An Evidence-Based Objective Study Protocol for Evaluating Cardiovascular and Cerebrovascular Indices Following Concussion: The Neary Protocol
Abstract
:1. Introduction
2. Objectives
2.1. Clinical Objectives
2.2. Scientific Objectives
- (1)
- It is hypothesized that changes at rest, in head orientation, at controlled respiration rates, during neurovascular coupling stimuli, and at mild exercise (i.e., squat-stand) will be different [2,33,34,36] when comparing the normally healthy baseline and injured state. Correlations between changes in cardiovascular and cerebrovascular activity will show a relationship that results in a decreased cerebral blood flow velocity (CBFV) and alterations in HRV [2,4,22,37,38], allowing these measurements to guide return-to-play and return-to-learn, and will assist in objectively confirming a concussion diagnosis;
- (2)
- Physiological responses are expected to increase in variability as the individual gradually begins a general return-to-play or learn protocol, followed by light aerobic exercise training. Their responses are hypothesized to return to levels similar to their initial baseline visit when they are ready to return-to-play or -learn. However, we acknowledge that some research suggests that there is a disconnect between the clinical impression and physiological outcomes, suggesting that some athletes can be cleared to return-to-play and -learn, when in fact, they may still be experiencing abnormal physiology [39]. Furthermore, neuropsychological testing has shown normal cognitive function despite dysfunctional motor learning tasks after athletes have been clinically cleared to return-to-play [40]. This illustrates that a multi-dimensional approach is required, including the physiological piece which has been missing and that we advocate in this protocol.
3. Methods and Analysis
3.1. Statistical Analysis
3.2. Recruitment Strategies
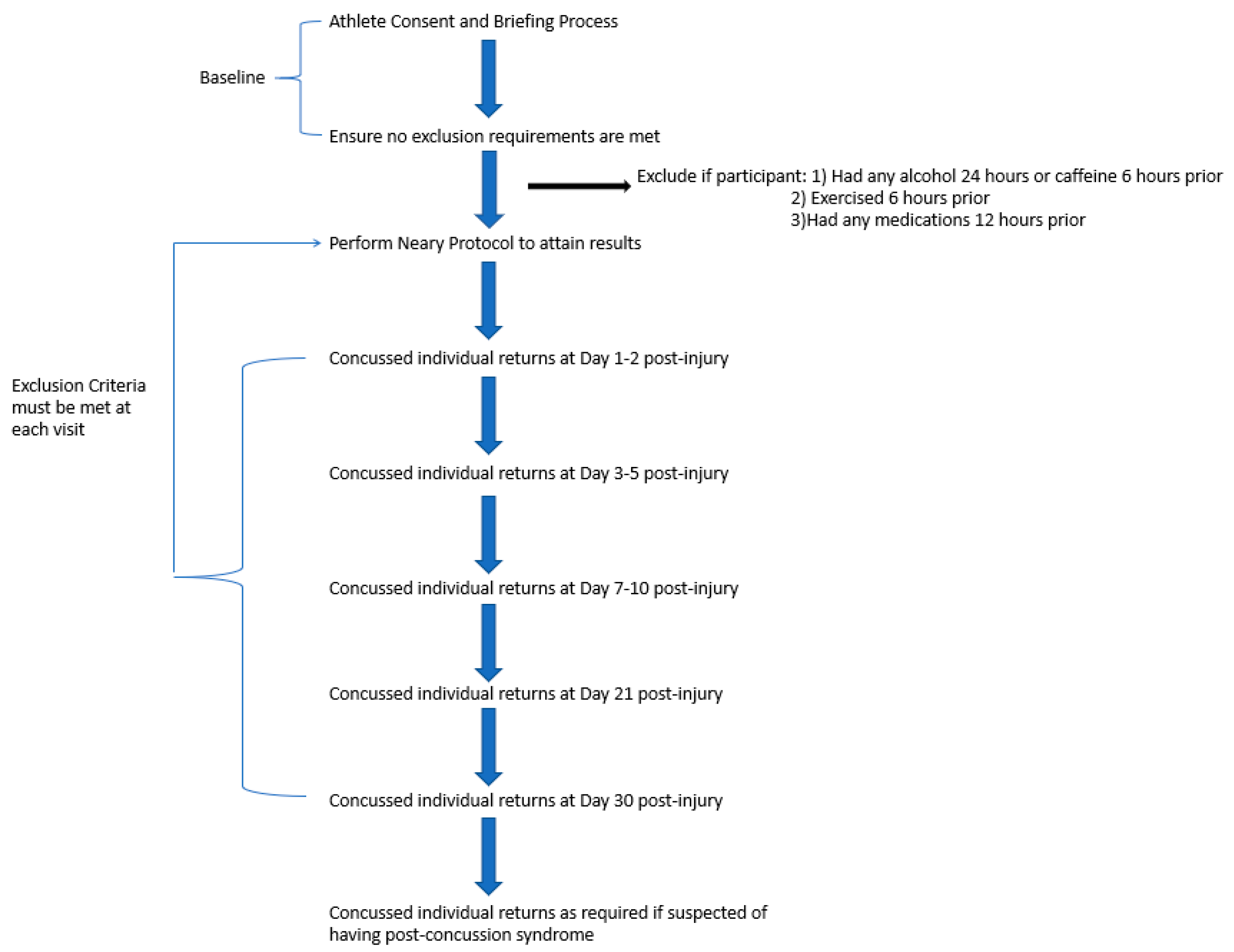
3.3. Overview of Protocol
4. Discussion
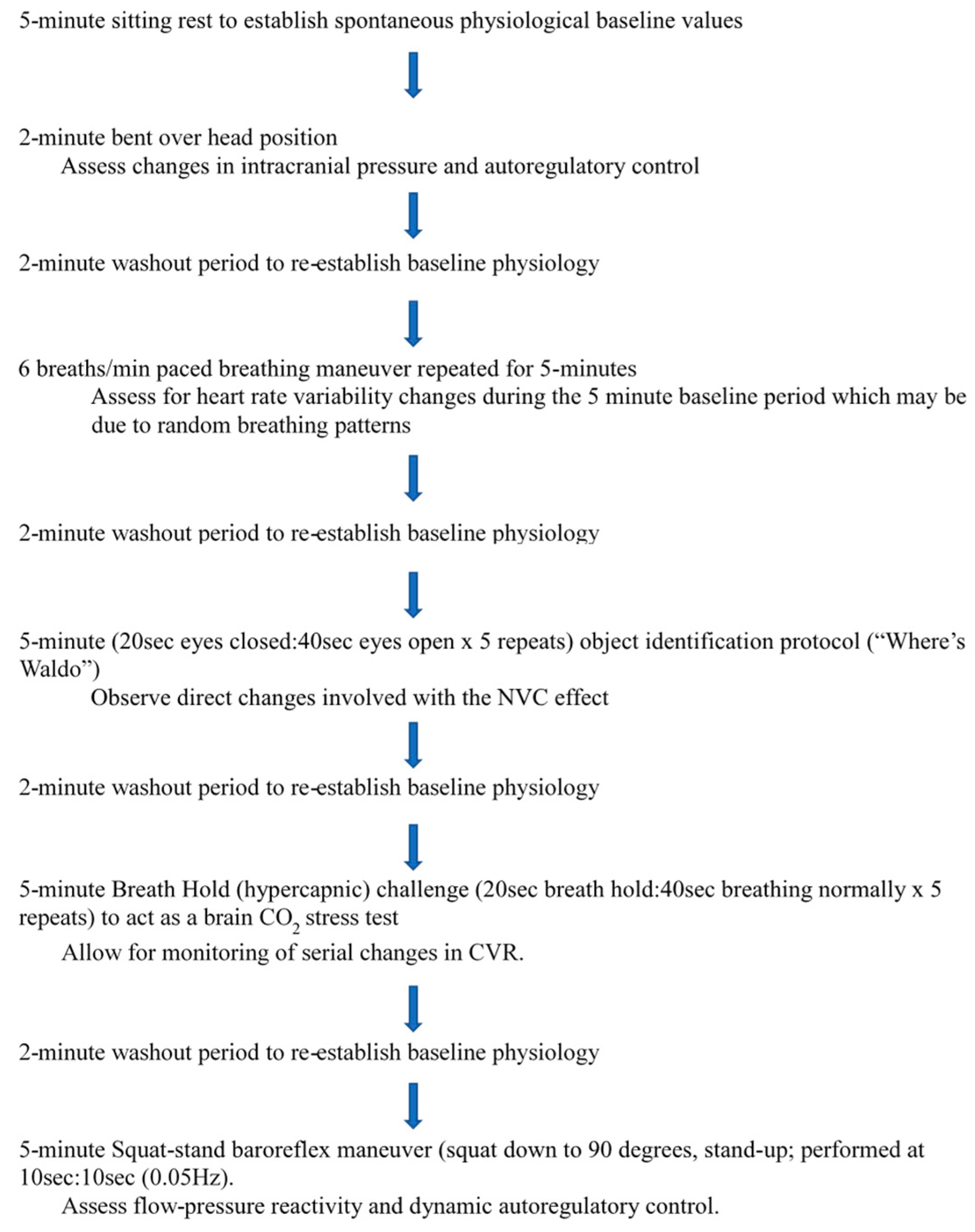
- (1)
- 5-min sitting rest to establish spontaneous physiological baseline values [43];
- (2)
- 2-min bent over head position (BOPT) [44];
- (3)
- 2-min washout period to re-establish baseline physiology;
- (4)
- six breaths per minute paced breathing maneuver repeated for 5-min [34];
- (5)
- 2-min washout period to re-establish baseline physiology;
- (6)
