A Review of Disparities and Unmet Newborn Screening Needs over 33 Years in a Cohort of Mexican Patients with Inborn Errors of Intermediary Metabolism
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Setting Location
2.3. Period of Data Collection
2.4. Case Definition, Confirmation, and Disorder Classification
2.5. Disorder Classification
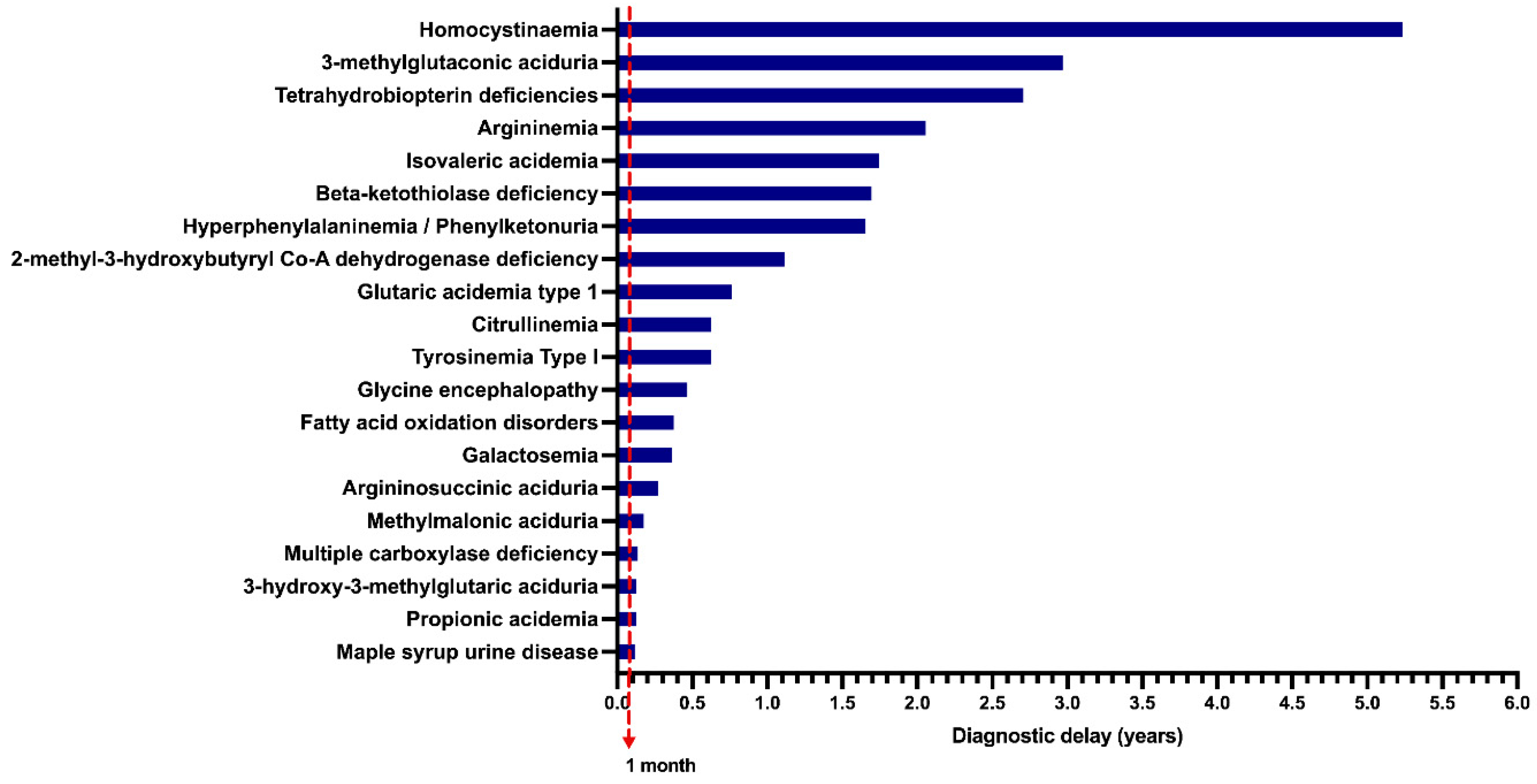
2.6. Diagnostic Delay, Travel from Habitual Address to Our Reference Center, and Mortality Rates
2.7. Statistical Methods
2.8. Ethical Considerations
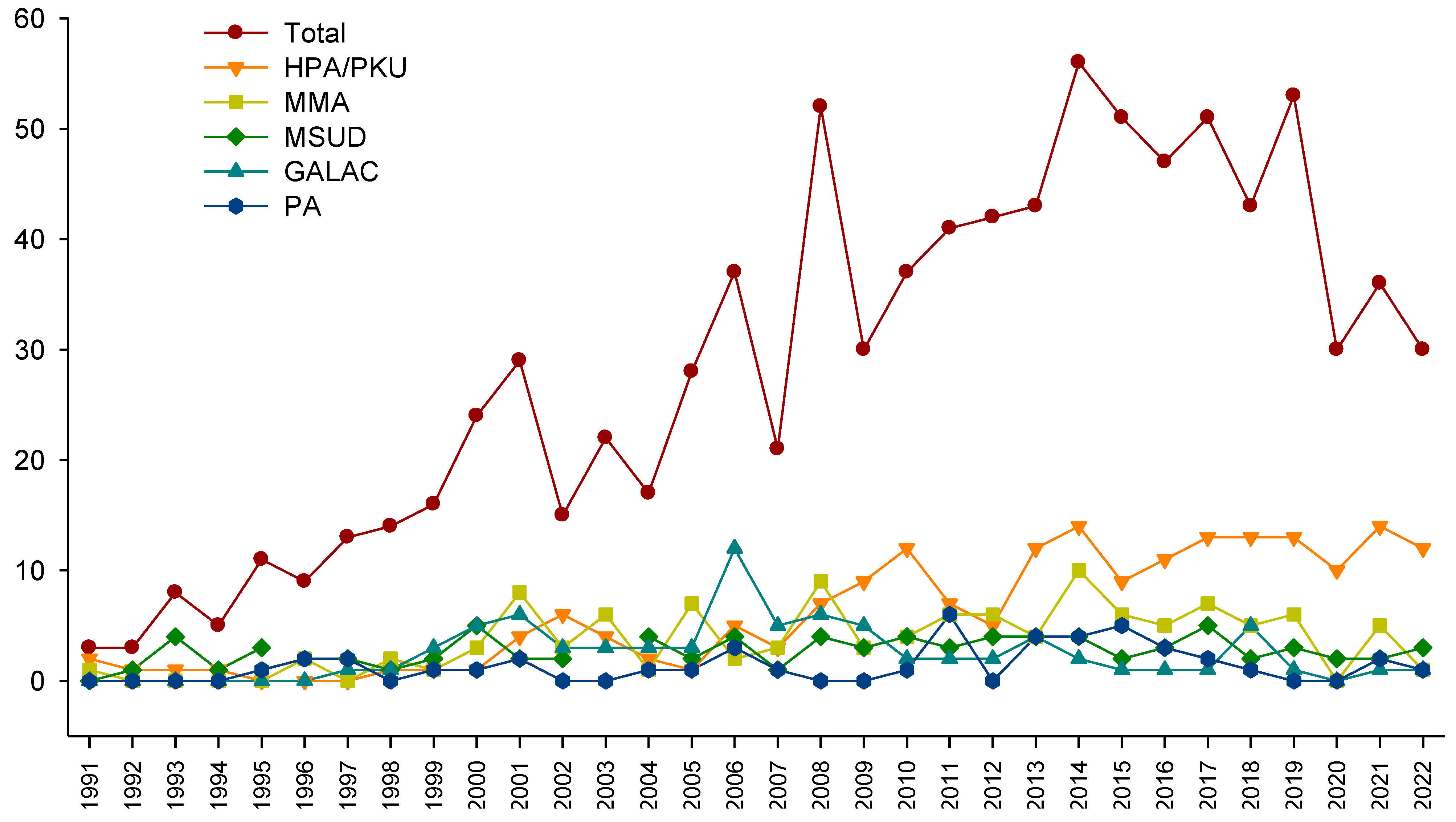
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saudubray, J.-M.; Garcia-Cazorla, À. Inborn errors of metabolism overview: Pathophysiology, manifestations, evaluation, and management. Pediatr. Clin. 2018, 65, 179–208. [Google Scholar]
- Jouvet, P.; Touati, G.; Lesage, F.; Dupic, L.; Tucci, M.; Saudubray, J.M.; Hubert, P. Impact of inborn errors of metabolism on admission and mortality in a pediatric intensive care unit. Eur. J. Pediatr. 2007, 166, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.; Adeloye, D.; Woolham, D.; Wastnedge, E.; Patel, S.; Rudan, I. Global birth prevalence and mortality from inborn errors of metabolism: A systematic analysis of the evidence. J. Glob. Health 2018, 8, 021102. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Nelson, C.; Brousseau, É.; Bilodeau-Bertrand, M.; Dewar, R.; Arbour, L. Extended Risk of Mortality in Children with Inborn Errors of Metabolism: A Longitudinal Cohort Study. J. Pediatr. 2023, 252, 16–21.e12. [Google Scholar] [CrossRef]
- Mathews, C.K.; Van Holde, K. Biochemistry, 2nd ed.; Benjamin/Cummings: San Francisco, CA, USA, 1996. [Google Scholar]
- García-Cazorla, À.; Saudubray, J.-M. Cellular neurometabolism: A tentative to connect cell biology and metabolism in neurology. J. Inherit. Metab. Dis. 2018, 41, 1043–1054. [Google Scholar] [CrossRef]
- van Karnebeek, C.D.; Stockler, S. Treatable inborn errors of metabolism causing intellectual disability: A systematic literature review. Mol. Genet. Metab. 2012, 105, 368–381. [Google Scholar] [CrossRef]
- Leal, A.F.; Fnu, N.; Benincore-Flórez, E.; Pachón, A.M.H.; Echeverri-Peña, O.Y.; Alméciga-Díaz, C.J.; Tomatsu, S. The landscape of CRISPR/Cas9 for inborn errors of metabolism. Mol. Genet. Metab. 2022, 138, 106968. [Google Scholar] [CrossRef]
- Arnold, G.L. Inborn errors of metabolism in the 21st century: Past to present. Ann. Transl. Med. 2018, 6, 467. [Google Scholar] [CrossRef]
- Gelb, M.H.; Basheeruddin, K.; Burlina, A.; Chen, H.-J.; Chien, Y.-H.; Dizikes, G.; Dorley, C.; Giugliani, R.; Hietala, A.; Hong, X. Liquid Chromatography–Tandem Mass Spectrometry in Newborn Screening Laboratories. Int. J. Neonatal Screen. 2022, 8, 62. [Google Scholar] [CrossRef]
- Shlomi, T.; Cabili, M.N.; Ruppin, E. Predicting metabolic biomarkers of human inborn errors of metabolism. Mol. Syst. Biol. 2009, 5, 263. [Google Scholar] [CrossRef]
- Borrajo, G.J. Newborn screening in Latin America: A brief overview of the state of the art. Am. J. Med. Genet. Part C Semin. Med. Genet. 2021, 187, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, R.; Castillo Taucher, S.; Hafez, S.; Oliveira, J.B.; Rico-Restrepo, M.; Rozenfeld, P.; Zarante, I.; Gonzaga-Jauregui, C. Opportunities and challenges for newborn screening and early diagnosis of rare diseases in Latin America. Front. Genet. 2022, 13, 3447. [Google Scholar] [CrossRef] [PubMed]
