Validity of Ultrasound Imaging Versus Magnetic Resonance Imaging for Measuring Anterior Thigh Muscle, Subcutaneous Fat, and Fascia Thickness
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
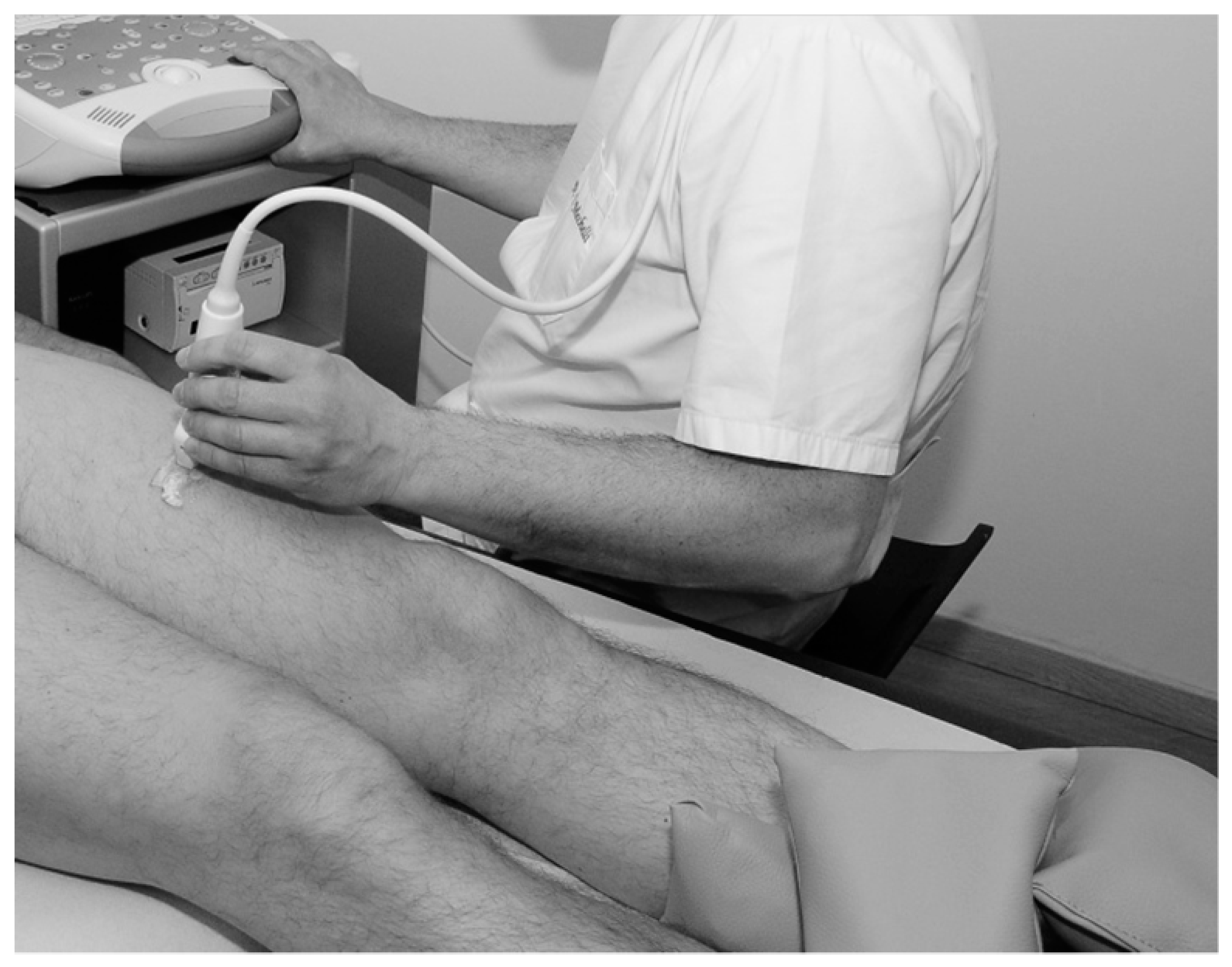
2.3. US Imaging Acquisition
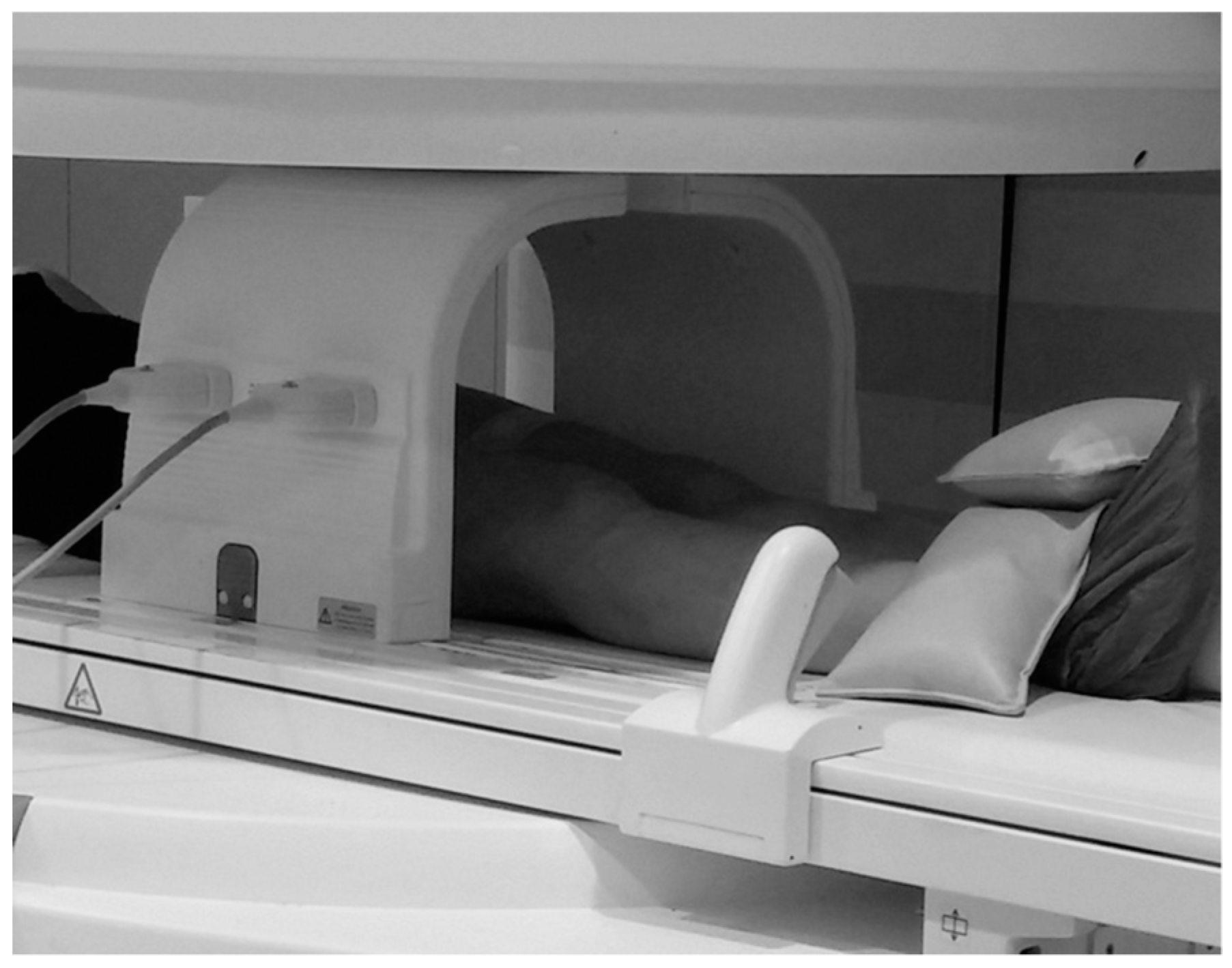
2.4. MRI Acquisition
2.5. Image Processing
2.6. Data Analysis
3. Results
3.1. Relative Measurements and Correlation Analysis
3.2. Agreement between Modalities
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petterson, S.C.; Barrance, P.; Buchanan, T.; Binder-Macleod, S.; Snyder-Mackler, L. Mechanisms Underlying Quadriceps Weakness in Knee Osteoarthritis. Med. Sci. Sports Exerc. 2008, 40, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.A.; McNair, P.J.; Lewis, G.N.; Dalbeth, N. Quadriceps arthrogenic muscle inhibition: The effects of experimental knee joint effusion on motor cortex excitability. Arthritis Res. Ther. 2014, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Rosager, S.; Aaboe, J.; Graven-Nielsen, T.; Bliddal, H. Experimental Knee Pain Reduces Muscle Strength. J. Pain 2011, 12, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.; Montgomery, H.; Moxham, J.; Harridge, S.; Hart, N. Structure to function: Muscle failure in critically ill patients: Muscle failure in critically ill patients. J. Physiol. 2010, 588, 4641–4648. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Padhke, R.; Dew, T.; Sidhu, P.S.; et al. Acute Skeletal Muscle Wasting in Critical Illness. JAMA 2013, 310, 1591. [Google Scholar] [CrossRef] [PubMed]
