Mapping Quantitative Trait Loci onto Chromosome-Scale Pseudomolecules in Flax
Abstract
1. Introduction
2. Materials and Methods
2.1. The Most Recent Release of the Chromosome-Scale Pseudomolecules
2.2. Marker Infomation of QTL in Flax
2.3. Mapping PCR-Based Markers to the Most Recent Release of the Chromosome-Scale Pseudomolecules
2.4. Mapping SNPs to the Most Recent Release of the Chromosome-Scale Pseudomolecules
2.5. Grouping QTL to Clusters
2.6. Candidate Gene Analysis Based on the Most Recent Release of the Chromosome-Scale Pseudomolecules
3. Results
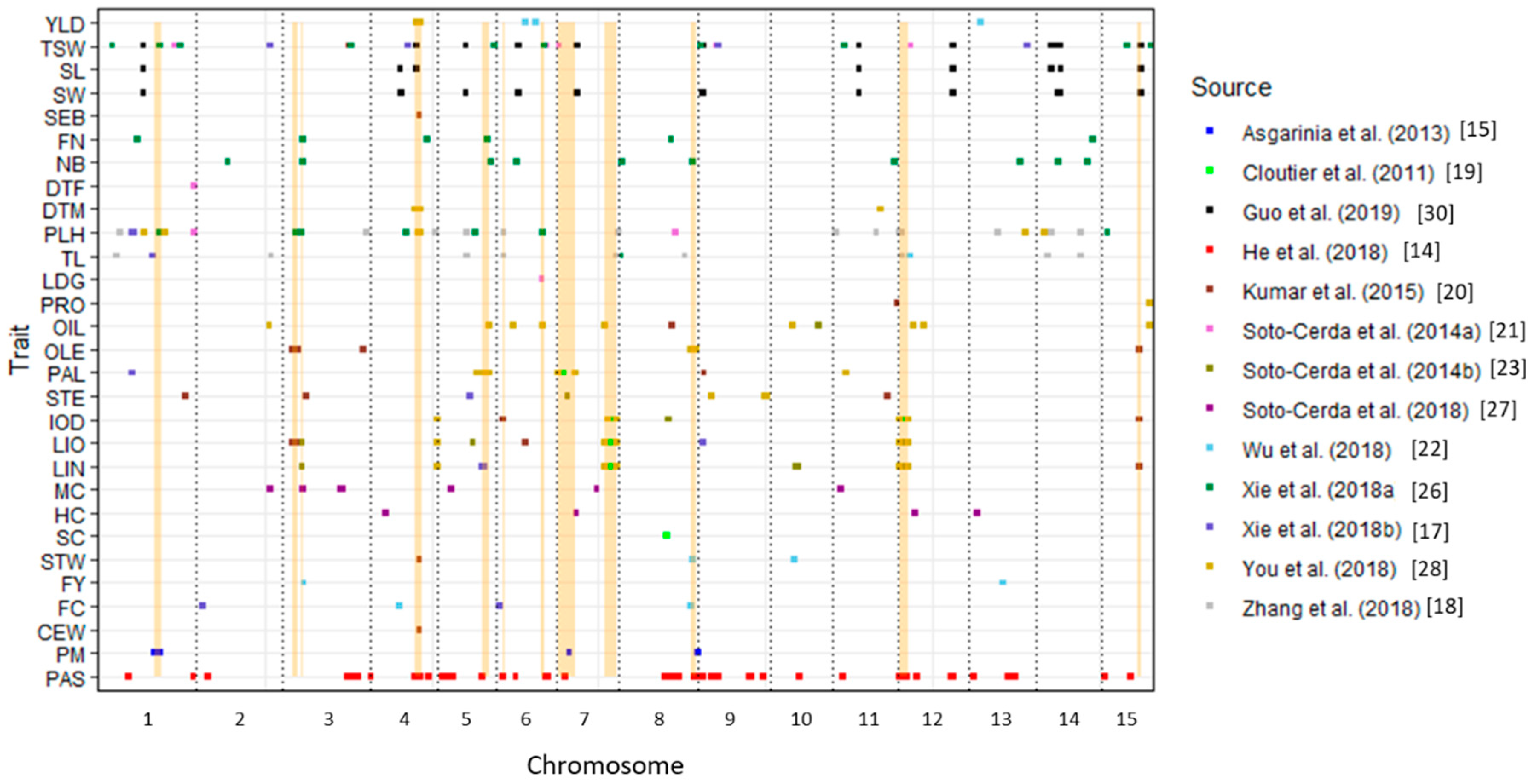
3.1. Mapping QTL onto the Most Recent Release of the Chromosome-Scale Pseudomolecules
3.2. Identical or Co-Located QTL
3.3. Candidate Genes for QTL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falconer, D.S. Introduction to Quantitative Genetics; Oliver & Boyd: Edinburgh/London, UK, 1960. [Google Scholar]
- Sehgal, D.; Singh, R.; Rajpal, V.R. Quantitative trait loci mapping in plants: Concepts and approaches. In Molecular Breeding for Sustainable Crop Improvement; Rajpal, V.R., Rao, S.R., Raina, S.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 11, pp. 31–59. [Google Scholar]
- Price, A.H. Believe it or not, QTLs are accurate! Trends Plant Sci. 2006, 11, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Holland, J.B.; McMullen, M.D.; Buckler, E.S. Genetic design and statistical power of nested association mapping in maize. Genetics 2008, 178, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Monir, M.M.; Zhu, J. Dominance and epistasis interactions revealed as important variants for leaf traits of maize NAM population. Front. Plant Sci. 2018, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Fang, X.; Jiang, P.; Zhang, G.; Hu, J.; Wang, X.; Meng, Q.; Cui, W.; Lan, S.; Ma, X.; et al. Genetic architecture of nitrogen-deficiency tolerance in wheat seedlings based on a nested association mapping (NAM) population. Front. Plant Sci. 2018, 9, 845. [Google Scholar] [CrossRef]
- Cavanagh, C.; Morell, M.; Mackay, I.; Powell, W. From mutations to MAGIC: Resources for gene discovery, validation and delivery in crop plants. Curr. Opin. Plant Biol. 2008, 11, 215–221. [Google Scholar] [CrossRef]
- Mackay, I.; Powell, W. Methods for linkage disequilibrium mapping in crops. Trends Plant Sci. 2007, 12, 57–63. [Google Scholar] [CrossRef]
- Camargo, A.V.; Mackay, I.; Mott, R.; Han, J.; Doonan, J.H.; Askew, K.; Corke, F.; Williams, K.; Bentley, A.R. Functional mapping of quantitative trait loci (QTLs) associated with plant performance in a wheat MAGIC mapping population. Front. Plant Sci. 2018, 9, 887. [Google Scholar] [CrossRef]
- Ongom, P.O.; Ejeta, G. Mating design and genetic structure of a multi-parent advanced generation intercross (MAGIC) population of sorghum (Sorghum bicolor (L.) Moench). Genes Genomes Genet. G3 2018, 8, 331–341. [Google Scholar] [CrossRef]
- Cloutier, S.; Miranda, E.; Ward, K.; Radovanovic, N.; Reimer, E.; Walichnowski, A.; Datla, R.; Rowland, G.; Duguid, S.; Ragupathy, R. Simple sequence repeat marker development from bacterial artificial chromosome end sequences and expressed sequence tags of flax (Linum usitatissimum L.). Theor. Appl. Genet. 2012, 125, 685–694. [Google Scholar] [CrossRef]
- Cloutier, S.; Niu, Z.; Datla, R.; Duguid, S. Development and analysis of EST-SSRs for flax (Linum usitatissimum L.). Theor. Appl. Genet. 2009, 119, 53–63. [Google Scholar] [CrossRef]
