Effectiveness of Silver Diammine Fluoride Applications for Dental Caries Cessation in Tribal Preschool Children in India: Study Protocol for a Randomized Controlled Trial
Abstract
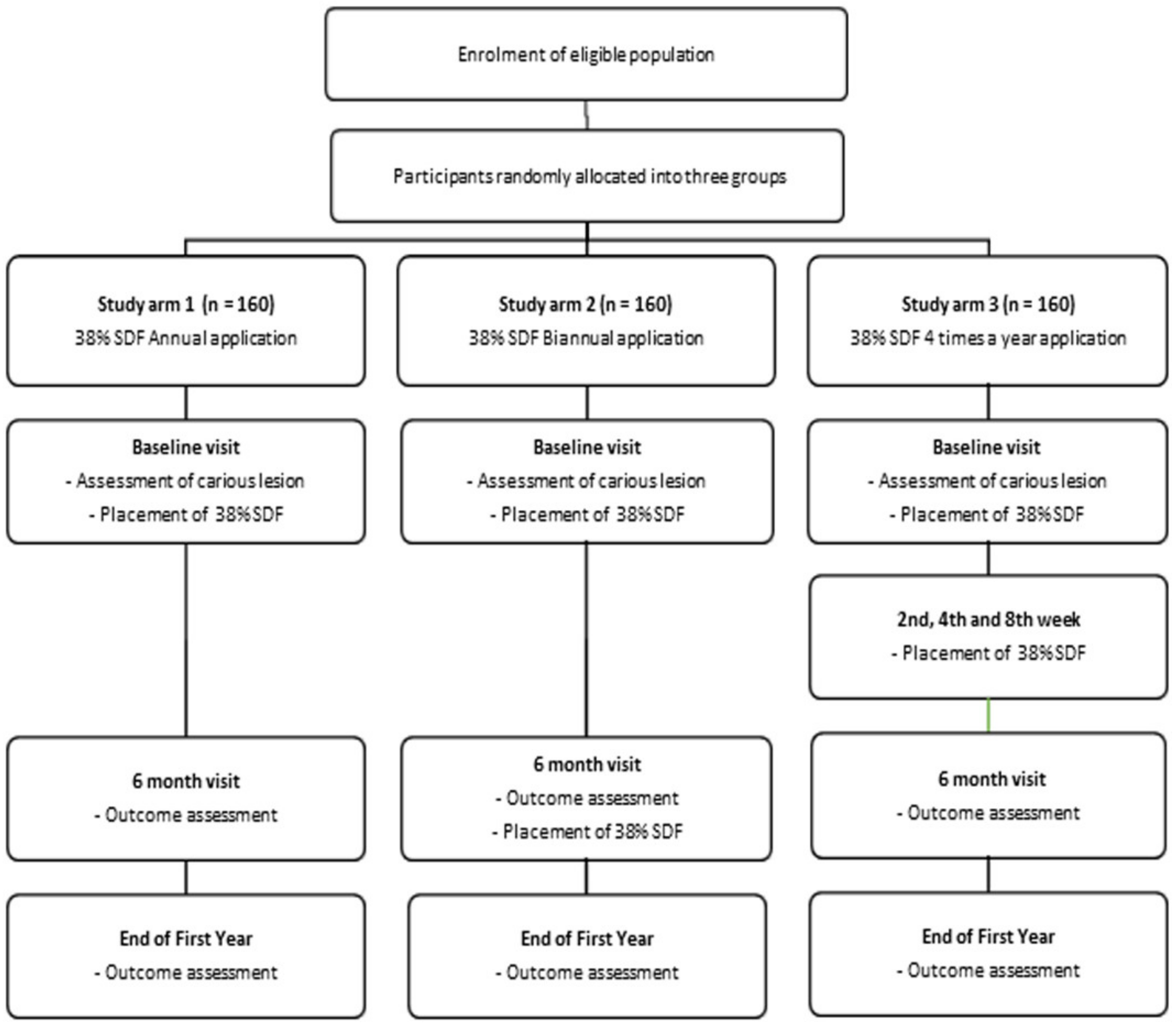
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Settings
2.3. Trial Registration
2.4. Study Population
2.5. Eligibility Criteria
2.5.1. Inclusion Criteria
- Children of any gender aged 2–6 years with the presence of cavitated caries lesion classified as stage 5–6 following the Modified International Caries Detection and Assessment System (ICDAS).