- Guleria, R.; Mohan, A.; Madan, K.; Mittal, S.; Kumar, R.; Hadda, V.; Khilnani, G.C.; Dhunguna, A.; Khan, M.A. Intra- and Inter-Observer Reliability of Quadriceps Muscle Thickness Measured with Bedside Ultrasonography by Critical Care Physicians. Indian J. Crit. Care Med. 2017, 21, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Galindo Martín, C.A.; Monares Zepeda, E.; Lescas Méndez, O.A. Bedside Ultrasound Measurement of Rectus Femoris: A Tutorial for the Nutrition Support Clinician. J. Nutr. Metab. 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Toledo, D.O.; Silva, D.C.L.E.; Santos, D.M.D.; Freitas, B.J.; Dib, R.; Cordioli, R.L.; Figueiredo, E.J.A.; Piovacari, S.M.F.; Silva, J.M., Jr. Bedside ultrasound is a practical measurement tool for assessing muscle mass. Rev. Bras. Ter. Intensiva 2017, 29, 476–480. [Google Scholar] [CrossRef]
- Tillquist, M.; Kutsogiannis, D.J.; Wischmeyer, P.E.; Kummerlen, C.; Leung, R.; Stollery, D.; Karvellas, C.J.; Preiser, J.-C.; Bird, N.; Kozar, R.; et al. Bedside Ultrasound Is a Practical and Reliable Measurement Tool for Assessing Quadriceps Muscle Layer Thickness. J. Parenter. Enter. Nutr. 2014, 38, 886–890. [Google Scholar] [CrossRef]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Wakefield, R.J.; Balint, P.V.; Szkudlarek, M.; Filippucci, E.; Backhaus, M.; D’Agostino, M.-A.; Sanchez, E.N.; Iagnocco, A.; Schmidt, W.A.; Bruyn, G.A.W.; et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J. Rheumatol. 2005, 32, 2485–2487. [Google Scholar] [PubMed]
