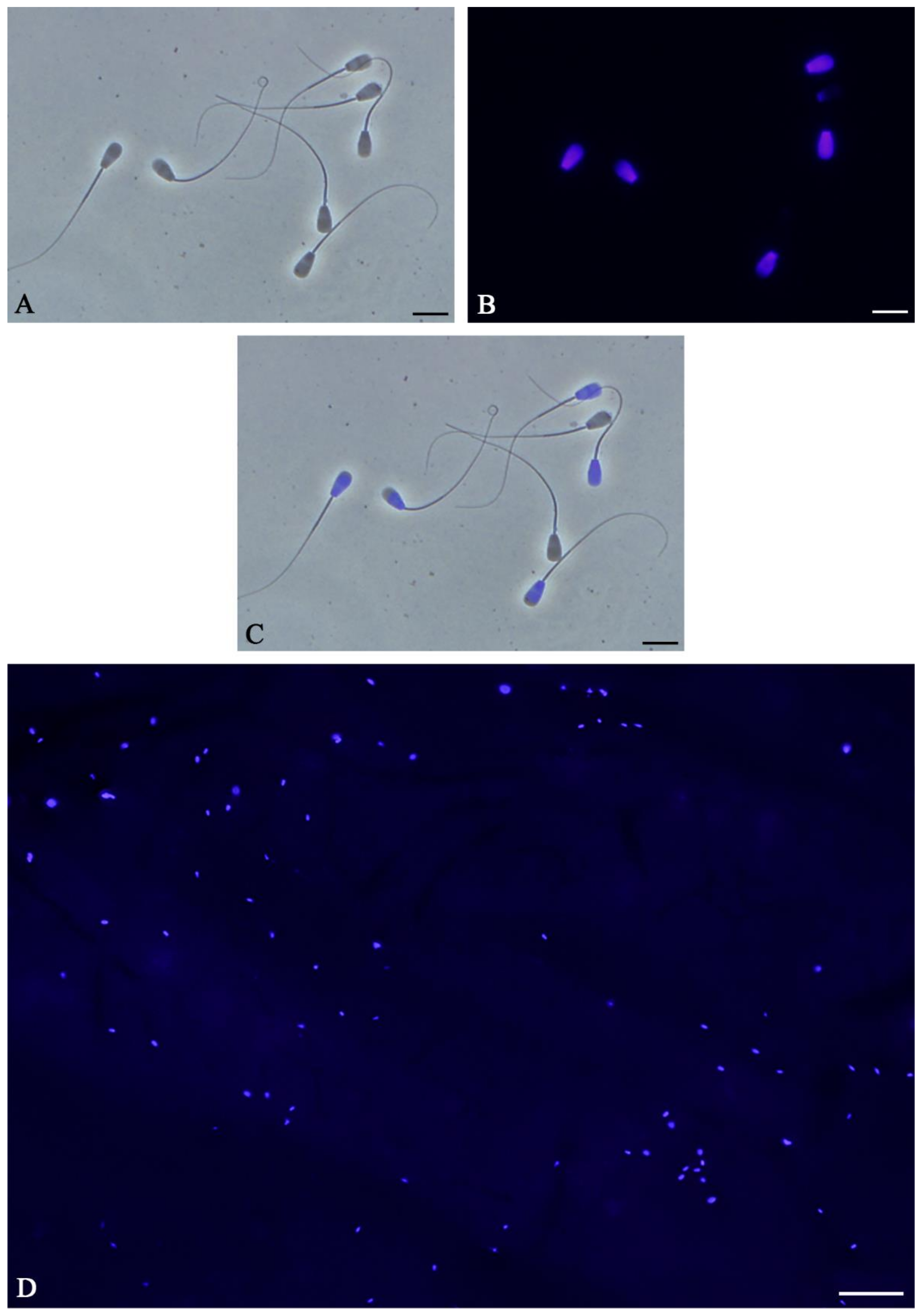
3.5. Sperm DNA Staining (15 min)
- 1.