- Kumar, S.; You, F.M.; Cloutier, S. Genome wide SNP discovery in flax through next generation sequencing of reduced representation libraries. BMC Genom. 2012, 13, 684. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xiao, J.; Rashid, K.Y.; Yao, Z.; Li, P.; Jia, G.; Wang, X.; Cloutier, S.; You, F.M. Genome-wide association studies for pasmo resistance in flax (Linum usitatissimum L.). Front. Plant Sci. 2018, 9, 1982. [Google Scholar] [CrossRef] [PubMed]
- Asgarinia, P.; Cloutier, S.; Duguid, S.; Rashid, K.; Mirlohi, A.; Banik, M.; Saeidi, G. Mapping quantitative trait loci for powdery mildew resistance in flax (Linum usitatissimum L.). Crop Sci. 2013, 53, 2462–2472. [Google Scholar] [CrossRef]
- Cloutier, S.; Ragupathy, R.; Miranda, E.; Radovanovic, N.; Reimer, E.; Walichnowski, A.; Ward, K.; Rowland, G.; Duguid, S.; Banik, M. Integrated consensus genetic and physical maps of flax (Linum usitatissimum L.). Theor. Appl. Genet. 2012, 125, 1783–1795. [Google Scholar] [CrossRef]
- Xie, D.; Dai, Z.; Yang, Z.; Tang, Q.; Sun, J.; Yang, X.; Song, X.; Lu, Y.; Zhao, D.; Zhang, L.; et al. Genomic variations and association study of agronomic traits in flax. BMC Genom. 2018, 19, 512. [Google Scholar] [CrossRef]
- Zhang, J.; Long, Y.; Wang, L.; Dang, Z.; Zhang, T.; Song, X.; Dang, Z.; Pei, X. Consensus genetic linkage map construction and QTL mapping for plant height-related traits in linseed flax (Linum usitatissimum L.). BMC Plant Biol. 2018, 18, 160. [Google Scholar] [CrossRef]
- Cloutier, S.; Ragupathy, R.; Niu, Z.; Duguid, S. SSR-based linkage map of flax (Linum usitatissimum L.) and mapping of QTLs underlying fatty acid composition traits. Mol. Breed. 2011, 28, 437–451. [Google Scholar] [CrossRef]
- Kumar, S.; You, F.M.; Duguid, S.; Booker, H.; Rowland, G.; Cloutier, S. QTL for fatty acid composition and yield in linseed (Linum usitatissimum L.). Theor. Appl. Genet. 2015, 128, 965–984. [Google Scholar] [CrossRef]
- Soto-Cerda, B.J.; Duguid, S.; Booker, H.; Rowland, G.; Diederichsen, A.; Cloutier, S. Genomic regions underlying agronomic traits in linseed (Linum usitatissimum L.) as revealed by association mapping. J. Integr. Plant Biol. 2014, 56, 75–87. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Q.; Zhang, L.; Li, S.; Ma, Y.; Pan, L.; Lin, H.; Wu, G.; Yuan, H.; Yu, Y.; et al. QTL mapping of fiber-related traits based on a high-density genetic map in flax (Linum usitatissimum L.). Front. Plant Sci. 2018, 9, 885. [Google Scholar] [CrossRef]
- Soto-Cerda, B.J.; Duguid, S.; Booker, H.; Rowland, G.; Diederichsen, A.; Cloutier, S. Association mapping of seed quality traits using the Canadian flax (Linum usitatissimum L.) core collection. Theor. Appl. Genet. 2014, 127, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Spielmeyer, W.; Green, A.G.; Bittisnich, D.; Mendham, N.; Lagudah, E.S. Identification of quantitative trait loci contributing to Fusarium wilt resistance on an AFLP linkage map of flax (Linum usitatissimum). Theor. Appl. Genet. 1998, 97, 633–641. [Google Scholar] [CrossRef]
- Wang, Z.; Hobson, N.; Galindo, L.; Zhu, S.; Shi, D.; McDill, J.; Yang, L.; Hawkins, S.; Neutelings, G.; Datla, R.; et al. The genome of flax (Linum usitatissimum) assembled de novo from short shotgun sequence reads. Plant J. 2012, 72, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Dai, Z.; Yang, Z.; Sun, J.; Zhao, D.; Yang, X.; Zhang, L.; Tang, Q.; Su, J. Genome-wide association study identifying candidate genes influencing important agronomic traits of flax (Linum usitatissimum L.) using SLAF-seq. Front. Plant Sci. 2018, 8, 2232. [Google Scholar] [CrossRef]
- Soto-Cerda, B.J.; Cloutier, S.; Quian, R.; Gajardo, H.A.; Olivos, M.; You, F.M. Genome-wide association analysis of mucilage and hull content in flax (Linum usitatissimum L.) seeds. Int. J. Mol. Sci. 2018, 19, 2870. [Google Scholar] [CrossRef]
