A Straightforward Hypoxic Cell Culture Method Suitable for Standard Incubators
Abstract
1. Introduction
2. Experimental Design
2.1. Materials
- 1.5 mL Safe-Lock tubes (Eppendorf, Hamburg, Germany, Cat. No.: 0030120086);
- 15 mL conical tubes (Greiner Bio-One, Frickenhausen, Germany; Cat. No.: 10384601);
- 2-Mercaptoethanol (Roth, Karlsruhe, Germany, Cat. No.: 4227.2);
- 50 mL conical tubes (Greiner Bio-One, Frickenhausen, Germany; Cat. No.: 10711212);
- 6-well plate (Greiner Bio-One, Frickenhausen, Germany; Cat. No.: 657160);
- Acrylamide/bis—Rotiphorese Gel 30 (Roth, Karlsruhe, Germany, Cat. No.: 3029.2);
- AgelessEye (oxygen indicator) (Mitsubishi Gas Chemical Company, Tokyo, Japan; Distributor: Long Life for Art, Cat. No.: O2INDAEYE);
- Amphotericin B (Thermo Fisher Scientific, Schwerte, Germany, Cat. No.: 15290018);
- Anti-GAPDH antibody (Merk, Darmstadt, Germany, Cat. No.: CB1001-500UG);
- Anti-HIF-1α antibody (Novus Biologicals, Centennial, USA, Distributor: Bio-Techne GmbH, Wiesbaden, Germany Cat. No.: NB100-479);
- Anti-mouse IgG-HRP (Jackson ImmunoResearch, West Grove, U.S.A., Distributor: Dianova, Hamburg, Germany, Cat. No.: 115-035-146);
- Anti-rabbit IgG-HRP (Jackson ImmunoResearch, West Grove, U.S.A., Distributor: Dianova, Hamburg, Germany, Cat. No.: 111-035-144);
- Anti-PDK1 antibody (Novus Biologicals, Centennial, USA, Distributor: Bio-Techne GmbH, Wiesbaden, Germany, Cat. No.: NB100-2384SS);
- APS (Ammonium peroxodisulfate) (Roth, Karlsruhe, Germany, Cat. No.: 9178.2);
- Blotting filter paper MN 218 B (Macherey-Nagel, Düren, Germany, Cat. No.: 742113);
- Bromophenol blue (Sigma-Aldrich, Munich, Germany, Cat. No.: B0126-25G);
- CoCl2 (cobalt chloride) Merk, Darmstadt, Germany; Cat. No.: 232696-5G);
- FCS (fetal calf serum), 0.2 µm sterile filtered (Pan Biotech, Aidenbach, Germany, Cat. No.: P30-3302);
- Formaldehyde, 37% (Roth, Karlsruhe, Germany, Cat. No.: 6967.2);
- Glycerin (Roth, Karlsruhe, Germany, Cat. No.: 6967.2);
- Glycine (Roth, Karlsruhe, Germany, Cat. No.: T873.2);
- HCl (hydrogen chloride), 37% (Roth, Karlsruhe, Germany, Cat. No.: 4625.1);
- HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (Serva, Heidelberg, Germany; Cat. No.: 25245.02);
- Image-iT Green Hypoxia Reagent (Fisher Scientific, Schwerte, Germany, Cat. No.: 15940773);
- IMDM (Iscove’s Modified Dulbecco’s) Medium (Fisher Scientific, Schwerte, Germany; Cat. No.: GibcoTM21980065);
- JAWS II (American Type Culture Collection (ATCC), Manassas, U.S.A., Cat. No.: CRL-11904);
- KCl (potassium chloride) (Roth, Karlsruhe, Germany, Cat. No.: 6781.1);
- KH2PO4 (potassium hydrogen phosphate) (Roth, Karlsruhe, Germany, Cat. No.: 3904.1);