- Cabello, J.F.; Novoa, F.; Huff, H.V.; Colombo, M. Expanded newborn screening and genomic sequencing in Latin America and the resulting social justice and ethical considerations. Int. J. Neonatal Screen. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- García-Flores, E.P.; Herrera-Maldonado, N.; Hinojosa-Trejo, M.A.; Vergara-Vázquez, M.; Halley-Castillo, M.E. Avances y logros del programa de tamiz metabólico neonatal (2012–2018). Acta Pediátrica México 2018, 39, 57–65. [Google Scholar] [CrossRef]
- Vela-Amieva, M.; Belmont-Martínez, L.; Fernández-Lainez, C.; Ramírez-Frías, C.; Ibarra-González, I. Frecuencia de enfermedades metabólicas congénitas susceptibles de ser identificadas por el tamiz neonatal. Acta Pediátrica México 2009, 30, 156–162. [Google Scholar]
- Fishler, P.K.; Euteneuer, J.C.; Brunelli, L. Ethical Considerations for Equitable Access to Genomic Sequencing for Critically Ill Neonates in the United States. Int. J. Neonatal Screen. 2022, 8, 22. [Google Scholar] [CrossRef]
- Stranneheim, H.; Lagerstedt-Robinson, K.; Magnusson, M.; Kvarnung, M.; Nilsson, D.; Lesko, N.; Engvall, M.; Anderlid, B.M.; Arnell, H.; Johansson, C.B.; et al. Integration of whole genome sequencing into a healthcare setting: High diagnostic rates across multiple clinical entities in 3219 rare disease patients. Genome Med. 2021, 13, 40. [Google Scholar] [CrossRef]
- Collins, F.S.; Doudna, J.A.; Lander, E.S.; Rotimi, C.N. Human Molecular Genetics and Genomics—Important Advances and Exciting Possibilities. N. Engl. J. Med. 2021, 384, 1–4. [Google Scholar] [CrossRef]
- Velázquez-Aragón, J.; Alcántara-Ortigoza, M.; Vela-Amieva, M.; Monroy, S.; Martínez-Cruz, V.; Todd-Quiñones, C.; González-del Angel, A. Low allelic heterogeneity in a sample of Mexican patients with classical galactosaemia. J. Inherit. Metab. Dis. Off. J. Soc. Study Inborn Errors Metab. 2008, 31, 333–337. [Google Scholar] [CrossRef]
- Méndez, S.T.; Vela-Amieva, M.; Velázquez-Arellano, A.; Ibarra, I.; Flores, M.E. Análisis de mutaciones en el gen de la metilmalonilCoA mutasa en diez pacientes mexicanos con acidemia metilmalónica aislada. Rev. Investig. Clin. 2012, 64, 255–261. [Google Scholar]
- Alcantara-Ortigoza, M.A.; Belmont-Martinez, L.; Vela-Amieva, M.; González-Del Angel, A. Analysis of the CTNS gene in nephropathic cystinosis Mexican patients: Report of four novel mutations and identification of a false positive 57-kb deletion genotype with LDM-2/exon 4 multiplex PCR assay. Genet. Test. 2008, 12, 409–414. [Google Scholar] [CrossRef]
- Ibarra-González, I.; Fernández-Lainez, C.; Belmont-Martínez, L.; Guillén-López, S.; Monroy-Santoyo, S.; Vela-Amieva, M. Caracterización de errores innatos del metabolismo intermediario en pacientes mexicanos. An. Pediatría 2014, 80, 310–316. [Google Scholar] [CrossRef]
- Ibarra-González, I.; Ridaura-Sanz, C.; Fernández-Lainez, C.; Guillén-López, S.; Belmont-Martínez, L.; Vela-Amieva, M. Hepatorenal Tyrosinemia in Mexico: A Call to Action. Adv. Exp. Med. Biol. 2017, 959, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Campos-Garcia, F.J.; Chacon-Camacho, O.F.; Contreras-Capetillo, S.; Cruz-Aguilar, M.; Medina-Escobedo, C.E.; Moreno-Graciano, C.M.; Rodas, A.; Herrera-Perez, L.D.A.; Zenteno, J.C. Characterization of novel GCDH pathogenic variants causing glutaric aciduria type 1 in the southeast of Mexico. Mol. Genet. Metab. Rep. 2019, 21, 100533. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-González, I.; Fernández-Lainez, C.; Guillén-López, S.; López-Mejía, L.; Belmont-Matínez, L.; Sokolsky, T.D.; Amin, V.R.; Kitchener, R.L.; Vela-Amieva, M.; Naylor, E.W. Molecular analysis using targeted next generation DNA sequencing and clinical spectrum of Mexican patients with isovaleric acidemia. Clin. Chim. Acta 2020, 501, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Vela-Amieva, M.; Alcántara-Ortigoza, M.A.; Ibarra-González, I.; González-del Angel, A.; Fernández-Hernández, L.; Guillén-López, S.; López-Mejía, L.; Carrillo-Nieto, R.I.; Belmont-Martínez, L.; Fernández-Lainez, C. An Updated PAH Mutational Spectrum of Phenylketonuria in Mexican Patients Attending a Single Center: Biochemical, Clinical-Genotyping Correlations. Genes 2021, 12, 1676. [Google Scholar] [CrossRef]