- Müller, W.; Lohman, T.G.; Stewart, A.D.; Maughan, R.J.; Meyer, N.L.; Sardinha, L.B.; Kirihennedige, N.; Reguant-Closa, A.; Risoul-Salas, V.; Sundgot-Borgen, J.; et al. Subcutaneous fat patterning in athletes: Selection of appropriate sites and standardisation of a novel ultrasound measurement technique: Ad hoc working group on body composition, health and performance, under the auspices of the IOC Medical Commission. Br. J. Sports Med. 2016, 50, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Störchle, P.; Müller, W.; Sengeis, M.; Ahammer, H.; Fürhapter-Rieger, A.; Bachl, N.; Lackner, S.; Mörkl, S.; Holasek, S. Standardized Ultrasound Measurement of Subcutaneous Fat Patterning: High Reliability and Accuracy in Groups Ranging from Lean to Obese. Ultrasound Med. Biol. 2017, 43, 427–438. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Bentman, S.; Bennett, K.; Stokes, M. Rehabilitative Ultrasound Imaging of the Lower Trapezius Muscle: Technical Description and Reliability. J. Orthop. Sports Phys. Ther. 2007, 37, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Temes, W.; Temes Clifton, A.; Hilton, V.; Girard, L.; Strait, N.; Karduna, A. Reliability and Validity of Thickness Measurements of the Supraspinatus Muscle of the Shoulder: An Ultrasonography Study. J. Sport Rehabil. 2014, 23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, L.O.P.; Maher, C.G.; Latimer, J.; Smeets, R.J.E.M. Reproducibility of Rehabilitative Ultrasound Imaging for the Measurement of Abdominal Muscle Activity: A Systematic Review. Phys. Ther. 2009, 89, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Wallwork, T.L.; Hides, J.A.; Stanton, W.R. Intrarater and Interrater Reliability of Assessment of Lumbar Multifidus Muscle Thickness Using Rehabilitative Ultrasound Imaging. J. Orthop. Sports Phys. Ther. 2007, 37, 608–612. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Emery, C.A. Sonographic Measures of the Gluteus Medius, Gluteus Minimus, and Vastus Medialis Muscles. J. Orthop. Sports Phys. Ther. 2014, 44, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Mechelli, F.; Arendt-Nielsen, L.; Stokes, M.; Agyapong-Badu, S. Inter-rater and intra-rater reliability of ultrasound imaging for measuring quadriceps muscle and non-contractile tissue thickness of the anterior thigh. Biomed. Phys. Eng. Express 2019, 5, 037002. [Google Scholar]
- Lee, J.-P.; Tseng, W.-Y.I.; Shau, Y.-W.; Wang, C.-L.; Wang, H.-K.; Wang, S.-F. Measurement of segmental cervical multifidus contraction by ultrasonography in asymptomatic adults. Man. Ther. 2007, 12, 286–294. [Google Scholar] [CrossRef]
- Juul-Kristensen, B.; Bojsen-Møller, F.; Holst, E.; Ekdahl, C. Comparison of muscle sizes and moment arms of two rotator cuff muscles measured by Ultrasonography and Magnetic Resonance Imaging. Eur. J. Ultrasound 2000, 11, 161–173. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Meaney, J.; Boyle, G.; Gormley, J.; Stokes, M. The validity of Rehabilitative Ultrasound Imaging for measurement of trapezius muscle thickness. Man. Ther. 2009, 14, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.; Wilson, S.; Stanton, W.; McMahon, S.; Keto, H.; McMahon, K.; Bryant, M.; Richardson, C. An MRI Investigation into the Function of the Transversus Abdominis Muscle During “Drawing-In” of the Abdominal Wall. Spine 2006, 31, E175–E178. [Google Scholar] [CrossRef] [PubMed]