- L-lactate-assay-kit (Sigma-Aldrich, Cat. No.: MAK329-1KT);
- MINI-Vertical Spacer, 0.75 mm (Roth, Karlsruhe, Germany Cat. No.: N624.1);
- Mouse GM-CSF (granulocyte-macrophage colony-stimulating factor), recombinant protein (Thermo Fisher Scientific, Schwerte, Germany, Cat. No.: PMC2011);
- Na2HPO4 (sodium hydrogen phosphate) (Roth, Karlsruhe, Germany, Cat. No.: 4984.1);
- NaCl (sodium chloride) (Roth, Karlsruhe, Germany, Cat. No.: 3957.1);
- Nitrocellulose membrane, 0.45µm (Hartenstein, Würzburg, Germany, Cat. No.: 10600002);
- Oxygen absorber (O2frepak, Guangdong, China; Distributor: Amazon, Germany, Cat. No.: O2frepak 100CC);
- Penicillin–streptomycin (5000 U/mL) (Thermo Fisher Scientific, Schwerte, Germany, Cat. No.: 15070063);
- Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Schwerte, Germany, Cat. No.: 32209);
- Pipette Tips, 1–200 μL (Thermo Fisher Scientific, Schwerte, Germany, Cat. No.: 11923446);
- Pipette Tips, 200–1000 μL (Greiner Bio-One, Frickenhausen, Germany, Cat. No.: 10557071);
- Polyoxyethylene (20)-sorbitan monolaurate (Tween 20) (VWR, Darmstadt, Germany, Cat. No.: M147-1L);
- Ponceau S (Roth, Karlsruhe, Germany, Cat. No.: 5938.2);
- Prestained protein ladder (Thermo Fisher Scientific, Schwerte, Germany Cat. No.: 26616);
- ProLong™ Gold antifade reagent with DAPI (mounting medium) (Thermo Fisher Scientific/Invitrogen, Schwerte, Germany, Cat. No.: P36931);
- Protease inhibitor (cOmplete ULTRA Tablets, EDTA-free) (Roche, Basel, Switzerland, Distributor: Sigma-Aldrich, Munich, Germany, Cat. No.: 5892953001);
- Scalpel (Roth Karlsruhe, Germany, Cat. No.: X004.1);
- SDS (sodium dodecyl sulfate) (Roth, Karlsruhe, Germany, Cat. No.: 2326.2);
- Skim milk powder (Heirler-Cenovis, Radolfzell, Germany, Cat. No.: 4010318030305);
- TEMED (N, N, N′, N′- tetramethylethylene-diamine) (Roth, Karlsruhe, Germany, Cat. No.: 2367.3);
- Transparent foil (Roth Karlsruhe, Germany, Cat. No.: 1255.1);
- Trichloroacetic acid (TCA)-Deproteinization Kit—Deproteinizing Sample Preparation Kit II (Sigma-Aldrich, Munich, Germany, Cat. No.: MAK342-1KT);
- Tris (Thermo Fisher Scientific, Schwerte, Germany, Cat. No.: 15504020);
- Trypan blue (Roth, Karlsruhe, Germany, Cat. No.: CN76.2);
- Trypsin-EDTA (Ethylenediaminetetraacetic acid) solution (Sigma-Aldrich, Munich, Germany, Cat. No.: E8008-100ML);
- Urea (Roth Karlsruhe, Germany, Cat. No.: 7638.1);
- Vacuum bags (Shenzhen Green Electrical Appliance Co., Ltd, Guangdong, China; Distributor: Amazon; Cat. No.: 11 × 16/100);
- X-ray film, UV/blue sensitive Super RX-N (Fujifilm Europe GmbH, Düsseldorf, Germany, Distributor Hartenstein, Würzburg, Germany, Cat. No.: RF11).