- You, F.M.; Xiao, J.; Li, P.; Yao, Z.; Jia, G.; He, L.; Kumar, S.; Soto-Cerda, B.; Duguid, S.D.; Booker, H.M.; et al. Genome-wide association study and selection signatures detect genomic regions associated with seed yield and oil quality in flax. Int. J. Mol. Sci. 2018, 19, 2303. [Google Scholar] [CrossRef]
- You, F.M.; Xiao, J.; Li, P.; Yao, Z.; Jia, G.; He, L.; Zhu, T.; Luo, M.-C.; Wang, X.; Deyholos, M.K.; et al. Chromosome-scale pseudomolecules refined by optical, physical, and genetic maps in flax. Plant J. 2018, 95, 371–384. [Google Scholar] [CrossRef]
- Guo, D.; Jiang, H.; Yan, W.; Yang, L.; Ye, J.; Wang, Y.; Yan, Q.; Chen, J.; Gao, Y.; Duan, L.; et al. Resequencing 200 flax cultivated accessions identifies candidate genes related to seed size and weight and reveals signatures of artificial selection. Front. Plant Sci. 2019, 10, 1682. [Google Scholar] [CrossRef]
- Schuler, G.D. Sequence mapping by electronic PCR. Genome Res. 1997, 7, 541–550. [Google Scholar] [CrossRef]
- Appels, R.; Eversole, K.; Feuillet, C.; Keller, B.; Rogers, J.; Stein, N.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; Poland, J.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
| Category | No | Trait | Abbreviation | Total QTL Identified | Total Unique QTL | Source |
|---|---|---|---|---|---|---|
| Seed yield and agronomic traits | 1 | Seed yield | YLD | 5 | 4 | [20,22,28] |
| 2 | Thousand seed weight (g) | TSW | 45 | 44 | [17,21,22,26,30] | |
| 3 | Seed length (mm) | SL | 10 | 10 | [30] | |
| 4 | Seed width (mm) | SW | 15 | 15 | [30] | |
| 5 | Seeds per boll | SEB | 1 | 1 | [20] | |
| 6 | Fruit (boll) number | FN | 9 | 8 | [17,26] | |
| 7 | Branching score | BSC | 1 | 1 | [21] | |
| 8 | Number of branches | NB | 13 | 13 | [26] | |
| 9 | Days to flowering | DTF | 1 | 1 | [21] | |
| 10 | Days to maturity | DTM | 3 | 2 | [20,28] | |
| 11 | Plant height (cm) | PLH | 33 | 30 | [18,21,22,26,28] | |
| 12 | Technical length (cm) | TL | 17 | 13 | [17,18,22,26] | |
| 13 | Lodging | LDG | 2 | 1 | [21] | |
| Seed quality | 14 | Iodine value | IOD | 8 | 7 | [19,20,23,28] |
| 15 | Protein content (%) | PRO | 2 | 2 | [20,28] | |
| 16 | Oil content (%) | OIL | 10 | 10 | [20,23,28] | |
| 17 | Oleic (%) | OLE | 4 | 4 | [20,28] | |
| 18 | Palmitic (%) | PAL | 7 | 5 | [17,19,20,28] | |
| 19 | Stearic (%) | STE | 8 | 7 | [17,20,23,28] | |
| 20 | Linoleic (%) | LIO | 11 | 9 | [17,19,20,23,28] | |
| 21 | Linolenic (%) | LIN | 12 | 10 | [17,19,20,23,28] | |
| 22 | Seed mucilage content | MC | 7 | 7 | [27] | |
| 23 | Seed hull content | HC | 4 | 4 | [27] | |
| 24 | Seed colour | SC | 2 | 1 | [19] | |
| Fibre | 25 | Straw weight (g) | STW | 4 | 4 | [20,22] |
| 26 | Fibre yield (g) | FY | 2 | 2 | [22] | |
| 27 | Fibre content (%) | FC | 4 | 4 | [17,22] | |
| 28 | Cell walls (%) | CEW | 1 | 1 | [20] | |
| Disease | 29 | Fusarium wilt rating | FW | 2 | 2 | [24] |
| 30 | Powdery mildew rating | PM | 3 | 3 | [15] | |
| 31 | Pasmo rating | PAS | 67 | 67 | [14] |
| Population | Pop Size | Markers | Method 1 | Ref 2 | Total QTL | No. of QTL Identified/Trait 3 | Source |
|---|---|---|---|---|---|---|---|
| DH | 59 | 8 RFLPs, 213 AFLPs | LM | GM | 2 | 2/FW | [24] |
| DH | 78 | 113 SSRs, 5 SNPs, 4 genes | LM | GM | 9 | 2/LIO, LIN, IOD; 1/PAL; 2/SC | [19] |
| F3-F4 | 300 | 143 SSRs | LM | GM | 3 | 3/PM | [15] |
| Core collection | 390 | 464 SSRs | AM | GM | 11 | 5/TSW; 1/DTF; 2/PLH; 1/BSC; 2/LDG | [21] |
| Core collection | 390 | 460 SSRs | AM | GM | 9 | 1/OIL; 1/STE; 3/LIO; 3/LIN; 1/IOD | [23] |