- 5-min (20 s eyes closed: 40 s eyes open × 5 repeats) object identification protocol (“Where’s Waldo”) to act as a complicated visual search paradigm that involves searching on a computer screen for an object character of a specific color and shape (“Waldo”) that is hidden in a field of distracters of similar colors and shapes to assess neurovascular coupling [33];
- (7)
- 2-min washout period to re-establish baseline physiology;
- (8)
- 5-min hypercapnic challenge (20 s breath hold: 40 s breathing normally × 5 repeats) to act as a brain CO2 stress test [22];
- (9)
- 2-min washout period to re-establish baseline physiology;
- (10)
5. Measures and Physiological Assessment Equipment
6. Potential Limitations
Author Contributions
Funding
Conflicts of Interest
Ethics Statement
References
- Halstead, M.E.; Walter, K.D. Sport-Related Concussion in Children and Adolescents. Pediatrics 2010, 126, 597–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, S.; Dech, R.; Baker, T.; Butz, M.; Aravinthan, K.; Neary, J.P. Parasympathetic baroreflexes and heart rate variability during acute stage of sport concussion recovery. Brain Inj. 2017, 31, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The new neurometabolic cascade of concussion. Neurosurgery 2014, 75 (Suppl. 4), S24–S33. [Google Scholar] [CrossRef] [PubMed]
- Gall, B.; Parkhouse, W.; Goodman, D. Heart rate variability of recently concussed athletes at rest and exercise. Med. Sci. Sports Exerc. 2004, 36, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- La Fountaine, M.F.; Hohn, A.N.; Testa, A.J.; Weir, J.P. Attenuation of Spontaneous Baroreceptor Sensitivity following Concussion. Med. Sci. Sports Exerc. 2018. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.; Meeuwisse, W.; Johnston, K.; Dvorak, J.; Aubry, M.; Molloy, M.; Cantu, R. Consensus Statement on Concussion in Sport: The 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br. J. Sports Med. 2009, 43, i76–84. [Google Scholar] [CrossRef] [PubMed]
- Mckee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweifel, C.; Castellani, G.; Czosnyka, M.; Helmy, A.; Manktelow, A.; Carrera, E.; Brady, K.M.; Hutchinson, P.J.A.; Menon, D.K.; Pickard, J.D.; Smielewski, P. Noninvasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head-injured patients. J. Neurotrauma 2010, 27, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- West, T.A.; Marion, D.W. Current recommendations for the diagnosis and treatment of concussion in sport: A comparison of three new