2.2. Equipment
- Bio-Rad Trans-Blot Cell (Bio-Rad Laboratories GmbH, Feldkirchen, Germany, Cat. No.: 1703930);
- Biological safety cabinet—Berner Flow Safe (Berner, Elmshorn, Germany, Cat. No.: B-[MaxPro]2-130);
- Centrifuge—Eppendorf 5420 (Eppendorf, Hamburg, Germany, Cat. No.: 5420000318);
- Centrifuge—Hettich Rotina 380R (Hettich, Vlotho, Germany, Cat. No.: 1706);
- Digital camera—Panasonic DMC-TZ18 Lumix (Panasonic Germany, Wiesbaden, Germany, Cat. No.: EAN 5025232608850);
- ELISA Reader—Sunrise Remote (Tecan, Gröding, Austria, Cat. No.: F039300);
- Film developer—Compact 2 (PROTEC GmbH & Co. KG, Oberstenfeld, Germany, Cat. No.: 11951-1111-5810);
- Fluorescence microscope—Zeiss Axiovert 200 with ApoTome unit (Carl Zeiss Light Microscopy, Göttingen, Germany, Cat. No.: Axiovert 200 including ApoTome 423660-0000-00);
- Heating block—Thermomixer comfort 5355 (Eppendorf, Hamburg, Germany Cat. No.: 022670107);
- Hypoxia Cabin—Whitley H35 Hypoxystation (Don Whitley Scientific Pty Ltd, Bingley, GB, Cat. No.: MEA06060);
- Incubator—Sanyo MCO-19AIC CO2 (Sanyo E & E, Europe B.V., The Netherlands, Cat. No.: 5519188);
- Inverted microscope—ECLIPSE TS100-F (Nikon, Düsseldorf, Germany, Cat. No.: TS100-F);
- Microscope camera—DFK 21AU04.AS (The Imaging Source Europe GmbH, Bremen, Germany, Cat. No.: DFK 41AU02);
- Microscope LED illumination source—CoolLED pe-200 (CoolLED, Andover, G.B., Cat. No.: CoolLED pE-200);
- Neubauer counting chamber (Roth, Karlsruhe, Germany, Cat. No.: T728.1);
- pH meter—Hanna Checker (HANNA Instruments, Vöhringen, Germany, Cat. No.: Z35109);
- Pipette 100–1000 µL (Eppendorf, Hamburg, Germany, Cat. No.: 3123000063);
- Pipette 20–200 µL (Eppendorf, Hamburg, Germany, Cat. No.: 3123000055);
- Pipette 2–20 µL (Eppendorf, Hamburg, Germany, Cat. No.: 3123000098);
- Power supply—Bio-Rad PowerPac Universal Power Supply (Bio-Rad Laboratories GmbH, Feldkirchen, Germany Cat. No.: 1645070);
- Rocking platform—Heidolph Duomax 1030 (Heidolph Instruments GmbH & CO. KG, Schwabach, Germany, Cat. No.: 543-32205-00);
- Ultrasound cleaning unit—Elmasonic S40 (SKSONIC, Mörfelden-Walldorf, Germany, Cat. No.: 1004635);
- Vacuum device—Univapo 150 H & Unijet II Refrigerated Aspirator (Uniequip, Freital, Germany, Cat. No.: Univapo 150 H & Unijet II 20710);
- Vacuum sealer- Kitchenboss (Shenzhen Green Electrical Appliance Co., Ltd, Guangdong, China; Distributor: Amazon, Cat. No.: G200/silver);
- Vertical electrophoresis chamber-Mini-PROTEAN Tetra Cell (Bio-Rad Laboratories GmbH, Feldkirchen, Germany, Cat. No.: 1658000).
3. Procedure
3.1. Preparation and Performance of Hypoxic Cell Culture Experiment—Time for Completion: 5–72 h
- Seed 1 × 105 cells per well in degassed HEPES-buffered complete IMDM (with or without 100 µM CoCl2) in four wells of a 6-well plate. This first step ensures efficient cell adherence and adaptation. CoCl2 blocks any degradation of the hypoxia factor HIF-1α under normoxic conditions [15] when the hypoxic cell culture plate is opened later (see Section 3.2.2 and Section 3.2.3).