| RIL | 243 | 329 SNPs, 362 SSRs | LM | GM | 20 | 1/PAL; 3/STE; 3/OLE;2/LIO; 1/LIN; 2/IOD; 1/OIL; 1/PRO; 1/CEW; 1/STW; 1/TSW; 1/SEB; 1/YLD; 1/DTM | [20] |
| 2 RILs | 233 | 4,497 SNPs | LM | GM | 24 | 14/PLH; 10/TL | [18] |
| F2 | 112 | 2,339 SNPs | LM | GM | 12 | 1/PLH; 1/TL; 3/YLD; 3/STW; 2/FY; 2/FC | [22] |
| Core collection | 224 | 146,959 SNPs | AM | SS | 43 | 9/PLH; 3/TL; 13/NB; 8/FN; 10/TSW | [26] |
| Core collection | 224 | 584,987 SNPs | AM | SS | 23 | 2/PLH; 1/FN; 8/TSW; 3/TL; 1/PAL; 2/STE; 1/LIO; 3/LIN; 2/FC | [17] |
| Core collection | 200 | 771,914 SNPs | AM | PCPs | 11 | 7/MC; 4/HC | [27] |
| 2 RILs and 1 DH | 260 | 17,288 SNPs | AM | PCPs | 33 | 1/YLD; 8/OIL; 5/PLH; 4/PAL; 3/IOD, LIN, LIO, 2/DTM; 2/STE; 1/PRO; 1/OLE | [28] |
| Core collection | 370 | 258,873 SNPs | AM | RCPs | 67 | 67 PAS | [14] |
| Germplasm collection | 200 | 674,074 SNPs | AM | RCPs | 46 | 10/SL; 15/SW; 21/TSW | [30] |
| QTL No | Trait | QTL/Marker ID | LG/Scaffold | Flanking Markers | Chr | Coordinates on chr | Co-Location | Source |
|---|---|---|---|---|---|---|---|---|
| 1 | FW | afB13 | 6 | afB13 | NA | NA | NA | [24] |
| 2 | afXR6 | 10 | afXR6 | NA | NA | NA | ||
| 3 | LIO | QLio.crc-LG7 | 7 | FAD3A/Lu44E4 | 7 | 16089395-16092602 | 70 | [19] |
| 4 | QLio.crc-LG16 | 16 | Lu206-Lu765B | 12 | 2036216-2041030 | 109 | ||
| 5 | LIN | QLin.crc-LG7 | 7 | FAD3A/Lu44E4 | 7 | 16089395-16092602 | 70 | |
| 6 | QLin.crc-LG16 | 16 | Lu206-Lu765B | 12 | 2036216-2041030 | 109 | ||
| 7 | IOD | QIod.crc-LG7 | 7 | FAD3A/Lu44E4 | 7 | 16089395-16092602 | 70 | |
| 8 | QIod.crc-LG16 | 16 | Lu206-Lu765B | 12 | 2038322-2038517 | 109 | ||
| 9 | PAL | QPal.crc-LG9 | 9 | Lu741-Lu675 | 7 | 1518897-2017169 | 66 | |
| 10 | SC | QL*.crc-LG22 | 22 | Colour-Lu178 | 8 | 14838877-14839100 | 75 | |
| 11 | Qb*.crc-LG22 | 22 | Colour-Lu178 | 8 | 14838877-14839100 | 75 | ||
| 12 | PM | QPM-crc-LG1 | 1 | Lu2698-Lu2712 | 1 | 16920407-18739647 | 11 | [15] |
| 13 | QPM-crc-LG7 | 7 | Lu2810-Lu2832 | 7 | 3817603-3817863 | 66 | ||
| 14 | QPM-crc-LG9 | 9 | Lu1125a-Lu932 | 9 | 357191-357510 | 83 | ||
| 15 | TSW | 3 | Lu2164 | 1 | 22948222-22948580 | 13 | [21] | |
| 16 | 6 | Lu2555 | 6 | 14948801-14948986 | 65 | |||
| 17 | 7 | Lu2532 | 7 | 661757-662020 | 66 | |||
| 18 | 7 | Lu58a | 12 | 3802629-3802807 | 111 | |||
| 19 | 9 | Lu526 | 9 | 5936422-5936694 | 88 | |||
| 20 | DTF | 1 | Lu943 | 1 | 28800644-28800902 | 16 | ||
| 21 | PLH | 1 | Lu943 | 1 | 28800644-28800902 | 16 | ||
| 22 | Lu316 | 8 | 17106045-17106266 | 79 | ||||
| 23 | BSC | 22 | Lu2067a | NA | NA | |||
| 24 | LDG | 6 | Lu2560 | 6 | 13553559-13553779 | 63 | ||
| 25 | 6 | Lu2564 | 6 | 13620999-13621234 | 63 | |||
| 26 | OIL | QOil-LG9.1 | 9 | c31-s67_Lu181 | 10 | 14217309-14219605 | 95 | [23] |
| 27 | STE | QSte-LG7.1 | 7 | c175-s1216_Lu146 | 7 | 3308199-3308517 | 66 | |
| 28 | LIO | QLio-LG3.1 | 3 | c729-s156_Lu3262 | 3 | 6080016-6080189 | 24 | |
| 29 | QLio-LG5.2 | 5 | c30-s11_Lu164 | 5 | 10600927-10601125 | 47 | ||
| 30 | QLio-LG12.3 | 12 | c306-s98_Lu765B | 12 | 2036216-2041030 | 109 | ||
| 31 | LIN | QLin-LG3.1 | 3 | c729-s156_Lu3262 | 3 | 6080016-6080189 | 24 | |
| 32 | QLin-LG5.2 | 5 | c202-s39_Lu41 | 10 | 7602629-8066018 | 94 | ||
| 33 | QLin-LG12.3 | 12 | c306-s98_Lu765B | 12 | 2036216-2041030 | 109 | ||
| 34 | IOD | QIod-LG8.1 | 8 | c46-s505_Lu2102 | 8 | 15166626-15166926 | 76 | |
| 35 | PAL | QPal.BM.crc-LG7 | 7 | Lu402/Lu7-1820805 | 9 | 2026186-2026487 | 86 | [20] |
| 36 | STE | QSte.BM.crc-LG1 | 1 | Lu2183a/Lu1-2670961 | 1 | 26435050-26435329 | 15 | |
| 37 | QSte.BM.crc-LG3 | 3 | Lu3-8415336/Lu2164 | 3 | 7263087 | 28 | ||