- Vela-Amieva, M.; Alcántara-Ortigoza, M.A.; Ibarra-González, I.; González-Del Angel, A.; Fernández-Hernández, L.; Guillén-López, S.; López-Mejía, L.; Carrillo-Nieto, R.I.; Fiesco-Roa, M.O.; Fernández-Lainez, C. Genotypic spectrum underlying tetrahydrobiopterin metabolism defects: Experience in a single Mexican reference center. Front. Genet. 2022, 13, 993612. [Google Scholar] [CrossRef]
- Ibarra-González, I.F.-L.C.; Guillén-López, S.; López-Mejía, L.; Belmont-Martínez, L.; Nieto-Carrillo, R.I.; Vela-Amieva, M. Importance of Studying Older Siblings of Patients Identified by Newborn Screening: A Single-Center Experience in Mexico. J. Inborn Errors Metab. Screen. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Sontag, M.K.; Sarkar, D.; Comeau, A.M.; Hassell, K.; Botto, L.D.; Parad, R.; Rose, S.R.; Wintergerst, K.A.; Smith-Whitley, K.; Singh, S.; et al. Case Definitions for Conditions Identified by Newborn Screening Public Health Surveillance. Int. J. Neonatal Screen. 2018, 4, 16. [Google Scholar] [CrossRef]
- Watson, M.S.; Mann, M.Y.; Lloyd-Puryear, M.A.; Rinaldo, P.; Howell, R.R. American College of Medical Genetics Newborn screening: Toward a uniform screening panel and system—Executive summary. Pediatrics 2006, 117, S296–S307. [Google Scholar] [CrossRef]
- Sontag, M.K.; Miller, J.I.; McKasson, S.; Sheller, R.; Edelman, S.; Yusuf, C.; Singh, S.; Sarkar, D.; Bocchini, J.; Scott, J.; et al. Newborn screening timeliness quality improvement initiative: Impact of national recommendations and data repository. PLoS ONE 2020, 15, e0231050. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Rose, A.M.; Waisbren, S.; Ahmad, A.; Prosser, L.A. Newborn Screening and Treatment of Phenylketonuria: Projected Health Outcomes and Cost-Effectiveness. Children 2021, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Grünert, S.C.; Stucki, M.; Morscher, R.J.; Suormala, T.; Bürer, C.; Burda, P.; Christensen, E.; Ficicioglu, C.; Herwig, J.; Kölker, S. 3-methylcrotonyl-CoA carboxylase deficiency: Clinical, biochemical, enzymatic and molecular studies in 88 individuals. Orphanet J. Rare Dis. 2012, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qiang, R.; Song, C.; Ma, X.; Zhang, Y.; Li, F.; Wang, R.; Yu, W.; Feng, M.; Yang, L.; et al. Spectrum analysis of inborn errors of metabolism for expanded newborn screening in a northwestern Chinese population. Sci. Rep. 2021, 11, 2699. [Google Scholar] [CrossRef] [PubMed]
- Ramoser, G.; Caferri, F.; Radlinger, B.; Brunner-Krainz, M.; Herbst, S.; Huemer, M.; Hufgard-Leitner, M.; Kircher, S.G.; Konstantopoulou, V.; Löscher, W.; et al. 100 years of inherited metabolic disorders in Austria-A national registry of minimal birth prevalence, diagnosis, and clinical outcome of inborn errors of metabolism in Austria between 1921 and 2021. J. Inherit. Metab. Dis. 2022, 45, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Vela-Amieva, M.; Abreu-Gonzalez, M.; Gonzalez-del Angel, A.; Ibarra-Gonzalez, I.; Fernandez-Lainez, C.; Barrientos-Rios, R.; Monroy-Santoyo, S.; Guillén-López, S.; Alcántara-Ortigoza, M. Phenylalanine hydroxylase deficiency in Mexico: Genotype–phenotype correlations, BH4 responsiveness and evidence of a founder effect. Clin. Genet. 2015, 88, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Vela-Amieva, M.; Ibarra-González, I.; del Alba Herrera-Pérez, L.; Caamal-Parra, G.; Belmont-Martínez, L.; García-Flores, E.P. Epidemiología de la fenilcetonuria obtenida mediante tamiz neonatal. Acta Pediátrica México 2019, 39, 25–34. [Google Scholar] [CrossRef]