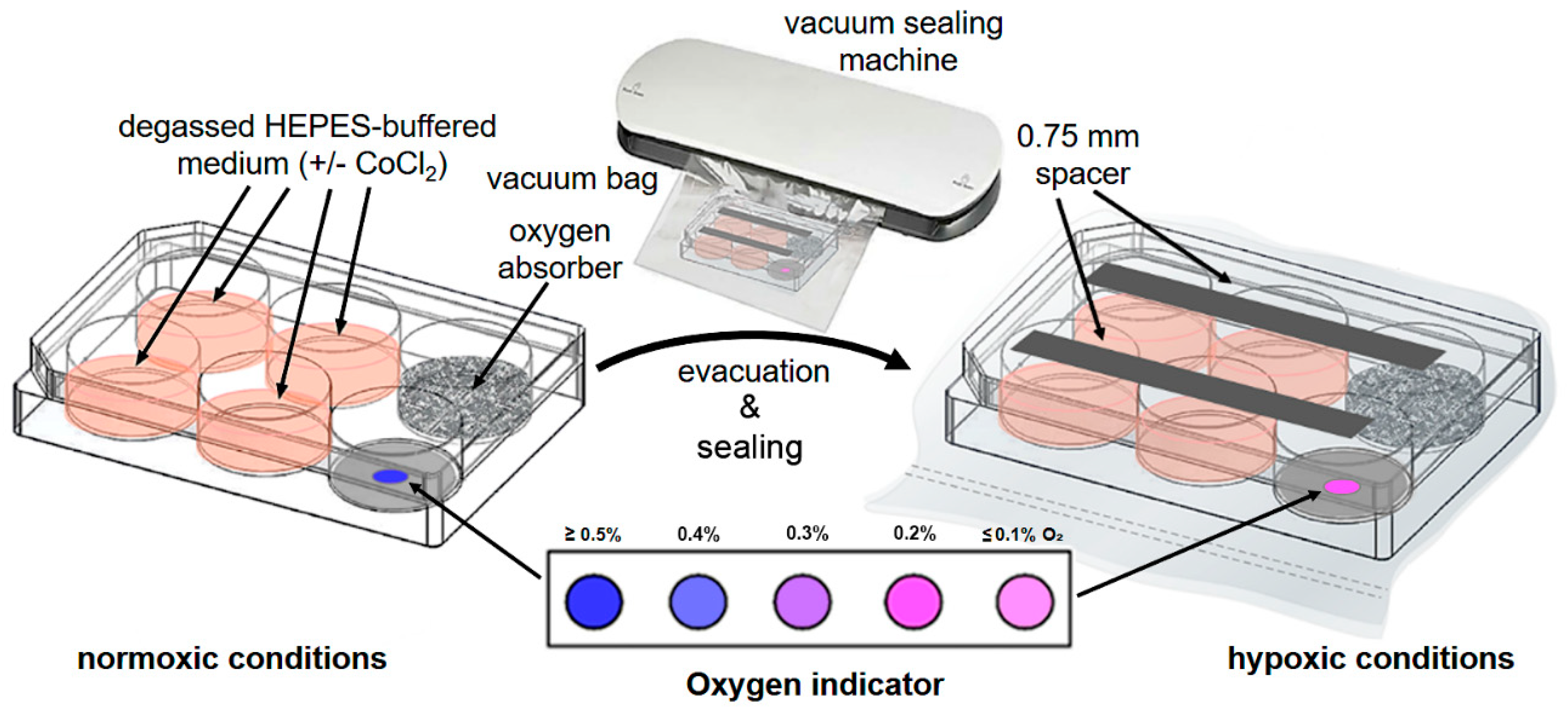
- After 1 h of incubation of the cells under normoxic conditions at 37 °C and 7.5% CO2, add 0.3 g of oxygen absorber to the fifth well and a single AgelessEye indicator to the sixth well of the 6-well plate (Figure 1).
- To allow gas exchange inside the 6-well plate, place two sterile 0.75 mm plastic spacers in a parallel alignment across the top and bottom three wells of the open cell culture plate and close it carefully with the corresponding lid (Figure 1).
- To microscopically verify the hypoxic conditions and the cultured cells’ hypoxic state, supplement one of the cell-containing wells with Image-iT Green Hypoxia Reagent (final concentration of 5 μM). This fluorogenic compound is live cell-permeable and emits a green fluorescence in hypoxic environments.
- Put the assembled cell culture plate into a vacuum bag. Insert the bag’s open end into the vacuum sealing machine and evacuate the air within the experimental unit to a low vacuum (Figure 1).
