Abstract
Tuberculosis (TB) remains as a major public health issue in developing countries. Accurate detection is essential for the proper management of patients with active disease. Here, we present a simple DNAzol-LAMP (loop-mediated isothermal amplification) procedure for the detection of Mycobacterium tuberculosis in sputum specimens. Twenty smear-positive sputum samples were analyzed as follows: (i) Genetic material was extracted by a standard DNAzol protocol, and (ii) mycobacterial DNA was detected by a typical TB-specific loop-mediated isothermal amplification method. Results and diagnostic test performance attests to the suitability of the proposed procedure.
1. Introduction
Tuberculosis (TB) continues among the major threats to public health. Worldwide, morbidity, and mortality rates indicate that eradication remains out of reach [1]. In 2015, the WHO estimated 10.4 million new cases [2], suggesting that both late detection and misdiagnosis sustain the disease prevalence [3]. Two core methods, smear microscopy, and bacterial culture, are the foremost means for the detection of Mycobacterium tuberculosis, the leading causative agent of TB in humans [4]. Smear microscopy is inexpensive, but has low sensitivity, while bacterial culture (the gold standard) is sensitive, but it takes up to six weeks to provide a reliable result. Despite their intrinsic disadvantages, to date, these methods are applied for routine mycobacteriology [5,6].
The development of accurate diagnostic systems is essential to reducing TB incidence [4]. Several nucleic acid amplification techniques (NAAT), such as polymerase chain reaction (PCR) methods have addressed this demand, and have proven their diagnostic value. Even so, the requirement of expensive equipment (i.e., thermal cyclers) restricts their inclusion as a routine method in clinical laboratories with budget restrictions [4,5,6,7]. The loop-mediated isothermal amplification (LAMP) method, an alternate NAAT, allows for the use of a heating block to carry out the reaction, conceding a simple adaptation to settings with limited resources [8,9]. Since LAMP has shown reliability for TB detection [10], the WHO Expert Group agreed that it has outstanding potential as a rapid diagnostic tool [11].
In 2015, Mexico reported 21 cases per 100,000 people [2], indicating that TB remains prevalent in the current population. Remarkably, the Northern States listed the highest incidence values, with Baja California at the forefront [12,13]. Here, we present a simple DNAzol–LAMP procedure for the detection of M. tuberculosis in sputum specimens. Also, endpoint PCR and bacterial culture results were available for comparison purposes, and the analysis of diagnostic test performance.
2. Materials and Methods
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the General Hospital of Tijuana (Project identification code 10/05/2016). No consent for participation was required. Sputum samples, DNA extracts, and bacterial cultures were analyzed anonymously. No human tissue or any other biological materials were used.
2.1. Materials
The BBL MycoPrep specimen digestion/decontamination kit was supplied by Becton, Dickinson, and Company (Sparks, MD, USA). The DNAzol BD reagent was acquired from Invitrogen (Waltham, MA, USA). Bst 2.0 DNA polymerase was available from New England Biolabs (Ipswich, MA, USA), while Taq DNA polymerase were from Qiagen (Germantown, MD, USA). DNA analysis reagents were provided by Bio-Rad Laboratories (Hercules, CA, USA). Unless otherwise mentioned, additional reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA). Synthetic oligonucleotides, based on the primers designed to target the IS6110 sequence [10], were obtained from Eurofins MWG (Louisville, KY, USA). All materials were of biochemical or biotechnological research grade.
2.2. Sputum Specimens
Sputum specimens were provided by patients suspected of having TB disease and collected by the personnel of the TB Diagnostics Lab at the General Hospital of Tijuana (GHT). Digestion-decontamination was completed using the BBL MycoPrep routine. For this study, 20 sputum samples positive for acid-fast bacilli (i.e., smear-positive) were analyzed. Sample handling and other subsequent procedures were performed in accordance with protocols approved by the Institutional Ethics Review Board (GHT).