- Wilcken, B. Fatty acid oxidation disorders: Outcome and long-term prognosis. J. Inherit. Metab. Dis. 2010, 33, 501–506. [Google Scholar] [CrossRef]
- Lüders, A.; Blankenstein, O.; Brockow, I.; Ensenauer, R.; Lindner, M.; Schulze, A.; Nennstiel, U. Neonatal screening for congenital metabolic and endocrine disorders: Results from Germany for the years 2006–2018. Dtsch. Ärzteblatt Int. 2021, 118, 101. [Google Scholar]
- Feuchtbaum, L.; Carter, J.; Dowray, S.; Currier, R.J.; Lorey, F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet. Med. 2012, 14, 937–945. [Google Scholar] [CrossRef]
- Iafolla, A.K.; Thompson Jr, R.J.; Roe, C.R. Medium–chain acyl-coenzyme A dehydrogenase deficiency: Clinical course in 120 affected children. J. Pediatr. 1994, 124, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Nennstiel-Ratzel, U.; Arenz, S.; Maier, E.M.; Knerr, I.; Baumkötter, J.; Röschinger, W.; Liebl, B.; Hadorn, H.-B.; Roscher, A.A.; von Kries, R. Reduced incidence of severe metabolic crisis or death in children with medium chain acyl-CoA dehydrogenase deficiency homozygous for c. 985A> G identified by neonatal screening. Mol. Genet. Metab. 2005, 85, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Dantés, O.; Flamand, L.; Cerecero-García, D.; Morales-Vazquez, M.; Serván-Mori, E. Origin, impacts and potential solutions to the fragmentation of the Mexican health system: A consultation to key actors. Health Res. Policy Syst. 2023, 21, 80. [Google Scholar] [CrossRef]
- Yusuf, C.; Sontag, M.K.; Miller, J.; Kellar-Guenther, Y.; McKasson, S.; Shone, S.; Singh, S.; Ojodu, J. Development of national newborn screening quality indicators in the United States. Int. J. Neonatal Screen. 2019, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Pandor, A.; Eastham, J.; Beverley, C.; Chilcott, J.; Paisley, S. Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: A systematic review. Health Technol Assess 2004, 8, 1–121. [Google Scholar] [CrossRef]
- Feuchtbaum, L.; Cunningham, G. Economic evaluation of tandem mass spectrometry screening in California. Pediatrics 2006, 117, S280–S286. [Google Scholar] [CrossRef]
- Bittles, A.H.; Black, M.L. Consanguinity, human evolution, and complex diseases. Proc. Natl. Acad. Sci. USA 2010, 107, 1779–1786. [Google Scholar] [CrossRef]
- Sass, J.; Hofmann, M.; Skladal, D.; Mayatepek, E.; Schwahn, B.; Sperl, W. Propionic acidemia revisited: A workshop report. Clin. Pediatr. 2004, 43, 837–843. [Google Scholar] [CrossRef]
- Yap, S.; Vara, R.; Morais, A. Post-transplantation Outcomes in Patients with PA or MMA: A Review of the Literature. Adv. Ther. 2020, 37, 1866–1896. [Google Scholar] [CrossRef]
- Barshes, N.R.; Vanatta, J.M.; Patel, A.J.; Carter, B.A.; O’Mahony, C.A.; Karpen, S.J.; Goss, J.A. Evaluation and management of patients with propionic acidemia undergoing liver transplantation: A comprehensive review. Pediatr. Transpl. 2006, 10, 773–781. [Google Scholar] [CrossRef]
- Forny, P.; Hörster, F.; Ballhausen, D.; Chakrapani, A.; Chapman, K.A.; Dionisi-Vici, C.; Dixon, M.; Grünert, S.C.; Grunewald, S.; Haliloglu, G.; et al. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: First revision. J. Inherit. Metab. Dis. 2021, 44, 566–592. [Google Scholar] [CrossRef]