| 38 | QSte.BM.crc-LG11 | 11 | Lu2128/Lu11-19000928 | 11 | 16797707-16797907 | 102 | ||
| 39 | OLE | QOle.BM.crc-LG3-1 | 3 | Lu3-3979616/Lu3-5950394 | 3 | 3231616-4799670 | 22 | |
| 40 | QOle.BM.crc-LG3-2 | 3 | Lu658/Lu3150 | 3 | 24238080-24238427 | 33 | ||
| 41 | QOle.BM.crc-LG5 | 5 | Lu5-9728492 | 15 | 11375006 | 131 | ||
| 42 | LIO | QLio.BM.crc-LG3 | 3 | Lu3-3979616/Lu3-5950394 | 3 | 3231616-4799670 | 22 | |
| 43 | QLio.BM.crc-LG6 | 6 | Lu2545 | 6 | 8616550-8616919 | 61 | ||
| 44 | LIN | QLin.BM.crc-LG5 | 5 | Lu5-9728492 | 15 | 11375006 | 131 | |
| 45 | IOD | QIod.BM.crc-LG5 | 5 | Lu5-9728492 | 15 | 11375006 | 131 | |
| 46 | QIod.BM.crc-LG6 | 6 | Lu6-2260313/Lu6-2330258 | 6 | 2018434-2088579 | 57 | ||
| 47 | OIL | QOil.BM.crc-LG8 | 8 | Lu8-22516618/Lu3189 | 8 | 16363106-16363334 | 78 | |
| 48 | PRO | QPro.BM.crc-LG11 | 11 | Lu11-21716266/Lu52 | 11 | 19594198-19594398 | 105 | |
| 49 | CEW | QCw.BM.crc-LG4 | 4 | Lu2031 | 4 | 14489225-14489333 | 40 | |
| 50 | STW | QSw.BM.crc-LG4 | 4 | Lu2031 | 4 | 14489225-14489333 | 40 | |
| 51 | TSW | QTsw.BM.crc-LG15 | 15 | Lu2010a/Lu2001 | 3 | 20394564-20394673 | 31 | |
| 52 | SEB | QSpb.BM.crc-LG4 | 4 | Lu2031 | 4 | 14489225-14489333 | 40 | |
| 53 | YLD | QYld.BM.crc-LG4 | 4 | Lu2031 | 4 | 14489225-14489333 | 40 | |
| 54 | DTM | QDm.BM.crc-LG4 | 4 | Lu2031 | 4 | 14489225-14489333 | 40 | |
| 55 | PLH | uq.C1–1 | Lu1_396428 | 1 | 6539309-6539089 | 3 | [18] | |
| 56 | uq.C3–1 | Lu3_693423 | 3 | 25295008-25294801 | 34 | |||
| 57 | uq.C4–1 | Lu4_300701 | 4 | 19453432-19453704 | 42 | |||
| 58 | uq.C5–1 | Lu5_8504 | 5 | 8681823-8682018 | 45 | |||
| 59 | uq.C6–1 | Lu6_639236 | 6 | 2175711-2175911 | 57 | |||
| 60 | uq.C8–2 | Lu8_185009 | 7 (4) | 6427466-6427621 (6238294-6238449) | ||||
| 61 | uq.C8–3 | Lu8_119488 | 8 | 28706-28938 | 72 | |||
| 62 | uq.C9–1 | Lu9_503128 | 14 | 4498680-4498955 | 122 | |||
| 63 | uq.C11–1 | Lu11_557617 | 11 | 1276828-1277143 | 96 | |||
| 64 | uq.C11–1 | Lu11_447048 | 11 | 13338945-13339276 | 100 | |||
| 65 | uq.C12–1 | Lu12_696508 | 12 | 1004697-1004929 | 108 | |||
| 66 | uq.C12–1 | Lu12_163596 | 12 | 351979-352221 | 106 | |||
| 67 | uq.C13–1 | Lu13_367183 | 13 | 8997700-8998007 | 115 | |||
| 68 | uq.C14–1 | Lu14_231853 | 14 | 13485754-13486113 | 126 | |||
| 69 | TL | uq.C1–1 | Lu1_695389 | 1 | 5664124-5664330 | 2 | ||
| 70 | uq.C2–2 | Lu2_597057 | 2 | 22508975-22508683 | 21 | |||
| 71 | uq.C5–1 | Lu5_8504 | 5 | 8681823-8682018 | 45 | |||
| 72 | uq.C6–1 | Lu6_639236 | 6 | 2175711-2175911 | 57 | |||
| 73 | uq.C7–1 | Lu7_781312 | 7 | 18087445-18087733 | 71 | |||
| 74 | uq.C8–1 | Lu8_646184 | 8 | 20045574-20045815 | 80 | |||
| 75 | uq.C8–2 | Lu8_185009 | 7 (4) | 6427466-6427621 (6238294-6238449) | ||||
| 76 | uq.C9–2 | Lu9_618122 | 14 | 3378716-3378969 | 121 | |||
| 77 | uq.C12–1 | Lu12_696508 | 12 | 1004697-1004929 | 108 | |||
| 78 | uq.C14–1 | Lu14_231853 | 14 | 13485754-13486113 | 126 | |||
| 79 | PLH | Marker4371 | scaffold156 (LG1) | 3 | 6019156-6019499 | 24 | [22] | |
| 80 | TL | Marker747228 | scaffold2786 (LG8) | 12 | 3620608-3620934 | 110 | ||
| 81 | YLD | Marker799956 | scaffold319 (LG10) | 13 | 3856362-3856771 | 114 | ||
| 82 | Marker770415 | scaffold117 (LG12) | 6 | 11929857-11930253 | 62 | |||
| 83 | Marker1073071 | scaffold27 (LG12) | 6 | 8701939-8702324 | 61 | |||
| 84 | STW | Marker326151 | scaffold33 (LG5) | 8 | 22241866-22242226 | 81 | ||
| 85 | Marker2368217 | scaffold355 (LG15) | 10 | 7140622-7140988 | 92 | |||
| 86 | Marker614116 | scaffold355 (LG15) | 10 | 7219061-7219445 | 93 | |||
| 87 | FY | Marker2603286 | scaffold156 (LG1) | 3 | 6573623-6574023 | 27 | ||
