A Novelty System for Biotization of Plant Microshoots and Collection of Natural Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Culture System
2.2. Procedures
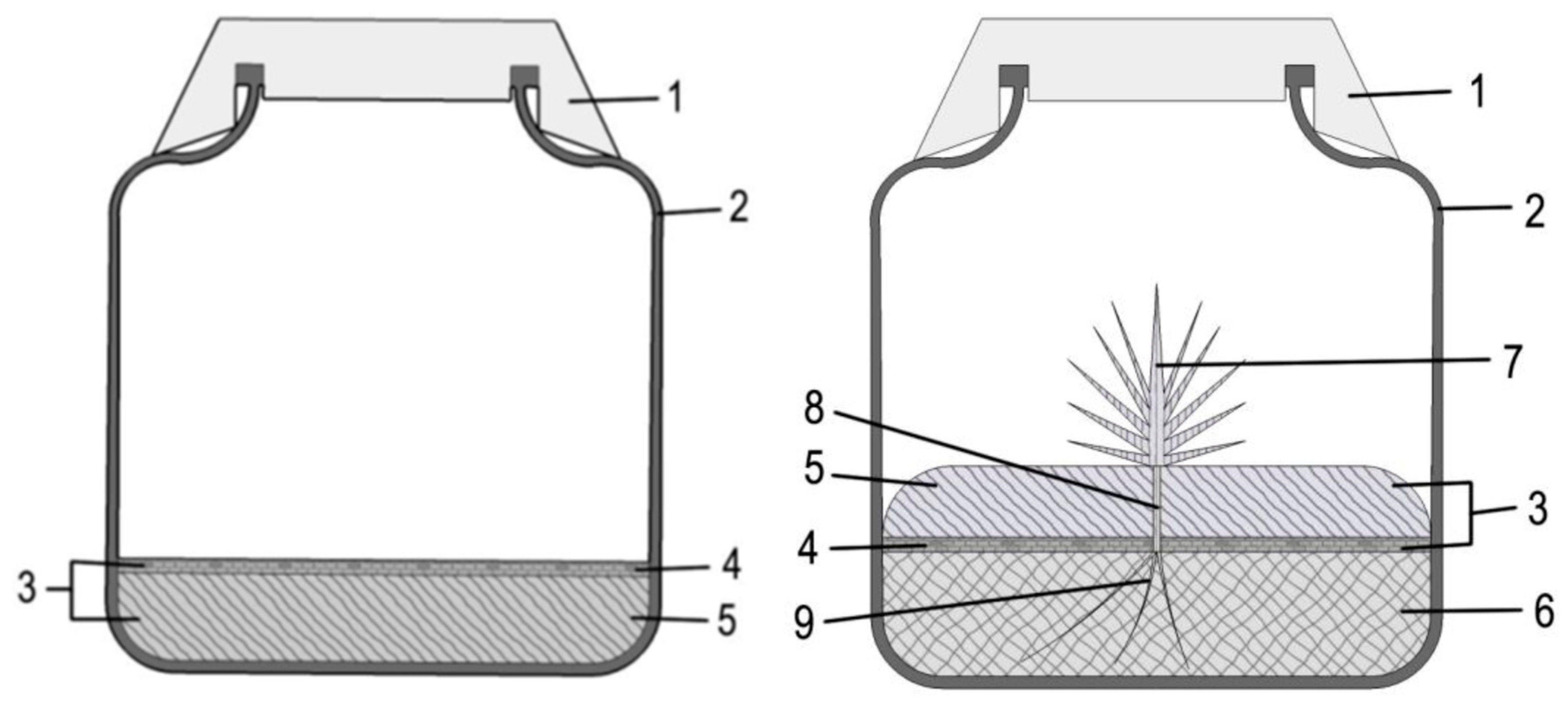
- The solid culture medium (5) and the liquid culture medium (6) are aseptically prepared—note 1;
- each flask is floored with the inert floater (4)—note 2;
- the medium supplemented with gelling agent is poured into the flasks. The inert floater will reach the surface before gelling of the medium (Figure 1, left);
- after gelling, the solid medium must be inverted; this is achieved by inserting the curved spatula between the solid medium and the inner flask surface, and carefully lifting it and turning it upside down, so that the inert floater faces downward;
- the curved spatula is then used to move the edge of the solid phase, creating an opening that allows pipet insertion of the liquid phase underneath the solid phase—note 3;
- the floating solid phase is pierced for insertion of the plant(s), by making an opening in the upper solid medium using a heat sterilized perforating tool (e.g., wide tweezers)—note 4;
- each plant is inserted in the hole that was made in the solid phase, such that the root system (9) is immersed into the liquid phase—note 5. At this point, the essential part of the system is set (Figure 1, right).