- Frazier, D.M.; Allgeier, C.; Homer, C.; Marriage, B.J.; Ogata, B.; Rohr, F.; Splett, P.L.; Stembridge, A.; Singh, R.H. Nutrition management guideline for maple syrup urine disease: An evidence- and consensus-based approach. Mol. Genet. Metab. 2014, 112, 210–217. [Google Scholar] [CrossRef]
- Häberle, J.; Burlina, A.; Chakrapani, A.; Dixon, M.; Karall, D.; Lindner, M.; Mandel, H.; Martinelli, D.; Pintos-Morell, G.; Santer, R.; et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J. Inherit. Metab. Dis. 2019, 42, 1192–1230. [Google Scholar] [CrossRef] [PubMed]
- Vela-Amieva, M.; Ibarra-González, I.; Monroy-Santoyo, S.; Fernández-Lainez, C.; Guillén López, S.; Belmont Martínez, L.; Hernández Montiel, A.; González del Angel, A.; Ruiz-García, M.; Sánchez-Pérez, M.C.; et al. Modelo de atención inicial de la fenilcetonuria y otras hiperfenilalaninemias en el Instituto Nacional de Pediatría. Acta Pediátrica México 2010, 6, 297. [Google Scholar]
- Vela-Amieva, M.; Ibarra-González, I.; Fernández-Lainez, C.; Belmont-Martínez, L. Fundamentos teórico-prácticos para la toma correcta de la muestra de sangre del talón para el tamiz neonatal. Acta Pediátrica México 2012, 33, 273–278. [Google Scholar] [CrossRef]
- López, S.G.; López-Mejía, L.; Carrillo-Nieto, R.I.; Martínez, L.B.; González, I.I.; Fernández-Lainez, C.; Vela-Amieva, M. Seguimiento bioquímico y dosis de medicamentos de EIM del metabolismo intermedio. Acta Pediátrica México 2023, 44, 75–82. [Google Scholar] [CrossRef]
- Sebastião, F.M.; Michelin-Tirelli, K.; Bender, F.; Lopes, F.F.; Moraes, I.; Kubaski, F.; Giugliani, R.; Burin, M. COVID-19 impact on the diagnosis of Inborn Errors of Metabolism: Data from a reference center in Brazil. Genet. Mol. Biol. 2021, 45, e20210253. [Google Scholar] [CrossRef] [PubMed]
- Özalp Akın, E.; Eminoğlu, F.T.; Doğulu, N.; Koç Yekeduz, M.; Öncül, U.; Akpınar, F.; Hayran, G. Unmet Needs of Children with Inherited Metabolic Disorders in the COVID-19 Pandemic. Turk. Arch. Pediatr. 2022, 57, 335–341. [Google Scholar] [CrossRef]
- Gray, J.M.; Patnick, J.; Blanks, R. Maximising benefit and minimising harm of screening. BMJ 2008, 336, 480–483. [Google Scholar] [CrossRef]
- Odenwald, B.; Brockow, I.; Hanauer, M.; Lüders, A.; Nennstiel, U. Is Our Newborn Screening Working Well? A Literature Review of Quality Requirements for Newborn Blood Spot Screening (NBS) Infrastructure and Procedures. Int. J. Neonatal Screen. 2023, 9, 35. [Google Scholar] [CrossRef]
- Maier, E.M.; Mütze, U.; Janzen, N.; Steuerwald, U.; Nennstiel, U.; Odenwald, B.; Schuhmann, E.; Lotz-Havla, A.S.; Weiss, K.J.; Hammersen, J. Collaborative evaluation study on 18 candidate diseases for newborn screening in 1.77 million samples. J. Inherit. Metab. Dis. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Feuchtbaum, L.; Yang, J.; Currier, R. Follow-up status during the first 5 years of life for metabolic disorders on the federal Recommended Uniform Screening Panel. Genet. Med. 2018, 20, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B. 3-Methylcrotonyl-CoA carboxylase deficiency: To screen or not to screen? J. Inherit. Metab. Dis. 2016, 39, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, S.; Fitzgerald, K.; Weiss, B.; Ficicioglu, C. Thirteen patients with MAT1A mutations detected through newborn screening: 13 years’ experience. JIMD Rep. 2014, 14, 71–76. [Google Scholar]
- Woerner, A.C.; Gallagher, R.C.; Vockley, J.; Adhikari, A.N. The Use of Whole Genome and Exome Sequencing for Newborn Screening: Challenges and Opportunities for Population Health. Front. Pediatr. 2021, 9, 663752. [Google Scholar] [CrossRef]
- Petrikin, J.E.; Cakici, J.A.; Clark, M.M.; Willig, L.K.; Sweeney, N.M.; Farrow, E.G.; Saunders, C.J.; Thiffault, I.; Miller, N.A.; Zellmer, L.; et al. The NSIGHT1-randomized controlled trial: Rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genom. Med. 2018, 3, 6. [Google Scholar] [CrossRef]