2.3. Extraction of DNA from Sputum Samples
Genetic material was extracted using the DNAzol BD reagent as recommended. Briefly, 0.2 mL sediment suspension was mixed with 0.5 mL of 1× Dulbecco’s PBS solution. Bacterial lysis was achieved heating at 80 °C for 10 min and cooling for 1 min on ice. One milliliter of DNAzol was added and mixed thoroughly. Cell debris was removed by centrifugation at 10,000× g for 10 min. The supernatant was mixed with 0.5 mL of cold ethanol and centrifuged at 14,000× g for 10 min. After washing, the precipitated DNA was dissolved in 30 μL of 8 mM NaOH.
2.4. Typical LAMP Assay
Reactions (25 μL) were completed in 1× isothermal buffer containing 1.6 μM FIP/BIP (forward/backward inner primers), 0.2 μM FOP/BOP (forward/backward outer primers), 0.8 μM FLP/BLP (forward/backward loop primers), 0.8 M betaine, 2 mM deoxynucleotide (dNTP) solution mix, 4 mM MgSO4, 8 units Bst 2.0 DNA polymerase, and 1 μL template [10]. Isothermal conditions were 90 min at 63 °C, and 5 min at 90 °C. All reactions were performed using a VWR Digital Dry Block Heater (i.e., heating block). Loss of volume was avoided by supplementing the reaction tube with 25 μL of mineral oil.
2.5. Standard Endpoint PCR Assay
Reactions (20 μL) were achieved in 1× Taq PCR Master Mix containing 1 μM FOP/BOP and 1 μL template. Endpoint amplifications were performed in a LabNet Multigene Thermal Cycler with the following conditions: 2 min at 94 °C; 45 cycles of exponential amplification (20 s at 94 °C, 20 s at 55 °C, 20 s at 72 °C); and 7 min at 72 °C.
2.6. Analysis of Amplification Products
Amplification products were separated by agarose gel electrophoresis, stained with ethidium bromide, visualized under UV light, and documented using a Bio-Rad GelDoc EZ System. Also, LAMP products were visually-analyzed using hydroxy naphthol blue dye as a chromogenic indicator [14]. However, judging positive (blue) or negative (violet) represented an experimental endeavor, due to color resemblance; hence this approach was not considered.
2.7. Assay Settings
Reference DNA was used as a template to validate both LAMP and PCR methods, and it served as the positive control throughout routine assays of sputum samples. Regularly, a reaction lacking a template was regarded as the negative control (i.e., blank reaction). Cross-contamination was prevented by using separate areas for routine work, such as the extraction of DNA and the preparation of responses. Samples were analyzed by duplication when a doubtful outcome was obtained.
2.8. Statistical Analysis of Data
Degrees of agreement between assays was pondered by kappa (κ) statistics using the GraphPad module for quick calculations [15]. Diagnostic performance: sensitivity, specificity, and predictive values, were calculated using the OpenEpi module for diagnostic test evaluation [16].
3. Results and Discussion
3.1. Assessment of the LAMP Assay Performed in a Heating Block
Reliable isothermal amplification reactions validated the reproducibility of the TB-specific LAMP assay as performed in a heating block. Using genetic material of the M. tuberculosis H37Rv strain as the template (reference DNA), positive reactions produced the typical ladder-like pattern (Figure 1a). Likewise, endpoint PCR reactions yielded the expected 233 bp sized product, (Figure 1b).
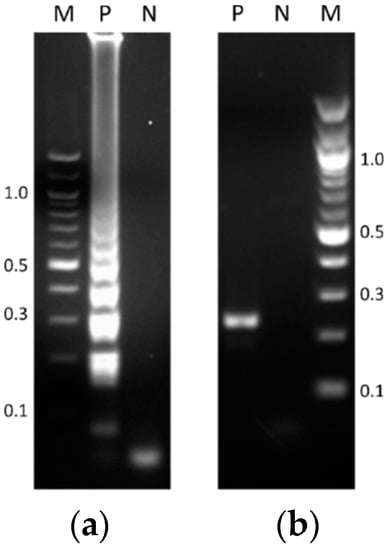
Figure 1.