guidelines. J. Neurotrauma 2014, 31, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.; Fralick, M.; Nejatbakhsh, N.; Tartaglia, M.C.; Tator, C.H. In search of evidence-based treatment for concussion: Characteristics of current clinical trials. Brain Inj. 2015, 29, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, J.L., 2nd; Dargo, L.; Ragan, B.G.; Kang, M. Reliability of Computerized Neurocognitive Tests for Concussion Assessment: A Meta-Analysis. J. Athl. Train. 2017, 52, 826–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J.; et al. Consensus Statement on Concussion in Sport—The 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br. J. Sports Med. 2017, 51, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Figley, C.R.; Mutch, W.A.; Massicotte, E.; Mikulis, D.J.; Essig, M.; Tator, C.H. Neuroimaging in sports-related concussion management: Current status and future directions. Curr. Res. Concussion 2014, 1, 33–39. [Google Scholar]
- Alberts, J.L.; Linder, S.M. The Utilization of Biomechanics to Understand and Manage the Acute and Long-term Effects of Concussion. Kinesiol. Rev. 2015, 4, 39–51. [Google Scholar] [CrossRef]
- Gaetz, M. The neurophysiology of brain injury. Clin. Neurophysiol. 2004, 115, 4–18. [Google Scholar] [CrossRef] [Green Version]
- Ainslie, P.N.; Duffin, J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: Mechanisms of regulation, measurement, and interpretation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1473–R1495. [Google Scholar] [CrossRef] [PubMed]
- Haran, F.J.; Tierney, R.; Wright, W.G.; Keshner, E.; Silter, M. Acute changes in postural control after soccer heading. Int. J. Sports Med. 2013, 34, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Kirkwood, M.W.; Provance, A.; Iverson, G.L.; Meehan, W.P., 3rd. Using concurrent gait and cognitive assessments to identify impairments after concussion: A narrative review. Concussion 2018, 3, CNC54. [Google Scholar] [CrossRef] [PubMed]
- Esterov, D.; Greenwald, B.D. Autonomic Dysfunction after Mild Traumatic Brain Injury. Brain Sci. 2017, 7, E100. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.A.; Dech, R.T.; Guzik, P.; Neary, J.P. Heart rate variability and implication for sport concussion. Clin. Physiol. Funct. Imaging 2018, 38, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.A.; Neary, J.P. Autonomic, cerebrovascular and mild traumatic brain injury physiology: Linkages and future applications. Curr. Res. Concussion 2015, 2, 49–58. [Google Scholar]