- Optionally, other organisms are placed on the solid phase, at the interface with the air compartment.
- As the culture proceeds, samples from the liquid phase can be collected for biochemical analysis, by inserting a pipette between the solid phase and the flask inner surface.
2.3. Notes
- In step a, culture media formulation may vary depending on the species and culture purpose including the possibility of the same medium being used for both phases, except for the absence of carbohydrates in the liquid phase;
- in step b, the amount of inert floater depends on the size of the flask but should create a layer with approximately 4 to 5 mm thickness for stability purposes;
- in step e, use for pipetting a 5 or 10 mL tip;
- alternatively, step f can be done between steps d and e;
- in step g, careful operation is required to avoid damaging the root system.
3. Results
4. Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oliveira, P.; Barriga, J.; Cavaleiro, C.; Peixe, A.; Potes, A.Z. Sustained in vitro root development obtained in Pinus pinea inoculated with ectomycorrhizal fungi. Forestry 2003, 76, 579–587. [Google Scholar] [CrossRef]
- Ragonezi, C.A. In Vitro Plantlet Regeneration from Mature Zygotic Embryos of Pinus pinea L.: Overcoming the Rooting Problems. Ph.D. Thesis, University of Évora, Évora, Portugal, 2013. Available online: http://hdl.handle.net/10174/16017 (accessed on 1 August 2018).
- Castro, M.R.B.; Ragonezi Gomes Lopes, C.A.; de Oliveira, P.G.L.; Zavattieri, M.A. Sistema e Método de Cultura de Plantas In Vitro Para Análise de Metabolitos Libertados pelo Sistema Radicular. Portuguese Patent nº 105239, 31 October 2012. Available online: https://tinyurl.com/y77axlnf (accessed on 1 August 2018).
- Ragonezi, C.; Teixeira, D.; Caldeira, A.T.; Martins, M.R.; Santos-Silva, C.E.; Klimaszewska, K.; Zavattieri, M.A. O-coumaric acid ester, a potential early signaling molecule in Pinus pinea and Pisolithus arrhizus symbiosis established in vitro. J. Plant Interact. 2014, 9, 297–305. Available online: https://www.tandfonline.com/doi/full/10.1080/17429145.2013.831489 (accessed on 1 August 2018). [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davis, F.T., Jr.; Geneve, R.L. Plant Propagation: Principles and Practices, 7th ed.; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 2002. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, M.R.d.C.B.e.; Ragonezi, C.; Oliveira, P.G.L.d.; Zavattieri, M.A. A Novelty System for Biotization of Plant Microshoots and Collection of Natural Compounds. Methods Protoc. 2019, 2, 5. https://doi.org/10.3390/mps2010005
Castro MRdCBe, Ragonezi C, Oliveira PGLd, Zavattieri MA. A Novelty System for Biotization of Plant Microshoots and Collection of Natural Compounds. Methods and Protocols. 2019; 2(1):5. https://doi.org/10.3390/mps2010005
Chicago/Turabian StyleCastro, Mário Rui da Costa Basílio e, Carla Ragonezi, Paulo Guilherme Leandro de Oliveira, and Maria Amely Zavattieri. 2019. "A Novelty System for Biotization of Plant Microshoots and Collection of Natural Compounds" Methods and Protocols 2, no. 1: 5. https://doi.org/10.3390/mps2010005
APA StyleCastro, M. R. d. C. B. e., Ragonezi, C., Oliveira, P. G. L. d., & Zavattieri, M. A. (2019). A Novelty System for Biotization of Plant Microshoots and Collection of Natural Compounds. Methods and Protocols, 2(1), 5. https://doi.org/10.3390/mps2010005