- Mendis, M.D.; Wilson, S.J.; Stanton, W.; Hides, J.A. Validity of Real-Time Ultrasound Imaging to Measure Anterior Hip Muscle Size: A Comparison with Magnetic Resonance Imaging. J. Orthop. Sports Phys. Ther. 2010, 40, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Worsley, P.R.; Kitsell, F.; Samuel, D.; Stokes, M. Validity of measuring distal vastus medialis muscle using rehabilitative ultrasound imaging versus magnetic resonance imaging. Man. Ther. 2014, 19, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Maas, H.; Sandercock, T.G. Force Transmission between Synergistic Skeletal Muscles through Connective Tissue Linkages. J. Biomed. Biotechnol. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Wilke, J.; Schleip, R.; Yucesoy, C.A.; Banzer, W. Not merely a protective packing organ? A review of fascia and its force transmission capacity. J. Appl. Physiol. 2018, 124, 234–244. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Warner, M.B.; Stokes, M. Comparison of the Sonographic Features of the Abdominal Wall Muscles and Connective Tissues in Individuals with and without Lumbopelvic Pain. J. Orthop. Sports Phys. Ther. 2013, 43, 11–19. [Google Scholar] [CrossRef]
- Yucesoy, C.A. Epimuscular Myofascial Force Transmission Implies Novel Principles for Muscular Mechanics. Exerc. Sport Sci. Rev. 2010, 38, 128–134. [Google Scholar] [CrossRef]
- Fleckenstein, J.L.; Crues, J.V.; Reimers, C.D. Muscle Imaging in Health and Disease; Springer: New York, NY, USA, 1996; ISBN 978-1-4612-2314-6. [Google Scholar]
- Saupe, N.; Prüssmann, K.P.; Luechinger, R.; Bösiger, P.; Marincek, B.; Weishaupt, D. MR Imaging of the Wrist: Comparison between 1.5- and 3-T MR Imaging—Preliminary Experience. Radiology 2005, 234, 256–264. [Google Scholar] [CrossRef]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report; Part C-7; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Carlson, R.V.; Boyd, K.M.; Webb, D.J. The revision of the Declaration of Helsinki: Past, present and future. Br. J. Clin. Pharmacol. 