- Len, T.K.; Neary, J.P.; Asmundson, G.J.G.; Candow, D.G.; Goodman, D.G.; Bjornson, B.; Bhambhani, Y.N. Serial monitoring of CO2 reactivity following sport concussion using hypocapnia and hypercapnia. Brain Inj. 2013, 27, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Churchill, N.; Schweizer, T.A.; Rhind, S.G.; Richards, D.; Baker, A.J.; Hutchison, M.G. Blood biomarkers are associated with brain function and blood flow following sport concussion. J. Neuroimmunol. 2018, 319, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.L.; Sing, D.C.; Rugg, C.M.; Feeley, B.T.; Senter, C. The Rise of Concussions in the Adolescent Population. Orthop. J. Sport Med. 2016, 4, 2325967116662458. [Google Scholar] [CrossRef] [PubMed]
- Forrest, R.H.J.; Henry, J.D.; McGarry, P.J.; Marshall, R.N. Mild traumatic brain injury in New Zealand: Factors influencing post-concussion symptom recovery time in a specialised concussion service. J. Prim. Health Care 2018, 10, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.; Sveen, U.; Alvsaker, K.; Bautz-Holter, E. Post-concussion symptoms after mild traumatic brain injury: Influence of demographic factors and injury severity in a 1-year cohort study. Disabil. Rehabil. 2009, 31, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- King, N.S. Post-concussion syndrome: Clarity amid the controversy? Br. J. Psychiatry 2003, 183, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Leddy, J.; Willer, B. Multi-Disciplinary Management of Athletes with Post-Concussion Syndrome: An Evolving Pathophysiological Approach. Front. Neurol. 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Smirl, J.D.; Bryk, K.; Fraser, S.; Jakovac, M.; van Donkelaar, P. Sport-Related Concussion Alters Indices of Dynamic Cerebral Autoregulation. Front. Neurol. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Min, S.D. Feasibility study for the non-invasive blood pressure estimation based on ppg morphology: Normotensive subject study. Biomed. Eng. Online 2017, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Len, T.K.; Neary, J.P.; Asmundson, G.J.G.; Goodman, D.G.; Bjornson, B.; Bhambhani, Y.N. Cerebrovascular reactivity impairment after sport-induced concussion. Med. Sci. Sports Exerc. 2011, 43, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Piekos, C.; Pierog, O.; Fenech, N.; Krauze, T.; Piskorski, J.; Wykretowicz, A. Classic electrocardiogram-based and mobile technology derived approaches to heart rate variability are not equivalent. Int. J. Cardiol. 