| 88 | Marker1722134 | scaffold127 (LG11) | 13 | 10603161-10603485 | 116 | |||
| 89 | FC | Marker1051901 | scaffold680 (LG5) | 8 | 21807786-21808148 | 81 | ||
| 90 | Marker1561746 | scaffold376 (LG11) | 4 | 8748431-8748795 | 36 | |||
| 91 | PLH | scaffold112_114241 | scaffold112 | scaffold112_114241 | 1 | 18444086 | 11 | [26] |
| 92 | scaffold1491_318496 | scaffold1491 | scaffold1491_318496 | 6 | 14006651 | 63 | ||
| 93 | scaffold31_1800846 | scaffold31 | scaffold31_1800846 | 3 | 3929932 | 22 | ||
| 94 | scaffold344_309662 | scaffold344 | scaffold344_309662 | 1 | 11008279 | 6 | ||
| 95 | scaffold51_1349321 | scaffold51 | scaffold51_1349321 | 4 | 10532424 | 37 | ||
| 96 | scaffold59_572553 | scaffold59 | scaffold59_572553 | 1 | 10051709 | 4 | ||
| 97 | scaffold156_641874 | scaffold156 | scaffold156_641874 | 3 | 5906791 | 23 | ||
| 98 | scaffold147_367986 | scaffold147 | scaffold147_367986 | 5 | 11288517 | 48 | ||
| 99 | scaffold859_123972 | scaffold859 | scaffold859_123972 | 15 | 1939372 | 129 | ||
| 100 | TL | scaffold297_275113 | scaffold297 | scaffold297_275113 | 1 | 16435852 | 9 | |
| 101 | scaffold361_14957 | scaffold361 | scaffold361_14957 | 1 | 16726904 | 10 | ||
| 102 | scaffold273_68457 | scaffold273 | scaffold273_68457 | 8 | 585113 | 73 | ||
| 103 | NB | scaffold116_30201 | scaffold116 | scaffold116_30201 | 2 | 9550662 | 18 | |
| 104 | scaffold156_1203677 | scaffold156 | scaffold156_1203677 | 3 | 6468562 | 26 | ||
| 105 | scaffold1863_545 | scaffold1863 | scaffold1863_545 | 8 | 1223698 | 74 | ||
| 106 | scaffold212_601171 | scaffold212 | scaffold212_601171 | 6 | 6380495 | 60 | ||
| 107 | scaffold353_773806 | scaffold353 | scaffold353_773806 | 5 | 16077893 | 54 | ||
| 108 | scaffold42_494571 | scaffold42 | scaffold42_494571 | 13 | 15861394 | 117 | ||
| 109 | scaffold464_754364 | scaffold464 | scaffold464_754364 | 14 | 15460919 | 127 | ||
| 110 | scaffold635_43971 | scaffold635 | scaffold635_43971 | 8 | 22494547 | 82 | ||
| 111 | scaffold977_784147 | scaffold977 | scaffold977_784147 | 11 | 18799131 | 104 | ||
| 112 | scaffold212_216830 | scaffold212 | scaffold212_216830 | 6 | 5996154 | 59 | ||
| 113 | scaffold359_282990 | scaffold359 | scaffold359_282990 | 14 | 6711296 | 124 | ||
| 114 | scaffold359_289139 | scaffold359 | scaffold359_289139 | 14 | 6705147 | 123 | ||
| 115 | scaffold977_469888 | scaffold977 | scaffold977_469888 | 11 | 18484872 | 103 | ||
| 116 | FN | scaffold137_111000 | scaffold137 | scaffold137_111000 | 1 | 11869417 | 7 | |
| 117 | scaffold225_427119 | scaffold225 | scaffold225_427119 | 8 | 15994154 | 77 | ||
| 118 | scaffold687_121617 | scaffold687 | scaffold687_121617 | 14 | 16813947 | 128 | ||
| 119 | scaffold156_761294 | scaffold156 | scaffold156_761294 | 3 | 6026211 | 24 | ||
| 120 | scaffold413_1116527 | scaffold413 | scaffold413_1116527 | 4 | 16914228 | 41 | ||
| 121 | scaffold156_1203677 | scaffold156 | scaffold156_1203677 | 3 | 6468562 | 26 | ||
| 122 | scaffold413_388319 | scaffold413 | scaffold413_388319 | 5 | 14910709 | 52 | ||
| 123 | scaffold687_123666 | scaffold687 | scaffold687_123666 | 14 | 16811898 | 128 | ||
| 124 | TSW | scaffold101_354340 | scaffold101 | scaffold101_354340 | 3 | 20942454 | 32 | |
| 125 | scaffold112_184204 | scaffold112 | scaffold112_184204 | 1 | 18514049 | 11 | ||
| 126 | scaffold1143_190268 | scaffold1143 | scaffold1143_190268 | 1 | 4375935 | 1 | ||
| 127 | scaffold1155_171787 | scaffold1155 | scaffold1155_171787 | 15 | 7690615 | 130 | ||
| 128 | scaffold123_1191347 | scaffold123 | scaffold123_1191347 | 11 | 3875819 | 98 | ||