- Place the assembled and evacuated hypoxic cell culture plate into a standard incubator and cultivate the cells for 5 h (or longer, e.g., 48 or 72 h) at 37 °C. As a control, a 6-well plate with corresponding cells (in the presence and absence of Image-iT Green Hypoxia Reagent) can be cultivated with complete IMDM in parallel for the same time under normoxic conditions. Further, for initial validation of the new hypoxic cell culture method, we additionally used a Whitley H35 hypoxystation (standard setting: ≤2% O2, 7.5% CO2, 90.5% N2) in control experiments.
- After 30 min of incubation, control the oxygen concentration in the sealed culture plates visually by the color of the AgelessEye (it should be constant at 0.2–0.3%, corresponding to the range of oxygen values found in tissues and cells in vivo [1]).
3.2. Experimental Verification of Hypoxic Cell Culture Conditions by Immunofluorescence, Lactate Assay, and Immune Blot Analysis—Time for Completion Depends on Cultivation Period and Respective Downstream Analyses
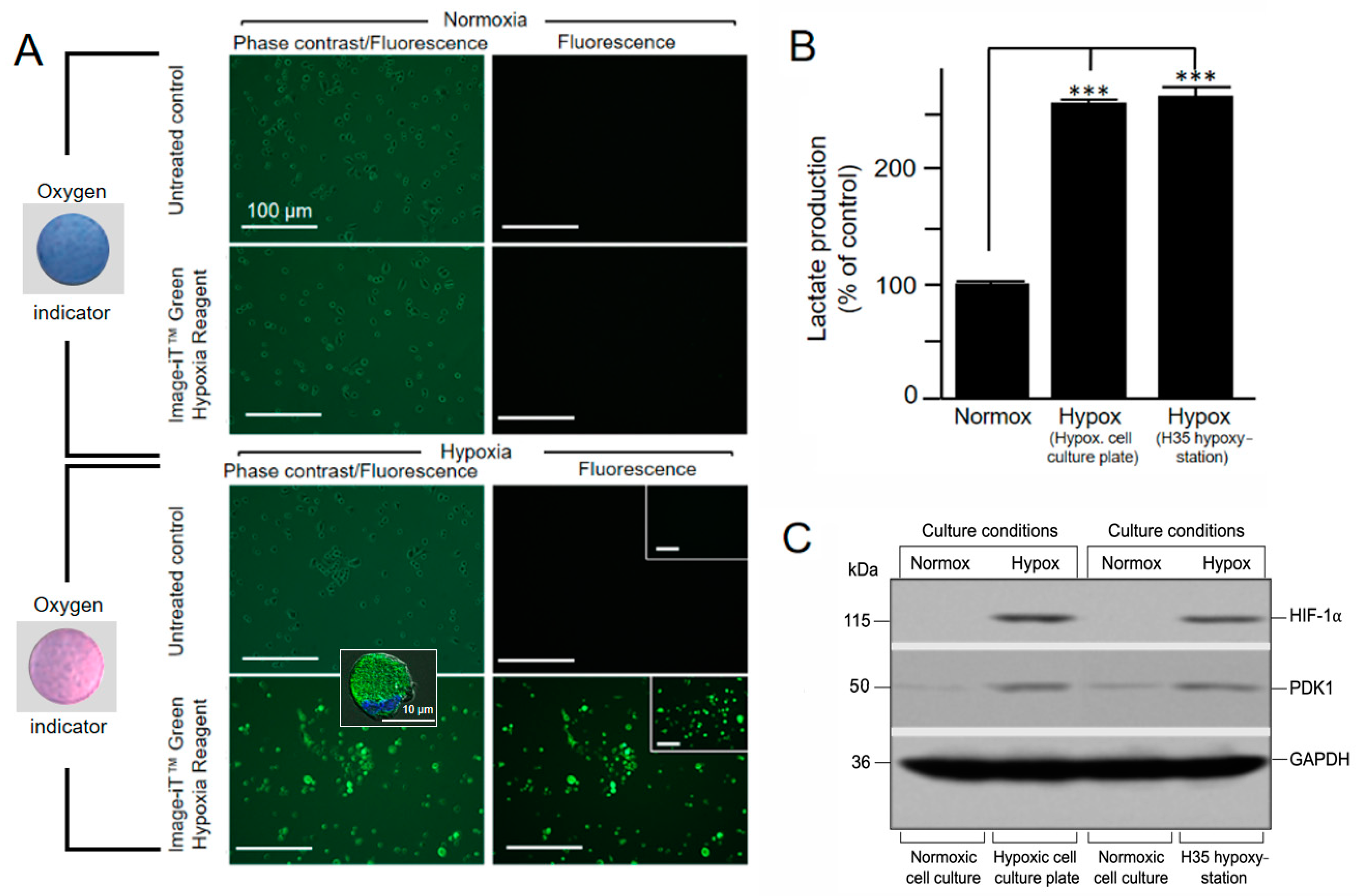
3.2.1. Detection of Low Oxygen Concentration in Living Cells by Hypoxia-Sensitive Fluorescence Dye after 5 h of Hypoxic Cultivation
- After 5 h of hypoxic cultivation, remove the hypoxic cell culture device from the incubator, cut the vacuum bag at the sealed end, and remove the 6-well plate. When exposed to oxygen, the AgelessEye will turn from pink to purple and then blue (≥0.5%).
- To distinguish living from dead cells, dispense 500 µL trypsin-EDTA solution into one of the culture vessels to completely cover the cells and place in the incubator at 37 °C for up to 5 min. This allows the cells to detach from the culture plate surface. This can be checked with an inverted microscope. When this is complete, all cells will be in suspension. Add complete IMDM (Iscove’s Modified Dulbecco’s Medium) containing FCS (Fetal Calf Serum) to the cell suspension to inhibit further tryptic activity. Mix one part of 0.4% trypan blue solution with one part cell suspension. Allow mixture to incubate for 3 min at room temperature and analyze by light microscopy. Do this for later analysis time points (e.g., for 48 and 72 h) as well.