2018, 258, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Smirl, J.D.; Wright, A.D.; Bryk, K.; van Donkelaar, P. Where’s Waldo? The utility of a complicated visual search paradigm for transcranial Doppler-based assessments of neurovascular coupling. J. Neurosci. Methods 2016, 270, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Piskorski, J.; Krauze, T.; Schneider, R.; Wesseling, K.H.; Wykretowicz, A.; Wysocki, H. Correlations between the Poincaré plot and conventional heart rate variability parameters assessed during paced breathing. J. Physiol. Sci. 2007, 57, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Smirl, J.D.; Hoffman, K.; Tzeng, Y.-C.; Hansen, A.; Ainslie, P.N. Methodological comparison of active- and passive-driven oscillations in blood pressure; implications for the assessment of cerebral pressure-flow relationships. J. Appl. Physiol 2015, 119, 487–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truijen, J.; Rasmussen, L.S.; Kim, Y.S.; Stam, J.; Stok, W.J.; Pott, F.C.; van Lieshout, J.J. Cerebral autoregulatory performance and the cerebrovascular response to head-of-bed positioning in acute ischaemic stroke. Eur. J. Neurol. 2018, 25, 1365-e117. [Google Scholar] [CrossRef] [PubMed]
- Piskorski, J.; Guzik, P. Geometry of the Poincaré plot of RR intervals and its asymmetry in healthy adults. Physiol. Meas. 2007, 28, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Loncar, G.; Bozic, B.; Lepic, T.; Dimkovic, S.; Prodanovic, N.; Radojicic, Z.; Cvorovic, V.; Markovic, N.; Brajovic, M.; Despotovic, N.; Putnikovic, B.; Popovic-Brkic, V. Relationship of reduced cerebral blood flow and heart failure severity in elderly males. Aging Male 2011, 14, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; De Beaumont, L.; Henry, L.C.; Boulanger, Y.; Evans, A.C.; Bourgouin, P.; Poirier, J.; Theoret, H.; Lassonde, M. Sports concussions and aging: A neuroimaging investigation. Cereb. Cortex 2013, 23, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- De Beaumont, L.; Tremblay, S.; Poirier, J.; Lassonde, M.; Theoret, H. Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb. Cortex 2012, 22, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Sport concussion assessment tool—5th edition. Available online: www.sportphysio.ca/wp-content/uploads/SCAT-5.pdf (accessed on 6 March 2019).