| 129 | scaffold1317_154716 | scaffold1317 | scaffold1317_154716 | 15 | 15275145 | 133 | ||
| 130 | scaffold132_713877 | scaffold132 | scaffold132_713877 | 1 | 24877317 | 14 | ||
| 131 | scaffold1491_58878 | scaffold1491 | scaffold1491_58878 | 6 | 14266269 | 64 | ||
| 132 | scaffold15_1207948 | scaffold15 | scaffold15_1207948 | 5 | 16914987 | 55 | ||
| 133 | scaffold1519_272169 | scaffold1519 | scaffold1519_272169 | 9 | 1027739 | 84 | ||
| 134 | FN | scaffold346-438191 | scaffold346 | scaffold346-438191 | 14 | 1083228 | 120 | [17] |
| 135 | TSW | scaffold43-1111162 | scaffold43 | scaffold43-1111162 | 2 | 21989104 | 19 | |
| 136 | scaffold51-598586 | scaffold51 | scaffold51-598586 | 4 | 11283142 | 39 | ||
| 137 | scaffold51-598611 | scaffold51 | scaffold51-598611 | 4 | 11283117 | 39 | ||
| 138 | scaffold51-699833 | scaffold51 | scaffold51-699833 | 4 | 11181895 | 38 | ||
| 139 | scaffold261-925068 | scaffold261 | scaffold261-925068 | 9 | 6419385 | 80 | ||
| 140 | scaffold373-545801 | scaffold373 | scaffold373-545801 | 13 | 17912691 | 119 | ||
| 141 | scaffold373-545816 | scaffold373 | scaffold373-545816 | 13 | 17912706 | 119 | ||
| 142 | scaffold107-300735 | scaffold107 | scaffold107-300735 | 2 | 22405177 | 20 | ||
| 143 | PAL | scaffold59-164258 | scaffold59 | scaffold59-164258 | 1 | 10459958 | 5 | |
| 144 | STE | scaffold11-96400 | scaffold11 | scaffold11-96400 | 5 | 9964973 | 46 | |
| 145 | scaffold11-96569 | scaffold11 | scaffold11-96569 | 5 | 9965142 | 46 | ||
| 146 | LIO | scaffold1253-27622 | scaffold1253 | scaffold1253-27622 | 9 | 1922095 | 85 | |
| 147 | LIN | scaffold416-80582 | scaffold416 | scaffold416-80582 | 5 | 13560525 | 50 | |
| 148 | scaffold302-224377 | scaffold302 | scaffold302-224377 | 5 | 13889425 | 51 | ||
| 149 | scaffold302-224395 | scaffold302 | scaffold302-224395 | 5 | 13889443 | 51 | ||
| 150 | FC | scaffold179-179593 | scaffold179 | scaffold179-179593 | 2 | 2253135 | 17 | |
| 151 | scaffold866-116645 | scaffold866 | scaffold866-116645 | 6 | 1083247 | 56 | ||
| 152 | PLH | scaffold344-309662 | scaffold344 | scaffold344-309662 | 1 | 11008279 | 6 | |
| 153 | scaffold59-572553 | scaffold59 | scaffold59-572553 | 1 | 10051709 | 4 | ||
| 154 | TL | scaffold297-275113 | scaffold297 | scaffold297-275113 | 1 | 16435852 | 9 | |
| 155 | scaffold297-275131 | scaffold297 | scaffold297-275131 | 1 | 16435834 | 9 | ||
| 156 | scaffold361-14957 | scaffold361 | scaffold361-14957 | 1 | 16726904 | 10 | ||
| 157 | MC | Lu2-22298066 | 2 | Lu2-22298066 | 2 | 22402960 | 20 | [27] |
| 158 | Lu3-25559600 | 3 | Lu3-25559600 | 3 | 17645461 | 29 | ||
| 159 | Lu3-26033342 | 3 | Lu3-26033342 | 3 | 18058033 | 30 | ||
| 160 | Lu3-7398487 | 3 | Lu3-7398487 | 3 | 6246253 | 25 | ||
| 161 | Lu5-3808878 | 5 | Lu5-3808878 | 5 | 4087340 | 44 | ||
| 162 | Lu7-13225294 | 7 | Lu7-13225294 | 7 | 12048040 | 68 | ||
| 163 | Lu11-2498303 | 11 | Lu11-2498303 | 11 | 2755439 | 97 | ||
| 164 | HC | Lu7-6577527 | 7 | Lu7-6577527 | 7 | 5834429 | 67 | |
| 165 | Lu10-21552161 | 10 | Lu10-21552161 | 4 | 4609469 | 35 | ||
| 166 | Lu12-5267706 | 12 | Lu12-5267706 | 12 | 5160897 | 112 | ||
| 167 | Lu13-2803224 | 13 | Lu13-2803224 | 13 | 2764903 | 113 | ||
| 168 | YLD | QYLD-Lu4.1 | 4 | Lu4-13594936-Lu4-14968389 | 4 | 13593668-14966967 | 40 | [28] |
| 169 | OIL | QOIL-Lu2.1 | 2 | Lu2-21913720-Lu2-21913720 | 2 | 21912675 | 19 | |
| 170 | QOIL-Lu5.2 | 5 | Lu5-15704607-Lu5-15705039 | 5 | 15703416-15703848 | 53 | ||
| 171 | QOIL-Lu6.3 | 6 | Lu6-4879632-Lu6-4879632 | 6 | 4879493 | 58 | ||
| 172 | QOIL-Lu6.4 | 6 | Lu6-13799180-Lu6-13970951 | 6 | 13798861-13970632 | 63 | ||