2004, 57, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Delaney, S.; Worsley, P.; Warner, M.; Taylor, M.; Stokes, M. Assessing contractile ability of the quadriceps muscle using ultrasound imaging. Muscle Nerve 2010, 42, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Ackland, T.R.; Lohman, T.G.; Sundgot-Borgen, J.; Maughan, R.J.; Meyer, N.L.; Stewart, A.D.; Müller, W. Current Status of Body Composition Assessment in Sport: Review and Position Statement on Behalf of the Ad Hoc Research Working Group on Body Composition Health and Performance, Under the Auspices of the I.O.C. Medical Commission. Sports Med. 2012, 42, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2000; ISBN 978-0-8385-2695-8. [Google Scholar]
- Walton, J.M.; Roberts, N.; Whitehouse, G.H. Measurement of the quadriceps femoris muscle using magnetic resonance and ultrasound imaging. Br. J. Sports Med. 1997, 31, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.; Maughan, R.J. The need for a novel approach to measure body composition: Is ultrasound an answer? Br. J. Sports Med. 2013, 47, 1001–1002. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.; Horn, M.; Fürhapter-Rieger, A.; Kainz, P.; Kröpfl, J.M.; Maughan, R.J.; Ahammer, H. Body composition in sport: A comparison of a novel ultrasound imaging technique to measure subcutaneous fat tissue compared with skinfold measurement. Br. J. Sports Med. 2013, 47, 1028–1035. [Google Scholar] [CrossRef]