- Image the cells that were pretreated with Image-iT Green Hypoxia Reagent in the vessel using the fluorescence microscope with excitation/emission 488/520 nm (a standard FITC/GFP (Fluorescein Isothiocyanate/Green Fluorescent Protein) excitation/emission filter set is recommended). If necessary, the fluorescent cells can also be fixed with 2% formaldehyde. Fluorescence of the fixed cells lasts max. 24 h (afterward, it is hardly detectable). If cells are also grown on coverslips, they can be viewed at higher magnification with an appropriate fluorescence microscope (e.g., ApoTome microscope) after respective fixation and embedding in mounting medium.
3.2.2. Measurement of Increased Glycolytic Lactate Production after 48 h of Hypoxic Cultivation
- After 48 h of cultivation under norm- and hypoxia, monitor the cells for increased glycolysis via an L-lactate assay.
- Remove the medium from the culture vessel by aspiration and wash cells with cold PBS.
- Dispense 500 µL trypsin-EDTA (Ethylenediamine Tetraacetic Acid) solution into the culture vessel and place it in the incubator at 37 °C for up to 5 min to detach the cells from the culture plate surface. When this is complete, add complete IMDM containing FCS to the cell suspension to inhibit further tryptic activity.
- Subsequently, determine the cell number by a Neubauer counting chamber.
- Wash the detached/harvested cells by centrifugation at 200× g for 5 min and resuspend them in PBS at a concentration of 1 × 105 cells/100 µL.
- After further centrifugation of 300 µL cell suspension at 200× g for 5 min, resuspend the cell pellet in 200 µL of lactate assay buffer provided with the kit.
- Homogenize the cells quickly by pipetting up and down ten times.
- Centrifuge for 5 min at 4 °C and 1000× g in a cold centrifuge to remove insoluble material.
- Deproteinize the resulting supernatants using a protein precipitating TCA (Tricarboxylic acid) kit according to the manufacturer’s protocol.
- For the further steps of the L-lactate assay, follow the detailed protocol of the manufacturer. The colorimetric response of the assay was measured with an ELISA (Enzyme-Linked Immunosorbent Assay) reader at a wavelength of 450 nm.