| 173 | QOIL-Lu7.4 | 7 | Lu7-14209179-Lu7-14209179 | 7 | 14208772 | 69 | ||
| 174 | QOIL-Lu10.5 | 10 | Lu10-6517448-Lu10-6517448 | 10 | 6517339 | 91 | ||
| 175 | QOIL-Lu12.6 | 12 | Lu12-4591214-Lu12-7491405 | 12 | 4591134-7490902 | 112 | ||
| 176 | QOIL-Lu15.7 | 15 | Lu15-14665900-Lu15-15429055 | 15 | 14665228-15428383 | 132 | ||
| 177 | PLH | QPLH-Lu1.1 | 1 | Lu1-13887715-Lu1-13930292 | 1 | 13887346-13929923 | 8 | |
| 178 | QPLH-Lu1.2 | 1 | Lu1-20012490-Lu1-20012490 | 1 | 20011813 | 12 | ||
| 179 | QPLH-Lu4.3 | 4 | Lu4-14305982-Lu4-15042104 | 4 | 14304616-15040682 | 40 | ||
| 180 | QPLH-Lu13.4 | 13 | Lu13-17243884-Lu13-17243884 | 13 | 17242916 | 118 | ||
| 181 | QPLH-Lu13.5 | 14 | Lu14-2320469-Lu14-2320469 | 14 | 2320188 | 121 | ||
| 182 | PAL | QPAL-Lu5.1 | 5 | Lu5-12062376-Lu5-12182441 | 5 | 12061283-12181348 | 49 | |
| 183 | QPAL-Lu5.2 | 5 | Lu5-13797851-Lu5-15668995 | 5 | 13796740-15667804 | 51 | ||
| 184 | QPAL-Lu7.3 | 7 | Lu7-624461-Lu7-5423691 | 7 | 624439-5423600 | 66 | ||
| 185 | QPAL-Lu11.4 | 11 | Lu11-4417685-Lu11-4429424 | 11 | 4417306-4429045 | 99 | ||
| 186 | IOD | QIOD-Lu4.1 | 4 | Lu4-19909467-Lu4-19909467 | 4 | 19907982 | 43 | |
| 187 | QIOD-Lu7.2 | 7 | Lu7-15346458-Lu7-17977459 | 7 | 15346004-17976903 | 70 | ||
| 188 | QIOD-Lu12.3 | 12 | Lu12-489561-Lu12-2981642 | 12 | 489561-2981562 | 107 | ||
| 189 | LIN | QLIN-Lu4.1 | 4 | Lu4-19909467-Lu4-19909467 | 4 | 19907982 | 43 | |
| 190 | QLIN-Lu7.2 | 7 | Lu7-14540719-Lu7-17977459 | 7 | 14540265-17976903 | 70 | ||
| 191 | QLIN-Lu12.3 | 12 | Lu12-489561-Lu12-2981642 | 12 | 489561-2981562 | 107 | ||
| 192 | LIO | QLIO-Lu4.1 | 4 | Lu4-19909467-Lu4-19909467 | 4 | 19907982 | 43 | |
| 193 | QLIO-Lu7.2 | 7 | Lu7-14540706-Lu7-17977459 | 7 | 14540252-17976903 | 70 | ||
| 194 | QLIO-Lu12.3 | 12 | Lu12-489561-Lu12-2981642 | 12 | 489561-2981562 | 107 | ||
| 195 | DTM | QDTM-Lu4.1 | 4 | Lu4-13171757-Lu4-15042104 | 4 | 13170489-15040682 | 40 | |
| 196 | QDTM-Lu11.2 | 11 | Lu11-14768686-Lu11-14768686 | 11 | 14767787 | 101 | ||
| 197 | STE | QSTE-Lu9.1 | 9 | Lu9-4229230-Lu9-4229230 | 9 | 4229031 | 87 | |
| 198 | QSTE-Lu9.2 | 9 | Lu9-20080531-Lu9-21636823 | 9 | 20079433-20654527 | 90 | ||
| 199 | PRO | QPRO-Lu15.1 | 15 | Lu15-14746288-Lu15-14746310 | 15 | 14745616-14745638 | 132 | |
| 200 | OLE | QOLE-Lu8.1 | 8 | Lu8-21782841-Lu8-23527563 | 8 | 21781910-23526575 | 81 |
| QTL No. | QTL | Chr | QTL Coordinates (bp) | RGA | Gene Location on chr (bp) | Gene Annotation |
|---|---|---|---|---|---|---|
| 12 | QPM-crc-LG1 | 1 | 16920407-18739647 | Lus10026756 | 17134471 | RLK |
| Lus10026761 | 17159664 | RLK | ||||
| Lus10026765 | 17189168 | NBS | ||||
| Lus10009703 | 18125241 | RLK | ||||
| 13 | QPM-crc-LG7 | 7 | 3817603-3817863 | Lus10023437 | 3725947 | TM-CC |
| 14 | QPM-crc-LG9 | 9 | 357191-357510 | Lus10001677 | 429431 | RLK |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, F.M.; Cloutier, S. Mapping Quantitative Trait Loci onto Chromosome-Scale Pseudomolecules in Flax. Methods Protoc. 2020, 3, 28. https://doi.org/10.3390/mps3020028
You FM, Cloutier S. Mapping Quantitative Trait Loci onto Chromosome-Scale Pseudomolecules in Flax. Methods and Protocols. 2020; 3(2):28. https://doi.org/10.3390/mps3020028
Chicago/Turabian StyleYou, Frank M., and Sylvie Cloutier. 2020. "Mapping Quantitative Trait Loci onto Chromosome-Scale Pseudomolecules in Flax" Methods and Protocols 3, no. 2: 28. https://doi.org/10.3390/mps3020028
APA StyleYou, F. M., & Cloutier, S. (2020). Mapping Quantitative Trait Loci onto Chromosome-Scale Pseudomolecules in Flax. Methods and Protocols, 3(2), 28. https://doi.org/10.3390/mps3020028