- The parent of the child understands the importance of SDF treatment to arrest prevent dental caries.
- Parent of a child willing to accompany the child at required intervals for evaluation.
2.5.2. Exclusion Criteria
- Children with ulcerative gingivitis or stomatitis.
- Developmental dental abnormalities such as enamel defects.
- Serious chronic medical conditions, such as congenital heart disease.
- Cavitated teeth that are nearing natural exfoliation time.
- Children with more than one-third of the crown missing, or pulpal involved (tooth with pulp exposure, presence of an abscess or a sinus, obvious discoloration, and premature hypermobility were regarded as pulpal involved tooth)
- Known silver allergy.
- Uncooperative children (after the failure of basic pediatric patient management protocols)
- Children with special health care needs.
2.6. Definition of Study Condition (Dental Caries)
2.7. Interventions
Silver Diammine Fluoride
- Study arm 1: annually (once a year; baseline visit);
- Study arm 2: bi-annually (twice a year at 6-month intervals; baseline visit and after 6 months);
- Study arm 3: four times a year (baseline visit, 2nd, 4th, and 8th week).
2.8. Primary Outcomes
- Any teeth with treated carious lesions that are lost due to exfoliation will be considered arrested throughout the lifetime of the tooth. For any reason, if the child’s teeth are restored before the trial period, censoring of the data depends on the duration of the trial period the child has participated.
- The following dental complications due to the evolution of caries will not be considered as caries arrested.
- ○
- The teeth extracted due to caries progression.
- ○
- If the SDF treated cavities, caries progressed beyond the dentine.
- ○
- If the tooth is extracted due to fracture, trauma will not be counted for the study.
2.9. Secondary Outcome
2.10. Participation Timeline
2.11. Sample Size
2.12. Study Implementation
2.13. Compliance with Trial Treatment
2.14. Adverse Events
3. Close-Out Procedures
4. Statistical Methods
5. Analysis Population and Missing Data
6. Patient and Public Involvement
7. Ethics and Dissemination
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nunn, J.H. The burden of oral ill health for children. Arch. Dis. Child. 2006, 91, 251–253. [Google Scholar] [CrossRef]
- Sheiham, A.; Williams, D.M.; Weyant, R.J.; Glick, M.; Naidoo, S.; Eiselé, J.-L.; Selikowitz, H.-S. Billions with oral disease: A global health crisis—A call to action. J. Am. Dent. Assoc. 2015, 146, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.; Anand, P.S. Early childhood caries: Prevalence, risk factors, and prevention. Front. Pediatr. 2017, 5. [Google Scholar] [CrossRef]
- Jose, B.; King, N.M. Early childhood caries lesions in preschool children in Kerala, India. Pediatr. Dent. 2003, 25, 594–600. [Google Scholar]
- Priyadarshini, H.R.; Hiremath, S.S.; Puranik, M.; Rudresh, S.M.; Nagaratnamma, T. Prevalence of early childhood caries among preschool children of low socioeconomic status in Bangalore city, India. J. Int. Soc. Prev. Community Dent. 2011, 1, 27–30. [Google Scholar] [CrossRef]
- Prakash, P.; Subramaniam, P.; Durgesh, B.H.; Konde, S. Prevalence of early childhood caries and associated risk factors in preschool children of urban Bangalore, India: A cross-sectional study. Eur. J. Dent. 2012, 6, 141–152. [Google Scholar] [CrossRef]
- Janakiram, C.; Antony, B.; Joseph, J. Association of undernutrition and early childhood dental caries. Indian Pediatr. 2018, 55, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Çolak, H.; Dülgergil, Ç.T.; Dalli, M.; Hamidi, M.M. Early childhood caries update: A review of causes, diagnoses, and treatments. J. Nat. Sci. Biol. Med. 2013, 4, 29–38. [Google Scholar] [CrossRef]