- Peterson, B.; Hernandez, E.J.; Hobbs, C.; Malone Jenkins, S.; Moore, B.; Rosales, E.; Zoucha, S.; Sanford, E.; Bainbridge, M.N.; Frise, E.; et al. Automated prioritization of sick newborns for whole genome sequencing using clinical natural language processing and machine learning. Genome Med. 2023, 15, 18. [Google Scholar] [CrossRef]
- Hörster, F.; Baumgartner, M.R.; Viardot, C.; Suormala, T.; Burgard, P.; Fowler, B.; Hoffmann, G.F.; Garbade, S.F.; Kölker, S.; Baumgartner, E. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut−, cblA, cblB). Pediatr. Res. 2007, 62, 225–230. [Google Scholar] [CrossRef]
- Hörster, F.; Tuncel, A.T.; Gleich, F.; Plessl, T.; Froese, S.D.; Garbade, S.F.; Kölker, S.; Baumgartner, M.R.; Additional Contributors from E-IMD. Delineating the clinical spectrum of isolated methylmalonic acidurias: cblA and mut. J. Inherit. Metab. Dis. 2021, 44, 193–214. [Google Scholar] [CrossRef]
- Chen, T.; Fan, C.; Huang, Y.; Feng, J.; Zhang, Y.; Miao, J.; Wang, X.; Li, Y.; Huang, C.; Jin, W. Genomic Sequencing as a First-Tier Screening Test and Outcomes of Newborn Screening. JAMA Netw. Open 2023, 6, e2331162. [Google Scholar] [CrossRef]
- Lalonde, E.; Rentas, S.; Lin, F.; Dulik, M.C.; Skraban, C.M.; Spinner, N.B. Genomic Diagnosis for Pediatric Disorders: Revolution and Evolution. Front. Pediatr. 2020, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Yubero, D.; Brandi, N.; Ormazabal, A.; Garcia-Cazorla, À.; Pérez-Dueñas, B.; Campistol, J.; Ribes, A.; Palau, F.; Artuch, R.; Armstrong, J. Targeted Next Generation Sequencing in Patients with Inborn Errors of Metabolism. PLoS ONE 2016, 11, e0156359. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Schlecht, H.; Heptinstall, L.E.; Bassett, J.K.; Cartwright, E.; Bhaskar, S.S.; Urquhart, J.; Broomfield, A.; Morris, A.A.; Jameson, E.; et al. Diagnosing childhood-onset inborn errors of metabolism by next-generation sequencing. Arch. Dis. Child. 2017, 102, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Sexto, A.; Gallego, D.; Leal, F.; Castejón-Fernández, N.; Navarrete, R.; Alcaide, P.; Couce, M.L.; Martín-Hernández, E.; Quijada-Fraile, P.; Peña-Quintana, L.; et al. Identification of Clinical Variants beyond the Exome in Inborn Errors of Metabolism. Int. J. Mol. Sci. 2022, 23, 12850. [Google Scholar] [CrossRef]
- Ibarra-González, I.; Fernández-Lainez, C.; Alcántara-Ortigoza, M.A.; González-Del Angel, A.; Fernández-Henández, L.; Guillén-López, S.; Belmont-Martínez, L.; López-Mejía, L.; Varela-Fascinetto, G.; Vela-Amieva, M. Mutational spectrum of Mexican patients with tyrosinemia type 1: In silico modeling and predicted pathogenic effect of a novel missense FAH variant. Mol. Genet. Genom. Med. 2019, 7, e937. [Google Scholar] [CrossRef]
- Taruscio, D.; Salvatore, M.; Lumaka, A.; Carta, C.; Cellai, L.L.; Ferrari, G.; Sciascia, S.; Groft, S.; Alanay, Y.; Azam, M. Undiagnosed diseases: Needs and opportunities in 20 countries participating in the Undiagnosed Diseases Network International. Front. Public Health 2023, 11, 1079601. [Google Scholar] [CrossRef]
- Brasil, S.; Leal, F.; Vega, A.; Navarrete, R.; Ecay, M.J.; Desviat, L.R.; Riera, C.; Padilla, N.; de la Cruz, X.; Couce, M.L.; et al. Improving the diagnosis of cobalamin and related defects by genomic analysis, plus functional and structural assessment of novel variants. Orphanet J. Rare Dis. 2018, 13, 125. [Google Scholar] [CrossRef]