- Add Hoechst 33342 to the sperm samples to a final concentration of 20 µM and incubate for 10 min at 37 °C;
 CRITICAL STEP: From this point on, keep the sperm samples in the dark to avoid photobleaching of Hoechst 33342.
CRITICAL STEP: From this point on, keep the sperm samples in the dark to avoid photobleaching of Hoechst 33342.- 2.
- Transfer 3–5 µL of the samples to a non-coated slide and cover slip. Image five regions of interest (ROIs, top left, bottom left, top right, bottom right and center) using a 20× lens under both phase contrast and fluorescent light using a DAPI filter. At least 200–400 spermatozoa should be imaged (see Section 4.2 for additional details);
- 2.
- Calculate the staining efficiency using the following formula:
- 4.
- Repeat steps 1–3 for all samples. Only include samples with a staining efficiency of >85% for further analyses.
3.6. Co-Incubation of Spermatozoa with Oviduct (12 min)
- 1.
- Remove a 1 cm segment of oviduct from the Hepes buffer and place it in on a heated plate or in an incubator (37°);
- 2.
- Using the smallest pipette tip possible to minimize damage to the oviductal epithelium, pipette 30 µL of a sperm sample and keep it at hand;
- 3.
- Using a forceps, gently open one end of an ampulla segment and deposit all 30 µL of the sperm sample into the middle region of the oviductal piece;
- 4.
- Incubate the ampulla segments for 10 min at 37 °C to allow the spermatozoa to bind to the oviductal epithelium.
 CRITICAL STEP: To accurately compare sperm binding capacity between different males, pieces from the same oviduct must be used to eliminate the variability of the individual female genital tract.
CRITICAL STEP: To accurately compare sperm binding capacity between different males, pieces from the same oviduct must be used to eliminate the variability of the individual female genital tract.- 5.
- Gently rinse the oviduct in PBS to wash off unbound sperm;
- 6.
- Gently transfer the tubal segment to a Falcon tube filled with 2.5% glutaraldehyde in Sorensen’s buffer solution. The bound spermatozoa will be fixed to the oviductal epithelium within 10 min;
 PAUSE STEP: The oviductal samples with sperm can be stored for up to 24 hrs at 4 °C before analysis.
PAUSE STEP: The oviductal samples with sperm can be stored for up to 24 hrs at 4 °C before analysis.- 7.
- Repeat steps 1–6 for all samples.
3.7. Imaging of Spermatozoa (15 min per Sample)
- Open the fixed oviduct segment longitudinally;
- Gently place the segment on a glass slide with the inner lining of the ampulla facing upwards;
- Place the segment under a fluorescence microscope and visualize the bound spermatozoa under a DAPI filter using a 20× lens. Image the spermatozoa in at least 5 ROIs or, alternatively, create consecutive images of the whole segment;
- Due to the 3D arrangement of the oviductal folds, it is necessary to finely adjust the objective to focus on various Z-planes at each region of interest;
- Repeat steps 1–4 for all samples.
3.8. Image Overlaying and True Sperm Number (20–60 min)
- Open Adobe Photoshop (Adobe Inc, San Jose, CA, USA);
- Import all the Z-plane images for the same region of interest as stacks (File → Scripts → Load files into stacks). This will convert the images into layers;
- Select all layers and overlay them (Edit → Auto-blend layers);
- In the new window, select Stack Images as the blend method. Ensure “Seamless tones and colors” and “Content aware fill transparent areas” are selected;
- Click OK. The software will detect which areas in each Z-stack are in focus and combine the focused areas into a master image. This process will take longer the more stacks are present. 12–14 Z-stacks per region of interest should be sufficient (see Section 4.3 for additional details);
- Count the number of fluorescent bound spermatozoa in master image and calculate the average number of spermatozoa per tubal sample;
- Since not all spermatozoa will fluoresce, the true number of bound spermatozoa can be calculated as follows:
- 8.
- Once the number of bound spermatozoa per region/segment has been calculated, the sperm binding capacity between different males can be compared.