3.2.3. Detection of CoCl2-Stabilized HIF-1a Levels and PDK1 (Pyruvate Dehydrogenase Kinase 1) Induction in Cell Extracts after 72 h of Hypoxic Cultivation
- For the preparation of cell lysates used for SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis)/immunoblot analysis, detach the cells with a trypsin-EDTA solution as described above.
- Collect the detached cells in a centrifuge tube and centrifuge at 400× g for 5 min at 4 °C.
- Resuspend the resulting cell pellet in 1 mL PBS centrifuge again at 400× g for 5 min at 4 °C. Subsequently, determine the cell number by a Neubauer counting chamber and resuspend the cell pellet in HIF-lysis buffer at a concentration of 1 × 105 cells/100 µL.
- Perform the cell lysis on ice for 30 min. Centrifuge the lysed cells at 1000× g and 4 °C for 30 min. Nuclei and cell debris will form a pellet so that the lysate supernatant can be transferred to a new reaction tube.
- For denaturation and complexation with SDS, add one volume of 2× SDS sample buffer to the cell lysates and boil for 10 min in a heating block at 95 °C.
- Load the polyacrylamide gel with the respective samples (containing an equal quantity of cell lysates (5 µL)) and a molecular weight marker (prestained protein ladder).
- Run the gel at 100 V until the dye front migrates from the stacking into the running gel (15 min) and increase to 200 V until the dye front reaches the bottom of the gel (45 min).
- Remove the gel from the apparatus, spacers, and glass plates and equilibrate it by soaking in transfer buffer for 2 min.
- Prepare the nitrocellulose membrane by wetting it in transfer buffer for 30 s. Handle the membrane carefully, ideally with rounded tweezers, to avoid scratching or puncturing the surface.
- Soak blotting filter papers and sponges in the transfer buffer for 5 min.
- Starting on the side facing the cathode, sequentially assemble the following components: sponge, filter paper, gel, nitrocellulose membrane, filter paper, sponge. Gently remove any air bubbles with a roller or serological pipette. Bubbles between the gel and the membrane will inhibit the transfer of proteins to the membrane.
- Place the completed transfer stack into a transfer cassette and perform wet transfer according to the manufacturer’s instructions for the blotting apparatus.
- After transfer, rinse the membrane briefly in distilled water. Gently mark the position of the molecular weight ladder bands with a pencil for size detection. Using a scalpel, cut the membrane horizontally at the level of the 40 and 70 KDa markers of the prestained protein ladder.
- Stain the membrane with Ponceau S for 30 s and then rinse briefly with distilled water to visualize protein bands and confirm that the protein transfer was successful. Wash away Ponceau S with several washes in PBS until the membrane is clear. Incubate membrane in PBS/0.1% (v/v) Tween 20/5% (w/v) milk powder solution for 1 h at room temperature with constant rocking.
- Dilute the primary antibodies to the working concentration (anti-HIF-1α, 1:500; anti-PDK1, 1:500; anti-GAPDH, 1:1000) in PBS/0.1% Tween 20/10% (v/v) FCS.
- Incubate the membrane in primary antibody solutions for 2 h at room temperature with gentle rocking (the upper part of the membrane with anti-HIF-1α, the middle part with anti-PDK1, and the lower part with anti-GAPDH).
- Wash the membrane with PBS/0.1% (v/v) Tween 20 solution three times for 10 min each with gentle rocking.
- Incubate the membrane with secondary antibody (goat anti-rabbit-HRP, 1:1000; goat anti-mouse-HRP, 1:1000) in PBS/0.1% (v/v) Tween 20 for 1 h at room temperature with gentle rocking (the upper/middle parts with anti-rabbit-HRP, and the lower part with anti-mouse-HRP).
- Wash the membrane in PBS/0.1% (v/v) Tween 20 three times for 10 min each with gentle rocking.
- Prepare the enhanced ECL (chemiluminescence) substrate just before use according to the manufacturer’s instructions.
- According to the manufacturer’s suggestions, incubate the membrane in the substrate (typical incubation times are 10 s to 2 min).