- Wagner, D.R. Ultrasound as a Tool to Assess Body Fat. J. Obes. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Narici, M.; Cooper, C.; Stokes, M. Anterior thigh composition measured using ultrasound imaging to quantify relative thickness of muscle and non-contractile tissue: A potential biomarker for musculoskeletal health. Physiol. Meas. 2014, 35, 2165–2176. [Google Scholar] [CrossRef]
- Takai, Y.; Ohta, M.; Akagi, R.; Kato, E.; Wakahara, T.; Kawakami, Y.; Fukunaga, T.; Kanehisa, H. Applicability of ultrasound muscle thickness measurements for predicting fat-free mass in elderly population. J. Nutr. Health Aging 2014, 18, 579–585. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Thiebaud, R.S.; Abe, T. Estimating Site-Specific Muscle Loss: A Valuable Tool for Early Sarcopenia Detection? Rejuvenation Res. 2014, 17, 496–498. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
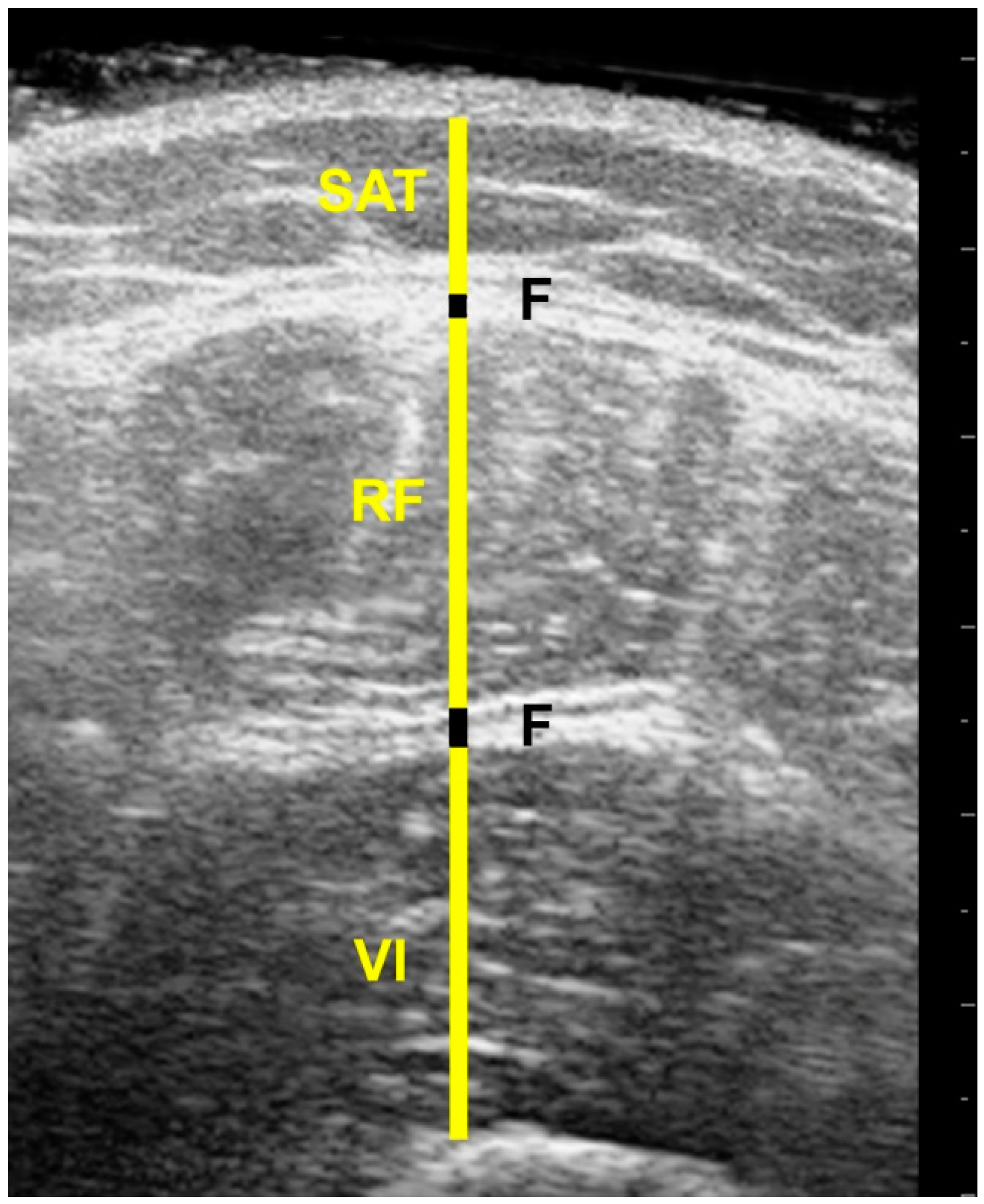
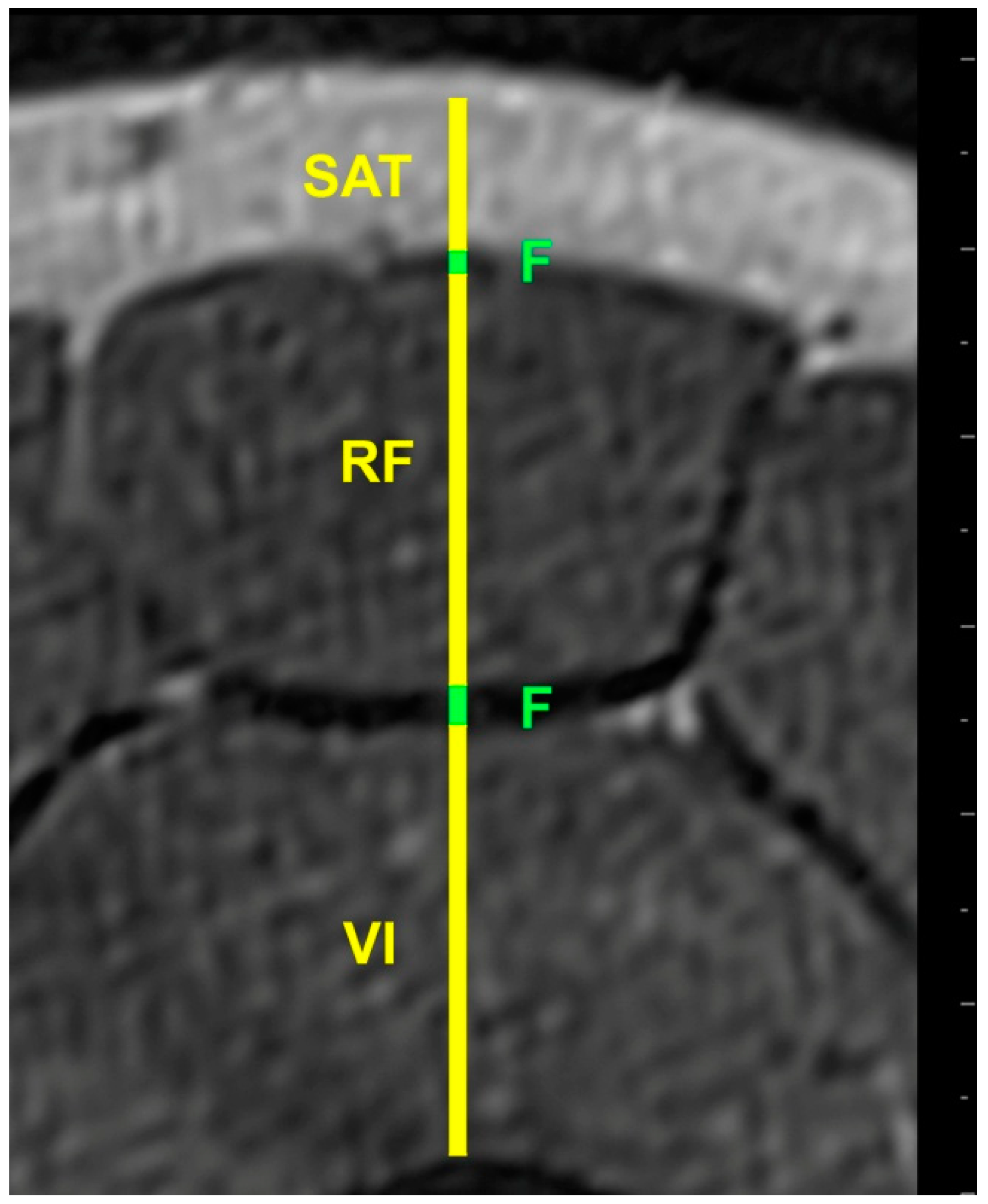
| Participants (n = 20) | Muscle Thickness (mm) | Subcutaneous Adipose Tissue (mm) | Non-Contractile Tissue (mm) | Fascia Thickness (mm) |
|---|---|---|---|---|
| Mean ± SD | ||||
| MRI | 28.6 ± 7.1 | 9.9 ± 4.7 | 12.7 ± 4.7 | 2.7 ± 0.3 |
| Ultrasound | 28.1 ± 6.9 | 10.5 ± 4.7 | 13.1 ± 4.7 | 2.6 ± 0.4 |
| Participants (n = 20) | r | p-Value |
|---|---|---|
| Muscle thickness (mm) | 0.99 | <0.01 * |
| Subcutaneous adipose tissue (mm) | 0.99 | <0.01 * |
| Non-contractile tissue (mm) | 0.99 | <0.01 * |
| Fascia thickness (mm) | 0.39 | 0.08 |
| Anterior Thigh Measurment n = 20 | ICC3,1 | 95% CI | SEM | Bland Altman Analysis | ||
|---|---|---|---|---|---|---|
| Mean Differences (mm) | Standard Deviation of Differences (mm) | 95% Limits of Agreement (mm) Mean ± 2SD | ||||