4. Expected Results
4.1. Sperm-Oviduct Interactions and Capacitation in Sperm Stained with Hoechst 33342
4.2. Staining Efficiency
4.3. Sperm Binding Capacity
5. Discussion
6. Conclusions
7. Reagents Setup
7.1. Preparation of Hepes Stock Solutions
- Add 10.16 g MgCl2-6H2O to 50 mL distilled water (final concentration = 1 M);
- Add 7.35 g CaCl2-2H2O to 50 mL distilled water (final concentration = 1 M).
7.2. Preparation of Hepes Buffer
- Add the reagents in Table 1 to 1 L distilled water;
- Adjust the pH of the solution to 7.4 using NaOH;
- Store the Hepes buffer at 4 °C for up to a month.
7.3. Preparation of Sorensen’s Buffer
- Prepare Solution A by adding 0.91 g KH2PO4 per 100 mL distilled water;
- Prepare Solution B by adding 1.19 g Na2HPO4-2H2O per 100 mL distilled water;
- Mix Solutions A and B in a 1:4 (v/v) ratio. This mixture is Sorensen’s buffer;
- Store the Sorensen’s buffer at 4 °C for up to a month.
7.4. Preparation of Fixing Solution
- Add 1 mL of 25% glutaraldehyde solution per 6.25 mL Sorensen’s buffer;
- Store fixing solution at 4 °C for up to a month.
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Dobrinski, I.; Ignotz, G.G.; Thomas, P.G.; Ball, B.A. Role of carbohydrates in the attachment of equine spermatozoa to uterine tubal (oviductal) epithelial cells in vitro. Am. J. Vet. Res. 1996, 57, 1635–1639. [Google Scholar] [PubMed]
- Green, C.E.; Bredl, J.; Holt, W.V.; Watson, P.F.; Fazeli, A. Carbohydrate mediation of boar sperm binding to oviductal epithelial cells in vitro. Reproduction 2001, 122, 305–315. [Google Scholar] [CrossRef]
- Lefebvre, R.; Lo, M.C.; Suarez, S.S. Bovine sperm binding to oviductal epithelium involves fucose recognition. Biol. Reprod. 1997, 56, 1198–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, S.A.; Kadirvel, G.; Daigneault, B.W.; Korneli, C.; Miller, P.; Bovin, N.; Miller, D.J. LewisX-containing glycans on the porcine oviductal epithelium contribute to formation of the sperm reservoir. Biol. Reprod. 2014, 91, 140. [Google Scholar] [CrossRef] [PubMed]
- Gwathmey, T.M.; Ignotz, G.G.; Mueller, J.L.; Manjunath, P.; Suarez, S.S. Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol. Reprod. 2006, 75, 501–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.J. Regulation of Sperm Function by Oviduct Fluid and the Epithelium: Insight into the Role of Glycans. Reprod. Domest. Anim. 2015, 50 (Suppl. S2), 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lamy, J.; Corbin, E.; Blache, M.C.; Garanina, A.S.; Uzbekov, R.; Mermillod, P.; Saint-Dizier, M. Steroid hormones regulate sperm-oviduct interactions in the bovine. Reproduction 2017, 154, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Topfer-Petersen, E.; Wagner, A.; Friedrich, J.; Petrunkina, A.; Ekhlasi-Hundrieser, M.; Waberski, D.; Drommer, W. Function of the mammalian oviductal sperm reservoir. J. Exp. Zool. 2002, 292, 210–215. [Google Scholar] [CrossRef]
- Hawk, H.W. Sperm survival and transport in the female reproductive tract. J. Dairy Sci. 1983, 66, 2645–2660. [Google Scholar] [CrossRef]
- Lee, J.-H. Female annual reproductive cycle of Rhinolophus ferrumequinum korai (Chiroptera: Rhinolophidae): Morphological changes and prolonged sperm storage and sperm fate of the female reproductive tract according to month. Eur. Zool. J. 2020, 87, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Pérez, F.A.; Roma, S.M.; Cabada, M.O.; Marini, P.E. Sperm binding glycoprotein is differentially present surrounding the lumen of isthmus and ampulla of the pig’s oviduct. Anat. Embryol. (Berl.) 2006, 211, 619–624. [Google Scholar] [CrossRef]