Agarose gel electrophoresis of products obtained by (a) the typical loop-mediated isothermal amplification (LAMP) reaction performed in a heating block, and (b) the standard endpoint polymerase chain reaction (PCR) assay. Lanes: M, molecular weight (MW) markers (100 bp DNA ladder, New England Biolabs); P, positive control (1 ng of reference DNA); N, negative control (blank reaction).
3.2. Detection of TB in Sputum Specimens by the DNAzol–LAMP Procedure
Patients who attended the TB Clinic of the GHT, suspected of having TB disease, provided the sputum specimens prior to starting any drug therapy. Immediately, BBL MycoPrep protocol fulfilled the proper digestion and decontamination of sputum samples. Afterwards, smear microscopy and bacterial culture completed the routine mycobacteriology analysis. Then, of the smear-positive pool, 20 samples served as the biological analytes for testing the DNAzol–LAMP procedure (Figure 2).
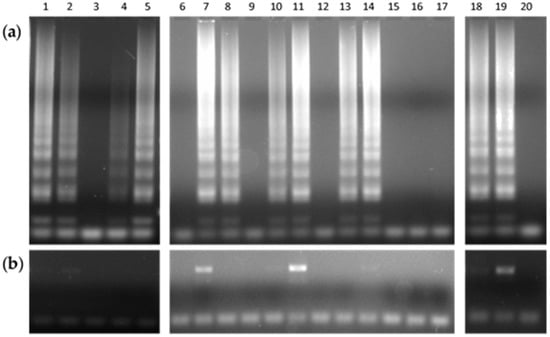
Figure 2.
Agarose gel electrophoresis of products obtained by (a) the typical LAMP reaction performed in a heating block, and (b) the standard endpoint PCR assay, from 20 sputum samples.
As observed (Table 1), 60% samples tested positive for both LAMP and bacterial culture, but only 20% rendered the same outcome for PCR.

Table 1.
Results of molecular and culture testing for detection of tuberculosis (TB) in smear-positive sputum samples.
Although the comparison LAMP/PCR revealed good values by kappa (κ) statistics (Table 2), the LAMP/culture showed superior agreement.

Table 2.
Results of agreement between LAMP assay and others (i.e., PCR or culture) for detection of TB in smear-positive sputum samples.
Together, these results suggest that LAMP (performed in a heating block) provided technical settings that were suitable for the isothermal amplification of mycobacterial DNA. Furthermore, the DNAzol–LAMP procedure exhibited reliable conditions for TB detection in sputum specimens, as demonstrated by the indicators of diagnostic test performance (Table 3).

Table 3.
Diagnostic performance of the TB-specific LAMP assay performed in a heating block.
As a final remark, it is fair notice that DNAzol–LAMP showed the ability of detection in sputum samples with apparent low-titers of live bacteria. Acknowledging that cell viability decreases with the storage time, it is reasonable to propose a thorough examination of such an advantage, as it represents an attractive improvement in the production of accurate results, and a significant reduction in the unpleasant practice of repeating the test or requesting a fresh sample.
4. Conclusions
Here, we report a simple DNAzol–LAMP procedure for the detection of M. tuberculosis in sputum specimens. We demonstrate the technical feasibility of performing the isothermal amplification in a heating block. By the reliable amplification of mycobacterial DNA, we confirm that the genetic material extracted from smear-positive sputum samples is a suitable template for the LAMP assay.
Author Contributions
Conceptualization, M.A.R.; Validation and Formal Analysis, All; Investigation, Á.R.-G.; Writing—Original Draft Preparation, M.A.R.; Writing—Review & Editing, All; Supervision, M.A.R., R.E.M. and A.F.L.-N.; Project Administration, M.A.R. and R.E.M.; Funding Acquisition, M.A.R. and P.L.A.M.
Funding
This research was funded in part by grants from the Mexican Council for Science and Technology (CONACyT; CB-2010/01-155714 and SSA/IMSS/ISSSTE-2011/01-161544) and the Autonomous University of Baja California (UABC; CPI/300/2/N/112/1).