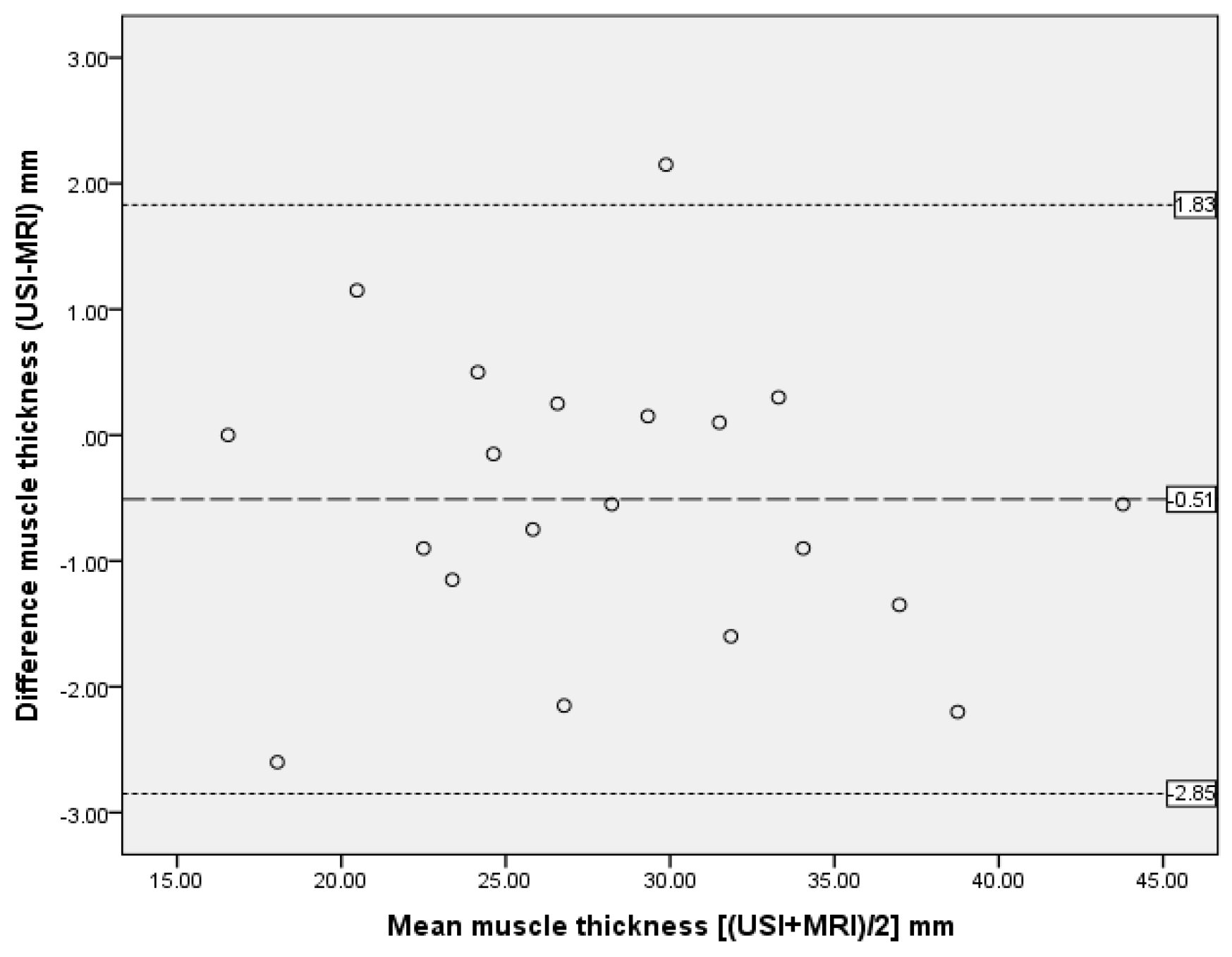
| Muscle thickness | 0.99 | 0.965–0.994 | 0.69 | −0.51 | 1.17 | −2.85 to 1.83 |
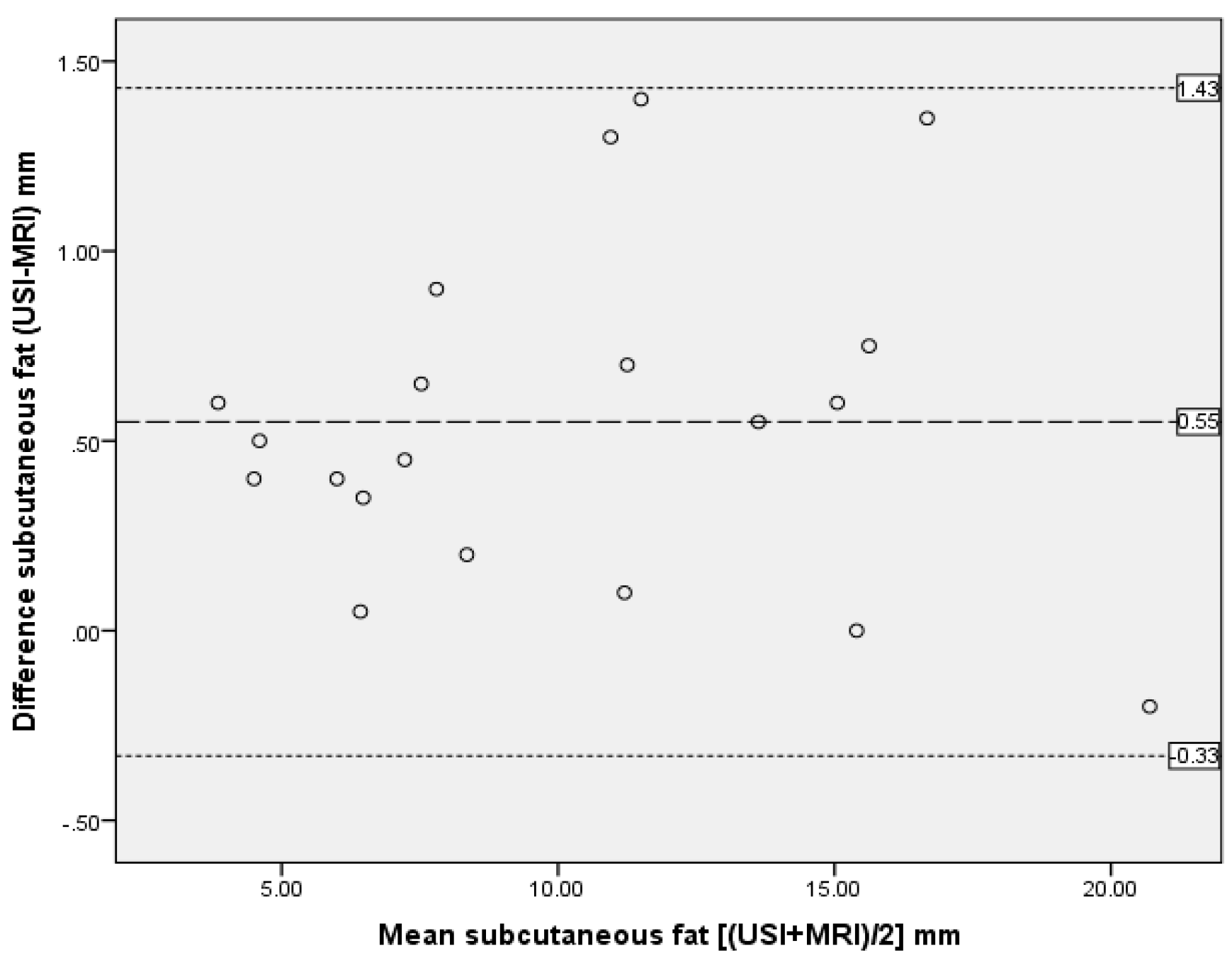
| Subcutaneous adipose tissue | 0.99 | 0.989–0.998 | 0.47 | 0.55 | 0.44 | −0.33 to 1.43 |
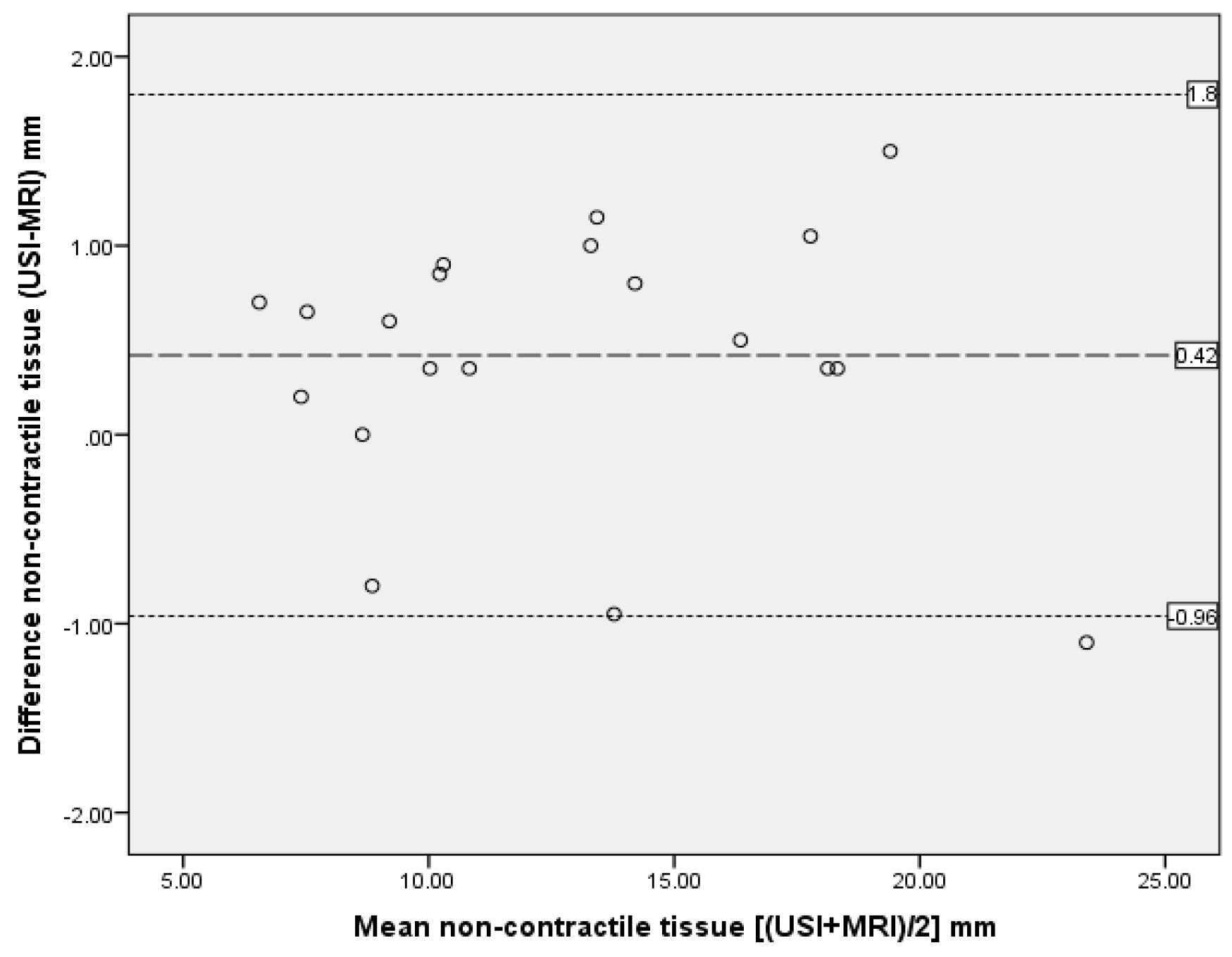
| Non-contractile tissue | 0.99 | 0.973–0.996 | 0.47 | 0.42 | 0.69 | −0.96 to 1.8 |
| Fascia | 0.36 | −0.084 to 0.687 | 0.29 | −0.13 | 0.42 | −0.97 to 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechelli, F.; Arendt-Nielsen, L.; Stokes, M.; Agyapong-Badu, S. Validity of Ultrasound Imaging Versus Magnetic Resonance Imaging for Measuring Anterior Thigh Muscle, Subcutaneous Fat, and Fascia Thickness. Methods Protoc. 2019, 2, 58. https://doi.org/10.3390/mps2030058
Mechelli F, Arendt-Nielsen L, Stokes M, Agyapong-Badu S. Validity of Ultrasound Imaging Versus Magnetic Resonance Imaging for Measuring Anterior Thigh Muscle, Subcutaneous Fat, and Fascia Thickness. Methods and Protocols. 2019; 2(3):58. https://doi.org/10.3390/mps2030058
Chicago/Turabian StyleMechelli, Filippo, Lars Arendt-Nielsen, Maria Stokes, and Sandra Agyapong-Badu. 2019. "Validity of Ultrasound Imaging Versus Magnetic Resonance Imaging for Measuring Anterior Thigh Muscle, Subcutaneous Fat, and Fascia Thickness" Methods and Protocols 2, no. 3: 58. https://doi.org/10.3390/mps2030058
APA StyleMechelli, F., Arendt-Nielsen, L., Stokes, M., & Agyapong-Badu, S. (2019). Validity of Ultrasound Imaging Versus Magnetic Resonance Imaging for Measuring Anterior Thigh Muscle, Subcutaneous Fat, and Fascia Thickness. Methods and Protocols, 2(3), 58. https://doi.org/10.3390/mps2030058