- Muro, Y.; Hasuwa, H.; Isotani, A.; Miyata, H.; Yamagata, K.; Ikawa, M.; Yanagimachi, R.; Okabe, M. Behavior of Mouse Spermatozoa in the Female Reproductive Tract from Soon after Mating to the Beginning of Fertilization. Biol. Reprod. 2016, 94, 80. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolle, S. Transport, Distribution and Elimination of Mammalian Sperm Following Natural Mating and Insemination. Reprod. Domest. Anim. 2015, 50 (Suppl. S3), 2–6. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Tienthai, P.; Atikuzzaman, M.; Vicente-Carrillo, A.; Ruber, M.; Alvarez-Rodriguez, M. The ubiquitous hyaluronan: Functionally implicated in the oviduct? Theriogenology 2016, 86, 182–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Aoki, K.; Doungkamchan, K.; Tiemeyer, M.; Bovin, N.; Miller, D.J. Sulfated Lewis A trisaccharide on oviduct membrane glycoproteins binds bovine sperm and lengthens sperm lifespan. J. Biol. Chem. 2019, 294, 13445–13463. [Google Scholar] [CrossRef]
- Yao, Y.Q.; Ho, P.C.; Yeung, W.S. Effects of human oviductal cell coculture on various functional parameters of human spermatozoa. Fertil. Steril. 1999, 71, 232–239. [Google Scholar] [CrossRef]
- Georgiou, A.S.; Snijders, A.P.; Sostaric, E.; Aflatoonian, R.; Vazquez, J.L.; Vazquez, J.M.; Roca, J.; Martinez, E.A.; Wright, P.C.; Fazeli, A. Modulation of the oviductal environment by gametes. J. Proteome Res. 2007, 6, 4656–4666. [Google Scholar] [CrossRef] [PubMed]
- Kolle, S.; Reese, S.; Kummer, W. New aspects of gamete transport, fertilization, and embryonic development in the oviduct gained by means of live cell imaging. Theriogenology 2010, 73, 786–795. [Google Scholar] [CrossRef]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Morrell, J.M.; Nongbua, T.; Valeanu, S.; Lima Verde, I.; Lundstedt-Enkel, K.; Edman, A.; Johannisson, A. Sperm quality variables as indicators of bull fertility may be breed dependent. Anim. Reprod. Sci. 2017, 185, 42–52. [Google Scholar] [CrossRef]
- Kastelic, J.P. Male involvement in fertility and factors affecting semen quality in bulls. Anim. Front. 2013, 3, 20–25. [Google Scholar] [CrossRef]
- Fair, S.; Lonergan, P. Review: Understanding the causes of variation in reproductive wastage among bulls. Animal 2018, 12, s53–s62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarevic, J.; Wikarczuk, M.; Somkuti, S.G.; Barmat, L.I.; Schinfeld, J.S.; Smith, S.E. Hyaluronan binding assay (HBA) vs. sperm penetration assay (SPA): Can HBA replace the SPA test in male partner screening before in vitro fertilization? J. Exp. Clin. Assist. Reprod. 2010, 7, 2. [Google Scholar]
- Gualtieri, R.; Talevi, R. In vitro-cultured bovine oviductal cells bind acrosome-intact sperm and retain this ability upon sperm release. Biol. Reprod. 2000, 62, 1754–1762. [Google Scholar] [CrossRef] [Green Version]
- Saraf, K.K.; Kumaresan, A.; Nayak, S.; Chhillar, S.; Sreela, L.; Kumar, S.; Tripathi, U.K.; Datta, T.K.; Mohanty, T.K. Development of an in vitro oviduct epithelial explants model for studying sperm-oviduct binding in the buffalo. Reprod. Domest. Anim. 2017, 52, 687–691. [Google Scholar] [CrossRef]
- Suarez, S.S. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2016, 363, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Yaniz, J.L.; Lopez-Gatius, F.; Santolaria, P.; Mullins, K.J. Study of the functional anatomy of bovine oviductal mucosa. Anat. Rec. 2000, 260, 268–278. [Google Scholar] [CrossRef]