Acknowledgments
The authors would like to thank Johanna Bernaldez of the Biomedical Innovation Department (CICESE) for offering a DNA sample of the M. tuberculosis H37Rv strain; and Rafael Laniado-Laborín, B.Sc. Walther Suárez, and all staff of the TB Diagnostics Laboratory (GHT) for their helpful assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Limitations
The reliability of outcome justifies the application of DNAzol–LAMP as a simple procedure for the routine diagnosis of TB in sputum samples. However, this notion must be assessed through a large-scale study to meet the sensitivity and specificity required clinical purposes.
References
- Raviglione, M.; Sulis, G. Tuberculosis 2015: Burden, challenges and strategy for control and elimination. Infect. Dis. Rep. 2016, 8, 6570. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Global Tuberculosis Report 2016; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Yuen, C.M.; Amanullah, F.; Dharmadhikari, A.; Nardell, E.A.; Seddon, J.A.; Vasilyeva, I.; Zhao, Y.; Keshavjee, S.; Becerra, M.C. Turning off the tap: Stopping tuberculosis transmission through active case-finding and prompt effective treatment. Lancet 2015, 386, 2334–2343. [Google Scholar] [CrossRef]
- Caulfield, A.J.; Wengenack, N.L. Diagnosis of active tuberculosis disease: From microscopy to molecular techniques. J. Clin. Tuberc. Other Mycobact. Dis. 2016, 4, 33–43. [Google Scholar] [CrossRef]
- Ou, X.; Li, Q.; Xia, H.; Pang, Y.; Wang, S.; Zhao, B.; Song, Y.; Zhou, Y.; Zheng, Y.; Zhang, Z.; et al. Diagnostic accuracy of the PURE-LAMP test for pulmonary tuberculosis at the county-level laboratory in China. PLoS ONE 2014, 9, e94544. [Google Scholar] [CrossRef] [PubMed]
- Gelaw, B.; Shiferaw, Y.; Alemayehu, M.; Bashaw, A.A. Comparison of loop-mediated isothermal amplification assay and smear microscopy with culture for the diagnostic accuracy of tuberculosis. BMC Infect. Dis. 2017, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Cudahy, P.; Shenoi, S.V. Diagnostics for pulmonary tuberculosis. Postgrad. Med. J. 2016, 92, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Soto, P.; Mvoulouga, P.O.; Akue, J.P.; Abán, J.L.; Santiago, B.V.; Sánchez, M.C.; Muro, A. Development of a highly sensitive loop-mediated isothermal amplification (LAMP) method for the detection of Loa loa. PLoS ONE 2014, 9, e94664. [Google Scholar] [CrossRef] [PubMed]
- Aryan, E.; Makvandi, M.; Farajzadeh, A.; Huygen, K.; Bifani, P.; Mousavi, S.L.; Fateh, A.; Jelodar, A.; Gouya, M.M.; Romano, M. A novel and more sensitive loop-mediated isothermal amplification assay targeting IS6110 for detection of Mycobacterium tuberculosis complex. Microbiol. Res. 2010, 165, 211–220. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). The Use of a Commercial Loop-Mediated Isothermal Amplification Assay (TB-LAMP) for the Detection of Tuberculosis: Expert Group Meeting Report; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- PAHO (Pan American Health Organization). Health in the Americas: 2012 Edition; Regional Outlook and Country Profiles; PAHO/WHO: Washington, DC, USA, 2012. [Google Scholar]
- Oren, E.; Fiero, M.H.; Barrett, E.; Anderson, B.; Nuῆez, M.; Gonzalez-Salazar, F. Detection of latent tuberculosis infection among migrant farmworkers along the US-Mexico border. BMC Infect. Dis. 2016, 16, 630. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Honda, E.; Ogura, A.; Nomoto, A.; Hanaki, K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques 2009, 46, 167–172. [Google Scholar] [CrossRef] [PubMed]
- GraphPad QuickCalcs. Available online: https://www.graphpad.com/quickcalcs/kappa1/ (accessed on 22 October 2018).
- Estadísticas de Código Abierto Para la Salud Pública. Available online: http://www.openepi.com/DiagnosticTest/DiagnosticTest.htm (accessed on 22 October 2018).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).