- Davies, G.M.; Worthington, H.V.; Ellwood, R.P.; Blinkhorn, A.S.; Taylor, G.O.; Davies, R.M.; Considine, J. An assessment of the cost effectiveness of a postal toothpaste programme to prevent caries among five-year-old children in the North West of England. Community Dent. Health 2003, 20, 207–210. [Google Scholar]
- Koh, R.; Pukallus, M.; Kularatna, S.; Gordon, L.G.; Barnett, A.G.; Walsh, L.J.; Seow, W.K. Relative cost-effectiveness of home visits and telephone contacts in preventing early childhood caries. Community Dent. Oral Epidemiol. 2015, 43, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Pukallus, M.; Plonka, K.; Kularatna, S.; Gordon, L.; Barnett, A.G.; Walsh, L.; Seow, W.K. Cost-effectiveness of a telephone-delivered education programme to prevent early childhood caries in a disadvantaged area: A cohort study. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Samnaliev, M.; Wijeratne, R.; Kwon, E.G.; Ohiomoba, H.; Ng, M.W. Cost-effectiveness of a disease management program for early childhood caries. J. Public Health Dent. 2014, 75, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Mariño, R.; Fajardo, J.; Morgan, M. Cost-effectiveness models for dental caries prevention programmes among Chilean schoolchildren. Community Dent. Health 2012, 29, 302–308. [Google Scholar] [PubMed]
- Duangthip, D.; Chen, K.J.; Gao, S.S.; Lo, E.C.M.; Chu, C.H. Managing early childhood caries with atraumatic restorative treatment and topical silver and fluoride agents. Int. J. Environ. Res. Public Health 2017, 14, 1204. [Google Scholar] [CrossRef]
- Wakshlak, R.B.-K.; Pedahzur, R.; Avnir, D. Antibacterial activity of silver-killed bacteria: The “zombies” effect. Sci. Rep. 2015, 5, 9555. [Google Scholar] [CrossRef] [PubMed]
- Slayton, R.L.; Urquhart, O.; Araujo, M.W.B.; Fontana, M.; Guzmán-Armstrong, S.; Nascimento, M.M.; Nový, B.B.; Tinanoff, N.; Weyant, R.J.; Wolff, M.S.; et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J. Am. Dent. Assoc. 2018, 149, 837–849.e19. [Google Scholar] [CrossRef] [PubMed]
- Hendre, A.D.; Taylor, G.W.; Chávez, E.M.; Hyde, S. A systematic review of silver diamine fluoride: Effectiveness and application in older adults. Gerodontology 2017, 34, 411–419. [Google Scholar] [CrossRef]
- Monse, B.; Heinrich-Weltzien, R.; Mulder, J.; Holmgren, C.; van Palenstein Helderman, W.H. Caries preventive efficacy of silver diammine fluoride (SDF) and ART sealants in a school-based daily fluoride toothbrushing program in the Philippines. BMC Oral Health 2012, 12, 52. [Google Scholar] [CrossRef]
- Contreras, V.; Toro, M.J.; Elías-Boneta, A.R.; Encarnación-Burgos, A. Effectiveness of silver diamine fluoride in caries prevention and arrest: A systematic literature review. Gen. Dent. 2017, 65, 22–29. [Google Scholar]
- Yee, R.; Holmgren, C.; Mulder, J.; Lama, D.; Walker, D.; van Palenstein Helderman, W. Efficacy of silver diamine fluoride for arresting caries treatment. J. Dent. Res. 2009, 88, 644–647. [Google Scholar] [CrossRef]
- Llodra, J.C.; Rodriguez, A.; Ferrer, B.; Menardia, V.; Ramos, T.; Morato, M. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J. Dent. Res. 2005, 84, 721–724. [Google Scholar] [CrossRef]
- Rosenblatt, A.; Stamford, T.C.M.; Niederman, R. Silver diamine fluoride: A caries “silver-fluoride bullet”. J. Dent. Res. 2009, 88, 116–125. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Niederman, R. Silver diamine fluoride treatment considerations in children’s caries management. Pediatr. Dent. 2016, 38, 466–471. [Google Scholar] [PubMed]
- Mattos-Silveira, J.; Floriano, I.; Ferreira, F.R.; Viganó, M.E.F.; Frizzo, M.A.; Reyes, A.; Novaes, T.F.; Moriyama, C.M.; Raggio, D.P.; Imparato, J.C.P.; et al. New proposal of silver diamine fluoride use in arresting approximal caries: Study protocol for a randomized controlled trial. Trials 2014, 15, 448. [Google Scholar] [CrossRef] [PubMed]
- Crystal, Y.O.; Marghalani, A.A.; Ureles, S.D.; Wright, J.T.; Sulyanto, R.; Divaris, K.; Fontana, M.; Graham, L. Use of silver diamine fluoride for dental caries management in children and adolescents, including those with special health care needs. Pediatr. Dent. 2017, 39, 135–145. [Google Scholar]