- Pupavac, M.; Tian, X.; Chu, J.; Wang, G.; Feng, Y.; Chen, S.; Fenter, R.; Zhang, V.W.; Wang, J.; Watkins, D.; et al. Added value of next generation gene panel analysis for patients with elevated methylmalonic acid and no clinical diagnosis following functional studies of vitamin B12 metabolism. Mol. Genet. Metab. 2016, 117, 363–368. [Google Scholar] [CrossRef]



| Detected in Our Center | Referred from Other Centers ** | ||||||
|---|---|---|---|---|---|---|---|
| n = 727 | n = 197 | ||||||
| Screened | Unscreened | Screened | Unscreened | Total Number of Patients | Gene | ICD-11 * | |
| n = 162 (22.3%) | n = 565 (77.7%) | n = 131 (66.4%) | n = 66 (33.5%) | n = 924 | |||
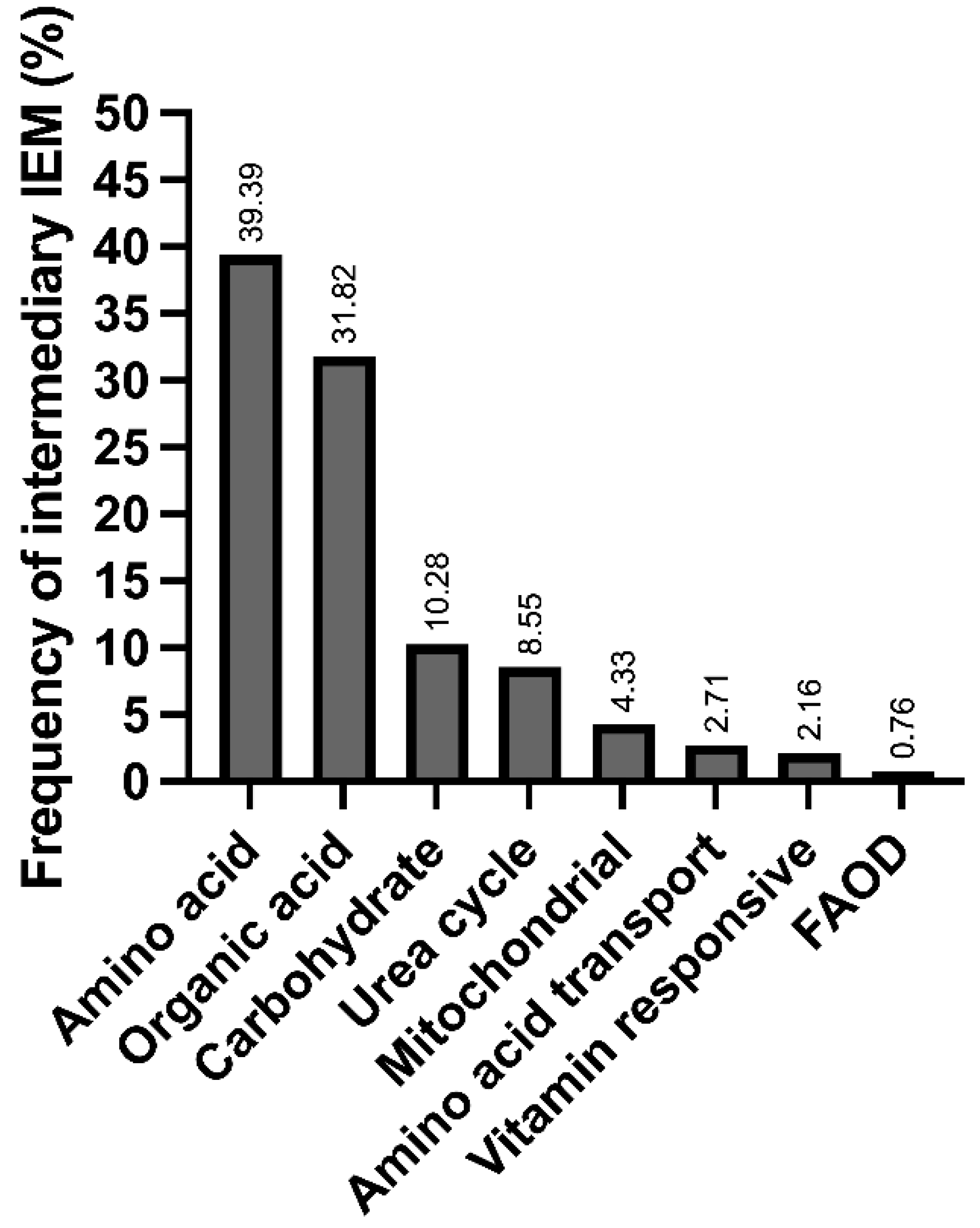
| Detectable by NBS (amino acids, acylcarnitines and succinylacetone) | |||||||
| Hyperphenylalaninemia/Phenylketonuria (HPA/PKU) | 92 | 43 | 52 | 8 | 195 | PAH | 5C50.00 |
| Methylmalonic aciduria (MMA) | 6 | 99 | 3 | 11 | 119 | MUT, MMAA, MMAB, MMACHC, MMADHC, MTRR, LMBRD1, MTR, ABCD4, MCEE, SUCLG1, SUCLA2, ACSF3 | 5C50.E |
| Maple syrup urine disease (MSUD) | 5 | 67 | 1 | 7 | 80 | BCKDHB, BCKDHA, DBT | 5C50.D0 |
| Galactosemia (GALAC) | 1 | 23 | 48 | 7 | 79 | GALT | 5C51.4 |
| Propionic acidemia (PA) | 1 | 37 | 6 | 44 | PCCB, PCCA | 5C50.E0 | |
| 3-Methylcrotonyl-CoA carboxylase 1 deficiency (MCC) | 21 | 9 | 7 | 37 | MCCC1 | 5C50.DY | |
| Isovaleric acidemia (IVA) | 2 | 24 | 1 | 6 | 33 | IVD | 5C50.DY |
| Homocystinaemia (HCY) | 24 | 3 | 27 | CBS | 5C50.B | ||
| Fatty acid oxidation disorders (FAOD) | 7 | 17 | 2 | 1 | 27 | SLC22A5, CPT1, SLC22A5, SLC25A20, SLC25A20, CPT2, ACADVL, ACADM, ACADS, ACAD9, ECHS1, HADHA, HADHB, HADHSC, NADK2, MECR, ETFA, ETFB, ETFDH | 5C52.0 |
| Citrullinemia (CIT-1) | 5 | 15 | 2 | 3 | 25 | ASS1 | 5C50.A3 |
| Tyrosinemia Type I (TYR-1) | 1 | 23 | 1 | 25 | FAH | 5C50.11 | |
| Argininemia (ARG) | 4 | 17 | 2 | 23 | ARG1 | 5C50.A2 | |
| Tetrahydrobiopterin deficiencies (BH4D) | 3 | 14 | 1 | 1 | 19 | PTS, QDPR, PCBD1 | 5C50.0Y/5C59 |
| Glutaric acidemia type 1 (GA) | 2 | 12 | 1 | 2 | 17 | GCDH | 5C50.E |
| 3-hydroxy-3-methylglutaric aciduria (HMG) | 3 | 12 | 1 | 16 | HMGCL | 5C52.02 | |
| Biotinidase deficiency (BTD) | 2 | 1 | 7 | 1 | 11 | BTD | 5C50.E0 |
| Multiple carboxylase deficiency (MCD) | 9 | 9 | HLCS | 5C50.E | |||
| Beta-ketothiolase deficiency (BKT) | 8 | 8 | ACAT1 | 5C50.DY | |||
| Argininosuccinic aciduria (ASA) | 1 | 4 | 1 | 1 | 7 | ASL | 5C50.A0 |
| Hypermethioninemia, isolated persistent (MET) | 5 | 1 | 6 | MAT1A | EC50.B | ||
| 2-methyl-3-hydroxybutyryl Co-A dehydrogenase deficiency (MHDB) | 3 | 3 | HSD17B10 | 5C52.01 | |||
| 3-Methylglutaconic aciduria (3MGA) | 3 | 3 | AUH | 5C50.DY | |||
| Ethylmalonic encephalopathy (EE) | 1 | 2 | 3 | ETHE1 | 5C50.E | ||
| Glycine encephalopathy (GE) | 3 | 3 | AMT, GLDC, GCSH | 5C50.70 | |||
| Gyrate atrophy of choroid and retina (GACR) | 3 | 3 | OAT | 5C50.9 | |||
| Succinyl CoA:3-oxoacid CoA transferase deficiency (SCOT) | 2 | 2 | OXCT1 | 5C52.02 | |||
| Tyrosinemia type 3 (TYRSN3) | 2 | 2 | HPD | 5C50.1 | |||
| 2-hydroxyglutaric aciduria (D2HGA1) | 1 | 1 | SLC25A1 | 5C50.E1 | |||
| Hyper-β-alaninemia (HBA) | 1 | 1 | Unknown | 5C55.1 | |||
| Not detectable to NBS (amino acids, acylcarnitines and succinylacetone) | |||||||
| Cystinosis (CTNS) | 24 | 24 | CTNS | 5C60.1 | |||
| Ornithine transcarbamylase deficiency (OTC) | 24 | 24 | OTC | 5C50.A3 | |||
| Glycogen storage disease (GSD) | 14 | 1 | 15 | G6PC, AGL, GBE1, PYGM, PHKA2 | 5C51.3 | ||
| Lactic acidemias and other mitochondrial disorders (MIT) | 11 | 2 | 13 | PDX1, SURF1, BCS1L | 5C53 | ||
| Lipoprotein lipase deficiency (LPL) | 7 | 7 | LPL | 5C80.1 | |||
| N-acetyl aspartic aciduria or Canavan disease (CD) | 6 | 6 | ASPA | 5C50.E1 | |||