- Carefully remove the membrane from the detection reagent and sandwich it between layers of plastic (i.e., a sheet protector or plastic wrap).
- Expose the membrane to autoradiography film in a dark room.
- After the exposure is complete, place the film into the developer and wait until it is processed.
- Once the film has been developed, overlay it back on your blot to mark the position of the protein ladder with a marker.
4. Expected Results
5. Reagents Setup
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wenger, R.H.; Kurtcuoglu, V.; Scholz, C.C.; Marti, H.H.; Hoogewijs, D. Frequently asked questions in hypoxia research. Hypoxia 2015, 3, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Palazon, A.; Goldrath, A.W.; Nizet, V.; Johnson, R.S. HIF Transcription factors, inflammation, and immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Krzywinska, E.; Stockmann, C. Hypoxia, metabolism and immune cell function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Werth, N.; Beerlage, C.; Rosenberger, C.; Yazdi, A.S.; Edelmann, M.; Amr, A.; Bernhardt, W.; von Eiff, C.; Becker, K.; Schafer, A.; et al. Activation of hypoxia inducible Factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE 2010, 5, e11576. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Xie, C.; Jiang, C.T. The role of hypoxia-inducible factors in metabolic diseases. Nat. Rev. Endocrinol. 2018, 15, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002, 16, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef]
- Braga, L.R.; Sarantopoulos, C.I.G.L.; Peres, L.; Braga, J.W.B. Evaluation of absorption kinetics of oxygen scavenger sachets using response surface methodology. Packag. Technol. Sci. 2010, 23, 351–361. [Google Scholar] [CrossRef]
- Miltz, J.; Perry, M. Evaluation of the performance of iron-based oxygen scavengers, with comments on their optimal applications. Packag. Technol. Sci. 2005, 18, 21–27. [Google Scholar] [CrossRef]
- Twigg, R.S. Oxidation-reduction aspects of resazurin. Nature 1945, 155, 401–402. [Google Scholar] [CrossRef]
- Dai, Z.J.; Gao, J.; Ma, X.B.; Yan, K.; Liu, X.X.; Kang, H.F.; Ji, Z.Z.; Guan, H.T.; Wang, X.J. Up-regulation of hypoxia inducible factor-1alpha by cobalt chloride correlates with proliferation and apoptosis in PC-2 cells. J. Exp. Clin. Cancer Res. 2012, 31, 28. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell. Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Bakmiwewa, S.M.; Heng, B.; Guillemin, G.J.; Ball, H.J.; Hunt, N.H. An effective, low-cost method for achieving and maintaining hypoxia during cell culture studies. Biotechniques 2015, 59, 223–229. [Google Scholar] [CrossRef]
- Martinez, C.A.; Cistulli, P.A.; Cook, K.M. A Cell culture model that mimics physiological tissue oxygenation using oxygen-permeable membranes. Bio Protoc. 2019, 9, e3371. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jin, F.; Zhong, H. A novel experimental hypoxia chamber for cell culture. Am. J. Cancer Res. 2014, 4, 53–60. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthiesen, S.; Jahnke, R.; Knittler, M.R. A Straightforward Hypoxic Cell Culture Method Suitable for Standard Incubators. Methods Protoc. 2021, 4, 25. https://doi.org/10.3390/mps4020025
Matthiesen S, Jahnke R, Knittler MR. A Straightforward Hypoxic Cell Culture Method Suitable for Standard Incubators. Methods and Protocols. 2021; 4(2):25. https://doi.org/10.3390/mps4020025
Chicago/Turabian StyleMatthiesen, Svea, Rico Jahnke, and Michael R. Knittler. 2021. "A Straightforward Hypoxic Cell Culture Method Suitable for Standard Incubators" Methods and Protocols 4, no. 2: 25. https://doi.org/10.3390/mps4020025
APA StyleMatthiesen, S., Jahnke, R., & Knittler, M. R. (2021). A Straightforward Hypoxic Cell Culture Method Suitable for Standard Incubators. Methods and Protocols, 4(2), 25. https://doi.org/10.3390/mps4020025