- Task Force. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Wszedybyl-Winklewska, M.; Frydrychowski, A.F.; Winklewski, P.J. Assessing changes in pial artery resistance and subarachnoid space width using a non-invasive method in healthy humans during the handgrip test. Acta Neurobiol. Exp. (Wars) 2012, 72, 80–88. [Google Scholar] [PubMed]
- Bailey, D.M.; Marley, C.J.; Brugniaux, J.V.; Hodson, D.; New, K.J.; Ogoh, S.; Ainslie, P.N. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 2013, 44, 3235–3238. [Google Scholar] [CrossRef] [PubMed]
- Mutch, W.A.C.; Ellis, M.J.; Graham, M.R.; Wourms, V.; Raban, R.; Fisher, J.A.; Mikulis, D.; Leiter, J.; Ryner, L. Brain MRI CO2 stress testing: A pilot study in patients with concussion. PLoS ONE 2014, 9, e102181. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.A.; Neary, J.P. Assessing prefrontal cortex oxygenation after sport concussion with near-infrared spectroscopy. Clin. Physiol. Funct. Imaging 2018, 38, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Smirl, J.D.; Bryk, K.; Fraser, S.K.; Grewal, H.S.; Jakovac, M.; Dierijck, J.; Donkelaar, P. van. Acute sport-related concussion induces transient impairment in dynamic cerebral auto regulation that is related to scat3 performance. Br. J. Sports Med. 2017, 51, A38. [Google Scholar] [CrossRef]
- Kontos, A.P.; Huppert, T.J.; Beluk, N.H.; Elbin, R.J.; Henry, L.C.; French, J.; Dakan, S.M.; Collins, M.W. Brain activation during neurocognitive testing using functional near-infrared spectroscopy in patients following concussion compared to healthy controls HHS Public Access. Brain Imaging Behav. 2014, 8, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Vogt, E.; Macquarrie, D.S.; Neary, J.P. Using ballistocardiography to measure cardiac performance: A brief review of its history and future significance. Clin. Physiol. Funct. Imaging 2012, 32, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Neary, J.P.; Macquarrie, D.S.; Jamnik, V.; Gledhill, N.; Gledhill, S.; Busse, E.F.G. Assessment of Mechanical Cardiac Function in Elite Athletes. Open Sport Med. J. 2011, 5, 26–37. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neary, J.P.; Singh, J.; Bishop, S.A.; Dech, R.T.; Butz, M.J.A.; Len, T.K. An Evidence-Based Objective Study Protocol for Evaluating Cardiovascular and Cerebrovascular Indices Following Concussion: The Neary Protocol. Methods Protoc. 2019, 2, 23. https://doi.org/10.3390/mps2010023
Neary JP, Singh J, Bishop SA, Dech RT, Butz MJA, Len TK. An Evidence-Based Objective Study Protocol for Evaluating Cardiovascular and Cerebrovascular Indices Following Concussion: The Neary Protocol. Methods and Protocols. 2019; 2(1):23. https://doi.org/10.3390/mps2010023
Chicago/Turabian StyleNeary, J. Patrick, Jyotpal Singh, Scott A. Bishop, Ryan T. Dech, Matthew J. A. Butz, and Trevor K. Len. 2019. "An Evidence-Based Objective Study Protocol for Evaluating Cardiovascular and Cerebrovascular Indices Following Concussion: The Neary Protocol" Methods and Protocols 2, no. 1: 23. https://doi.org/10.3390/mps2010023
APA StyleNeary, J. P., Singh, J., Bishop, S. A., Dech, R. T., Butz, M. J. A., & Len, T. K. (2019). An Evidence-Based Objective Study Protocol for Evaluating Cardiovascular and Cerebrovascular Indices Following Concussion: The Neary Protocol. Methods and Protocols, 2(1), 23. https://doi.org/10.3390/mps2010023