- Camara Pirez, M.; Steele, H.; Reese, S.; Kolle, S. Bovine sperm-oviduct interactions are characterized by specific sperm behaviour, ultrastructure and tubal reactions which are impacted by sex sorting. Sci. Rep. 2020, 10, 16522. [Google Scholar] [CrossRef]
- Einspanier, R.; Kettler, A.; Gabler, C.; Kloas, W.; Einspanier, A.; Schams, D. The Mammalian Oviduct: Aspects on Auto-and Paracrine Mechanisms. Reprod. Domest. Anim. 2000, 35, 125–128. [Google Scholar] [CrossRef]
- Mostek, A.; Janta, A.; Majewska, A.; Ciereszko, A. Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins. Int. J. Mol. Sci. 2021, 22, 7903. [Google Scholar] [CrossRef] [PubMed]
- Ickowicz, D.; Finkelstein, M.; Breitbart, H. Mechanism of sperm capacitation and the acrosome reaction: Role of protein kinases. Asian J. Androl. 2012, 14, 816–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, D.L. Hoechst 33342: The dye that enabled differentiation of living X-and Y-chromosome bearing mammalian sperm. Theriogenology 2009, 71, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 2008, 52, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Talevi, R.; Gualtieri, R. Molecules involved in sperm-oviduct adhesion and release. Theriogenology 2010, 73, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Kolle, S.; Dubielzig, S.; Reese, S.; Wehrend, A.; Konig, P.; Kummer, W. Ciliary transport, gamete interaction, and effects of the early embryo in the oviduct: Ex vivo analyses using a new digital videomicroscopic system in the cow. Biol. Reprod. 2009, 81, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolle, S. Live cell imaging of the oviduct. Methods Enzymol. 2012, 506, 415–423. [Google Scholar] [CrossRef]
- Suarez, S.S. Formation of a reservoir of sperm in the oviduct. Reprod. Domest. Anim. 2002, 37, 140–143. [Google Scholar] [CrossRef]
- Leahy, T.; Gadella, B.M. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction 2011, 142, 759–778. [Google Scholar] [CrossRef]
- Maxwell, W.M.C.; Johnson, L.A. Physiology of spermatozoa at high dilution rates: The influence of seminal plasma. Theriogenology 1999, 52, 1353–1362. [Google Scholar] [CrossRef]


| Chemical | Quantity | Final Concentration |
|---|---|---|
| KCl | 0.418 g | 5.6 mM |
| NaCl | 7.970 g | 136.4 mM |
| MgCl2-6H2O (1 M) | 1.000 mL | 1 mM |
| CaCl2-2H2O (1 M) | 2.200 mL | 2.2 mM |
| D-(+)-Glucose | 1.980 g | 11 mM |
| HEPES | 2.380 g | 10 mM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camara Pirez, M.; Li, S.; Koelle, S. Assessment of Sperm Binding Capacity in the Tubal Reservoir Using a Bovine Ex Vivo Oviduct Culture and Fluorescence Microscopy. Methods Protoc. 2021, 4, 67. https://doi.org/10.3390/mps4040067
Camara Pirez M, Li S, Koelle S. Assessment of Sperm Binding Capacity in the Tubal Reservoir Using a Bovine Ex Vivo Oviduct Culture and Fluorescence Microscopy. Methods and Protocols. 2021; 4(4):67. https://doi.org/10.3390/mps4040067
Chicago/Turabian StyleCamara Pirez, Miguel, Simeng Li, and Sabine Koelle. 2021. "Assessment of Sperm Binding Capacity in the Tubal Reservoir Using a Bovine Ex Vivo Oviduct Culture and Fluorescence Microscopy" Methods and Protocols 4, no. 4: 67. https://doi.org/10.3390/mps4040067
APA StyleCamara Pirez, M., Li, S., & Koelle, S. (2021). Assessment of Sperm Binding Capacity in the Tubal Reservoir Using a Bovine Ex Vivo Oviduct Culture and Fluorescence Microscopy. Methods and Protocols, 4(4), 67. https://doi.org/10.3390/mps4040067