| Alkaptonuria (AKU) | 2 | 2 | HGD | 5C50.10 | |||
| Glycerol kinase deficiency (GKD) | 2 | 2 | GK | 5C51.1 | |||
| Glucose-galactose malabsorption (GGM) | 1 | 1 | SLC5A1 | 5C61.0 | |||
| Hartnup disease (HND) | 1 | 1 | SLC6A19 | 5C60.Y | |||
| MTHFR deficiency (MTHFRD) | 1 | 1 | MTHFR | 5C63.1 | |||
| Mexican State | Approximate Travel Distance to the Reference Center at INP (km) | Number (%) IEiM Patients | |
|---|---|---|---|
| Short travel distance | CDMX | 0 | 177 (19.2) |
| México | 63 | 116 (12.6) | |
| Morelos | 75 | 12 (1.3) | |
| Hidalgo | 91 | 46 (5.0) | |
| Tlaxcala | 133 | 7 (0.8) | |
| Puebla | 147 | 30 (3.2) | |
| Long travel distance | Querétaro | 212 | 32 (3.5) |
| Michoacán | 298 | 34 (3.7) | |
| Veracruz | 308 | 37 (4.0) | |
| Guanajuato | 330 | 49 (5.3) | |
| Guerrero | 368 | 26 (2.9) | |
| San Luis Potosí | 424 | 13 (1.4) | |
| Oaxaca | 474 | 31 (3.4) | |
| Colima | 477 | 3 (0.32) | |
| Aguascalientes | 502 | 14 (1.5) | |
| Jalisco | 536 | 49 (5.3) | |
| Zacatecas | 635 | 10 (1.1) | |
| Chiapas | 705 | 16 (1.7) | |
| Nayarit | 740 | 4 (0.4) | |
| Tabasco | 762 | 68 (7.3) | |
| Durango | 772 | 6 (0.6) | |
| Coahuila | 818 | 2 (0.2) | |
| Campeche | 910 | 3 (0.3) | |
| Nuevo León | 917 | 12 (1.3) | |
| Tamaulipas | 954 | 10 (1.1) | |
| Sinaloa | 1218 | 18 (1.2) | |
| Yucatán | 1323 | 15 (1.6) | |
| Chihuahua | 1559 | 20 (2.1) | |
| Quintana Roo | 1621 | 7 (0.8) | |
| Baja California Sur | 1677 | 5 (0.5) | |
| Sonora | 1890 | 11 (1.2) | |
| Baja California | 2300 | 2 (0.2) | |
| No data | 39 (4.2) |
| Total of Patients n = 637 | Screened n = 162 | Unscreened n = 475 |
|---|---|---|
| Median age at arrival to our Center in months (IQR) a | 1.60 (0.93−2.82) **** | 10.10 (1.90−33.37) |
| Min (days) − Max (months) | 1−12.1 | 1−238.43 |
| General mortality | 8/162 (4.94%) **** | 123/475 (25.89%) |
| Adjusted mortality b | 7/44 (15.91%) * | 123/423 (29.08%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra-González, I.; Fernández-Lainez, C.; Vela-Amieva, M.; Guillén-López, S.; Belmont-Martínez, L.; López-Mejía, L.; Carrillo-Nieto, R.I.; Guillén-Zaragoza, N.A. A Review of Disparities and Unmet Newborn Screening Needs over 33 Years in a Cohort of Mexican Patients with Inborn Errors of Intermediary Metabolism. Int. J. Neonatal Screen. 2023, 9, 59. https://doi.org/10.3390/ijns9040059
Ibarra-González I, Fernández-Lainez C, Vela-Amieva M, Guillén-López S, Belmont-Martínez L, López-Mejía L, Carrillo-Nieto RI, Guillén-Zaragoza NA. A Review of Disparities and Unmet Newborn Screening Needs over 33 Years in a Cohort of Mexican Patients with Inborn Errors of Intermediary Metabolism. International Journal of Neonatal Screening. 2023; 9(4):59. https://doi.org/10.3390/ijns9040059
Chicago/Turabian StyleIbarra-González, Isabel, Cynthia Fernández-Lainez, Marcela Vela-Amieva, Sara Guillén-López, Leticia Belmont-Martínez, Lizbeth López-Mejía, Rosa Itzel Carrillo-Nieto, and Nidia Alejandra Guillén-Zaragoza. 2023. "A Review of Disparities and Unmet Newborn Screening Needs over 33 Years in a Cohort of Mexican Patients with Inborn Errors of Intermediary Metabolism" International Journal of Neonatal Screening 9, no. 4: 59. https://doi.org/10.3390/ijns9040059
APA StyleIbarra-González, I., Fernández-Lainez, C., Vela-Amieva, M., Guillén-López, S., Belmont-Martínez, L., López-Mejía, L., Carrillo-Nieto, R. I., & Guillén-Zaragoza, N. A. (2023). A Review of Disparities and Unmet Newborn Screening Needs over 33 Years in a Cohort of Mexican Patients with Inborn Errors of Intermediary Metabolism. International Journal of Neonatal Screening, 9(4), 59. https://doi.org/10.3390/ijns9040059