- Duangthip, D.; Wong, M.C.M.; Chu, C.H.; Lo, E.C.M. Caries arrest by topical fluorides in preschool children: 30-month results. J. Dent. 2018, 70, 74–79. [Google Scholar] [CrossRef]
- Gao, S.S.; Zhao, I.S.; Hiraishi, N.; Duangthip, D.; Mei, M.L.; Lo, E.C.M.; Chu, C.H. Clinical trials of silver diamine fluoride in arresting caries among children: A Systematic Review. JDR Clin. Transl. Res. 2016, 1, 201–210. [Google Scholar] [CrossRef]
- Fung, M.H.T.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J. Dent. Res. 2018, 97, 171–178. [Google Scholar] [CrossRef]
- Zhi, Q.H.; Lo, E.C.M.; Lin, H.C. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J. Dent. 2012, 40, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Chibinski, A.C.; Wambier, L.M.; Feltrin, J.; Loguercio, A.D.; Wambier, D.S.; Reis, A. Silver diamine fluoride has efficacy in controlling caries progression in primary teeth: A Systematic Review and Meta-Analysis. Caries Res. 2017, 51, 527–541. [Google Scholar] [CrossRef]
- Chu, C.H.; Lo, E.C.M.; Lin, H.C. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J. Dent. Res. 2002, 81, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Narain, J.P. Health of tribal populations in India: How long can we afford to neglect? Indian J. Med. Res. 2019, 149, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, B. ICDAS II criteria (International Caries Detection and Assessment System). J. Istanb. Univ. Fac. Dent. 2015, 49, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, J.; Thompson, A.; Milgrom, P.; Shirtcliff, M. Diammino silver fluoride arrestment of caries associated with anti-microbial action. In Proceedings of the 2010 IADR/PER General Session, Barcelona, Spain, 14–17 July 2010. [Google Scholar]
| Procedures | Timeline | ||||||
|---|---|---|---|---|---|---|---|
| Visit 1 | 2 | 3 | 4 | 5 | 6 | ||
| Baseline | 2 Weeks | 4 Weeks | 8 Weeks | 6 Months | 12 Months | ||
| Pre-screening consent | Yes | - | - | - | - | - | |
| Eligibility assessment | Yes | - | - | - | - | - | |
| Oral examination | Yes | Yes | Yes | Yes | Yes | Yes | |
| Informed consent | Yes | - | - | - | - | - | |
| Allocation to study arms | Yes | - | - | - | - | - | |
| Caries Excavation | Yes | ||||||
| Application of 38% SDF | Group 1 | Yes | No | No | No | No | No |
| Group 2 | Yes | No | No | No | Yes | No | |
| Group 3 | Yes | Yes | Yes | Yes | No | No | |
| Outcome assessment | Yes * | - | - | - | Yes | Yes | |
| Adverse event assessments | Yes | Yes | Yes | Yes | Yes | Yes | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janakiram, C.; Ramanarayanan, V.; Devan, I. Effectiveness of Silver Diammine Fluoride Applications for Dental Caries Cessation in Tribal Preschool Children in India: Study Protocol for a Randomized Controlled Trial. Methods Protoc. 2021, 4, 30. https://doi.org/10.3390/mps4020030
Janakiram C, Ramanarayanan V, Devan I. Effectiveness of Silver Diammine Fluoride Applications for Dental Caries Cessation in Tribal Preschool Children in India: Study Protocol for a Randomized Controlled Trial. Methods and Protocols. 2021; 4(2):30. https://doi.org/10.3390/mps4020030
Chicago/Turabian StyleJanakiram, Chandrashekar, Venkitachalam Ramanarayanan, and Induja Devan. 2021. "Effectiveness of Silver Diammine Fluoride Applications for Dental Caries Cessation in Tribal Preschool Children in India: Study Protocol for a Randomized Controlled Trial" Methods and Protocols 4, no. 2: 30. https://doi.org/10.3390/mps4020030
APA StyleJanakiram, C., Ramanarayanan, V., & Devan, I. (2021). Effectiveness of Silver Diammine Fluoride Applications for Dental Caries Cessation in Tribal Preschool Children in India: Study Protocol for a Randomized Controlled Trial. Methods and Protocols, 4(2), 30. https://doi.org/10.3390/mps4020030






